Research Article Open Access
Neurological Complications of Novel Influenza A (H1N1) pdm09 Infection: Report of Two Cases and a Systematic Review of the Literature
| Hussein Algahtani1*, Bader Shirah2, Sulaiman Alkhashan3 and Raafat Ahmad4 | |
| 1King Abdulaziz Medical City/King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia | |
| 2King Abdullah International Medical Research Center/King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia | |
| 3Department of Neurology, Farwanyia Hospital, Kuwait | |
| 4Faculty of Medicine, Suez Canal University, Egypt | |
| *Corresponding Author : | Dr. Hussein Algahtani P.O. Box: 12723, Jeddah, Saudi Arabia Tel: 00966556633130 E-mail: halgahtani@hotmail.com |
| Received: December 29, 2015; Accepted: January 28, 2016; Published: January 30, 2016 | |
| Citation: Algahtani H, Shirah B, Alkhashan S, Ahmad R (2016) Neurological Complications of Novel Influenza A (H1N1) pdm09 Infection: Report of Two Cases and a Systematic Review of the Literature. J Neuroinfect Dis 7:201. doi:10.4172/2314-7326.1000201 | |
| Copyright: © 2016 Algahtani H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Background: Neurological complications of Influenza A (H1N1) pdm09 in adults are rarely reported in the literature. The aim of this study is to report a case of Influenza A (H1N1) pdm09 acute necrotizing encephalitis (ANE) and another case of acute hemorrhagic leukoencephalopathy (AHLE) and to review the neurological complications of this disorder in adults. Methods: We conducted a search on Medline, Ovid, EMBASE, ProQuest and PubMed databases using the key words (neurological complications of H1N1 in adults). We also added to the search results those articles which were not found in the search but exist in the literature under different search words. Only papers written in English published from March 2009 to December 2015 were included. Demographic data and clinical diagnosis of neurological complications and outcomes in adults including death, neurological sequalea or recovery after influenza A (H1N1) pdm09 infection have been reviewed. Results: we included 37 articles presenting a total of 261 adult patients with neurologic complications following Influenza A (H1N1) pdm09 infection. In addition, a case of influenza A (H1N1) pdm09 acute necrotizing encephalitis (ANE) and another case of acute hemorrhagic leukoencephalopathy (AHLE) arising from our own neurological practice were also included. Conclusion: In conclusion, this study demonstrates that influenza A (H1N1) pdm09 is both a pandemic and a seasonal (sporadic) disease. We speculate that most of the neurological complications are immune mediated and calling for further studies to test for the potential benefits of early steroids use in the disease. We are calling for an international collaboration and registry for Influenza A (H1N1) pdm09 infection.
| Keywords |
| Influenza A; Neurological complications; H1N1 pandemic |
| Introduction |
| In June 2009, the World Health Organization (WHO) has declared a global pandemic of a new strain of swine-originated influenza A (H1N1) pdm09 with continuous occurrence of number of sporadic cases. This is considered the first pandemic of the 21st century, which began in Mexico, followed by cases reported in the United States and across the world [1]. Fortunately, most cases are mild with complete and uneventful recovery. However, several cases of severe infection with pulmonary and systemic complications including death have been reported [2]. Influenza-related neurological complications are rare in immuno competent adults with variable clinical signs and pathology. These complications include encephalitis, Reye syndrome, Guillain- Barré syndrome, transverse myelitis and acute disseminated encephalomyelitis (ADEM) [3]. In March 2010, almost all countries reported cases of influenza A (H1N1) and more than 17,700 deaths (laboratory confirmed cases) had been documented [1]. Up to date, there are no accurate data regarding the global frequency of neurological complications related to this disease [4]. The incidence of neurological complications in influenza A (H1N1) pdm09 was estimated to be 3.7% of the total number of patients confirmed to have influenza A (H1N1) pdm09 [5]. |
| Patients and Methods |
| Patient selection, data acquisition, and outcome measure. We report life-threatening specific complications of influenza A (H1N1) pdm09 infection in two adult patients that was responsible for grave consequences. We also conducted a systematic review in Ovid, Medline, EMBASE, ProQuest and PubMed database using the key word (neurological complications of H1N1 in adults). One clinical neurologist and one medical student performed a full-text review of the papers and extracted all relevant data retrospectively. Inclusion criteria for this review included studies reporting clinical and laboratory data on neurological complication of influenza A (H1N1) pdm09 in adults. Data extracted included patients demographics, past medical history, details of neurological complications, interventions and treatment, and patients' outcome. Disagreement on consensus on inclusion or exclusion was arbitrated by a third author. The search included all study types, including abstracts and pending publications available. No non-electronic-versions were requested since the pandemic is a recent event. Bibliographies were reviewed to identify additional articles and studies. Adult patients were considered as more than 18 years old and only papers written in English were included. Those papers written in other languages or poorly written with deficient relevant clinical and epidemiological data were excluded. |
| Case Report 1 |
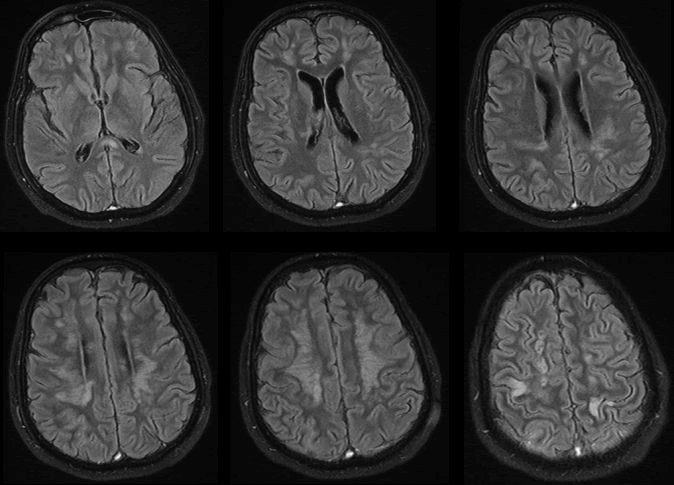
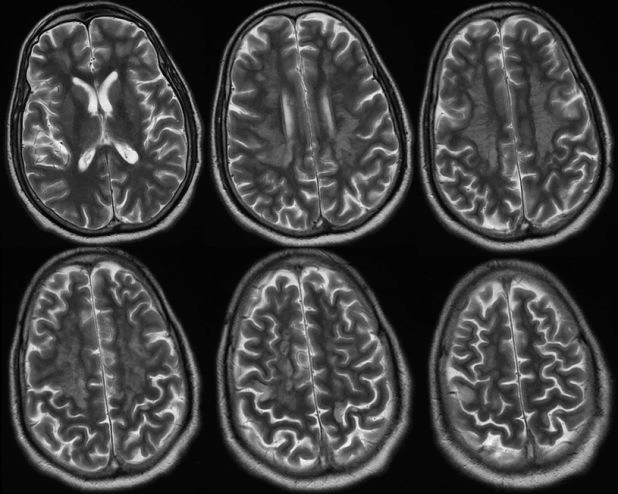
| 53 years old man, known hypertensive and diabetic, was brought to emergency department with left side weakness that started one day prior to presentation. In fact, his symptoms started one week ago with flu-like symptoms and headache. His conditions had deteriorated and he became drowsy and desaturated. He was intubated, mechanical inflated and shifted to ICU. Later on, he developed acute kidney injury and circulatory compromise requiring ionotropic supports. MRI of the brain showed diffused bilateral cortical, subcortical and periventricular white matter signal changes with involvement of the splenium of the corpus callosum. These changes were high signal changes on FLAIR, diffusion weighted imaging (DWI) and T2 sequences and low signals on T1. MRI findings were consistent with viral encephalitic changes with scattered foci of ischemia (Figures 1 and 2). |
| CSF analysis was unremarkable including cytology, biochemistry and microbiology. Chest radiograph showed evidence of infiltrate scatter in lower mid zones. Apart from impaired renal function test, the rest of his biochemical profile was unremarkable. H1N1 PCR from nasopharyngeal swap came back positive in two occasions. The patient was started broad-spectrum antibacterial therapy and supportive management. Unfortunately, his condition has deteriorated further and he deceased. |
| Case Report 2 |
| 46 years old previously healthy male, presented to emergency department with fever and shortness of breath of two days duration. Initial examination revealed a fully conscious and oriented gentleman with normal neurological examination. He was febrile (39.5 Co) and hypoxic (oxygen saturation 85%). On arrival, he was isolated, intubated, and mechanically ventilated. |
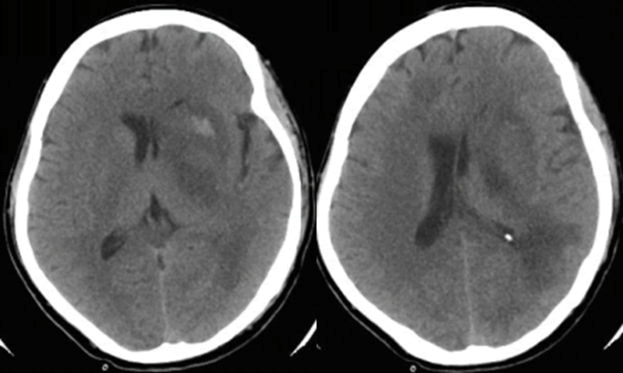
| His initial investigations revealed abnormal chest X-ray with bilateral multiple lung consolidations. His C-reactive protein and ESR were high and his white blood cells count showed neutrophilia and lymphopenia. The rest of his biochemical profile was normal. Dull septic screen was done including throat swab, blood, urine, and endotracheal secretion. H1N1PCR was positive and the rest of the septic workup was unremarkable. The patient was started on Oseltamivir, broad-spectrum antibiotics, and rehydration. Although he showed some improvements initially, his level of consciousness deteriorated on hospital day 13. His neurological examination revealed focal lateralizing signs indicating multifocal disease including asymmetrical weakness, brisk refluxes and bilateral Babinski sign. Immediate CT scan showed multiple hypodense and hyperdense areas involving frontal and parietal lobes and the basal ganglia with diffuse cerebral edema (Figure 3). |
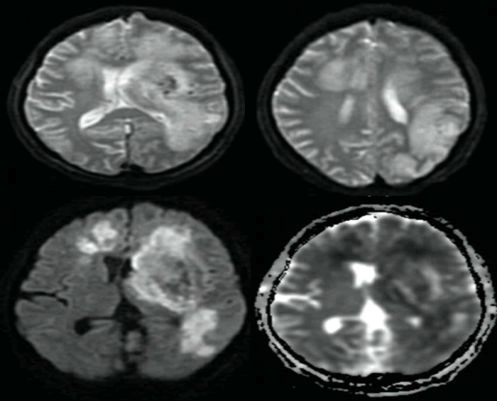
| The images were suggestive of the diagnosis of acute hemorrhagic leukoencephalopathy. MRI of the brain was done on day 15, and it showed more striking abnormalities with medline shift, blood signals, and areas of restricted diffusion in ADC images (Figure 4). |
| The patient was started on pulse steroid therapy and mannitol. Unfortunately, the patient condition deteriorated with progression of cerebral edema and tonsillar herniation, and six days later the patient deceased. |
| Results |
| Many articles were initially retrieved, but some were eliminated because of age (children). Finally, considering our inclusion criteria and adding other articles missed in the search, only 37 articles (261 patients) were included. After adding our patients, the total number of patients in our study was 263 patients. Mean age of infection-related neurologic complications was 39.29 ± 17.06. Twenty nine patients were male and twenty-one were female; there was no gender information on the 213 remaining patients. Neurological complication was grouped into Ten groups (Table 1). |
| The most common group was encephalopathy/encephalitis group followed by the meningitis group. Seizures and status epilepticus were not uncommon affecting 14 patients (5.32%). Among the catastrophic groups were clinical presentation with acute necrotizing encephalopathy and acute hemorrhagic leukoencephalopathy. Common premorbid conditions include asthma, cancer, and various other chronic diseases. Eleven patients died (4.18%) and eight (3.04%) developed permanent neurological damage (Table 2). |
| The most common cause of death in most studies was acute necrotizing encephalopathy, which is consistent with our study. Cerebrospinal fluid (CSF) analysis was done in 35 patients, and 19 (54.2%) were abnormal and 2 were positive for H1N1 PCR. Although CT scan of the brain was done in few studies, the gold standard neuroimaging tool for diagnosing the neurological complications of H1N1 was MRI. Important sequences requested were T1, T2, DWI, and FLAIR. |
| Discussion |
| Influenza A infection may cause a wide range of clinicopathological entities, many have been reported and well-recognized in the literature. While mostly diagnosed in children, neurological complications of pandemic influenza A (H1N1) pdm09 may also affect adults with occasional fatal consequences [6]. Neurological manifestations may affect any level of the neuraxis with varying severity and prognosis. |
| The course of these presentations ranges from full resolution of symptoms to an aggressive disease rapidly culminating in fatality [7]. Involvement of the central nervous system in influenza A virus infection may include seizures, encephalitis, myelitis, Reye syndrome, and Guillain-Barré syndrome. Other neurologic manifestations of influenza are now known to include acute disseminated encephalomyelitis (ADEM), and acute necrotizing encephalopathy (ANE) [8]. According to a study done be Cardenas et al, fatal cases and permanent neurological sequelea were reported in 11% of patients with influenza related neurological complications [4]. This number includes also post-vaccination complications and occurred in all age groups. In our study, we report only the neurological complications related to influenza A (H1N1) pdm09 virus infection in adults. The percentage in our study was 7.22%. |
| The pathogenesis of neurological complications is not well understood and has not been elucidated so far. It is most likely caused by a para- or post- infectious immune mediated mechanisms rather than direct viral invasion of the brain and the spinal cord [9]. |
| This mechanism is well supported by several observations including absence of the virus RNA in the CSF in most studies. In our study, this was also observed, and only 5.71% of CSF samples were positive for H1N1 virus RNA. Another observation is the delay of the onset of neurological complications after the occurrence of the first symptom of the viral infection. Another supporting hypothesis is the correlation of the severity of some neurological complications with the concentration of pro-inflammatory cytokines. These cytokines can induce vascular endothelial injury, increase blood-brain barrier permeability, induce apoptosis of cells (both neurons and glia) and cause acute edema and necrosis involving both grey and white matter [10]. The last observation is the occurrence of several cases of ADEM which is a well-known autoimmune demyelinating disease. |
| Mortality rate in our study was around 4% with a similar percentage of those who had incomplete recovery. Factors possibly contributing to mortality/morbidity include altered mental status at presentation, type of neurological complications (poor prognosis in patients affected by ANE and AHLE), obesity, and vascular insult. |
| Acute necrotizing encephalopathy (ANE), also called influenza associated encephalitis/encephalopathy, is a rare central nervous system complication characterized by altered mental status and seizures. Other symptoms include fever, vomiting, and respiratory symptoms [11]. As mentioned before, it is generally considered to be a post-infectious disease secondary to immune mediated response which is triggered by the virus [12]. Although ANE may affect adults, most cases of ANE reported in the literature are usually children [13]. It is not specific to H1N1 virus infection and association with other organisms such as mycoplasma, herpes simplex virus, and human herpes virus-6 that have been reported in the literature [14]. Laboratory findings in ANE include deranged liver enzymes, increase renal parameters, hypoproteinemia, and abnormal CSF analysis [15]. Neuroimaging usually demonstrates multifocal bilateral periventricular white matter, thalamic, brain stem, or cerebellar changes [16]. On pathology, affected areas of the brain are characterized by the presence of hemorrhages and necrosis [12]. ANE carries a bad prognosis and patients usually end up with either profound disability or death [17]. |
| Acute hemorrhagic leukoencephalopathy (AHLE), also known as Weston-Hurst disease, is an autoimmune condition characterized by an inflammatory response which leads to vascular injury, perivascular demyelination, hemorrhages and necrosis [18]. The exact etiology is unknown. However, a cross-reactivity between human myelin antigens and viral antigens are hypothesized to induce the excessive immunological response. Nemours infections have been reported to be associated with this fatal condition including influenza A, herpes simplex virus, and varicella zoster virus [19]. Due to similarities in etiology and pathogenesis, it is considered a severe variant of ADEM [20]. An animal murine model has been developed by injecting a viral capsid of the Theiler’s murine encephalomyelitis virus inducing a strong in vivo activation of CD8+ T cells and leading to the development of AHLE within one day of injection [21]. The early clinical signs and symptoms are headache, fever, neck pain and stiffness, seizures and altered level of consciousness. These are the same clinical features of ANE, which constitutes a diagnostic challenge for the neurologist. The CSF analysis is almost always abnormal with high protein but cultures and PCR for viruses are often negative. CT scan and MRI demonstrate abnormal widespread changes with hemorrhages and cerebral edema. The diagnosis can only be confirmed by pathology, which typically shows extensive perivascular demyelination, fibrinoid vascular necrosis and hemorrhages [22]. Treatment with plasmapheresis, intravenous immunoglobulin, pulse steroid therapy, and cyclofosfamide has been used with variable success [23]. AHLE is considered a catastrophic disease with poor prognosis and death occurring within days or weeks after the onset of symptoms [22]. |
| Conclusion |
| In conclusion, this study demonstrates that influenza A (H1N1) pdm09 is both a pandemic and a seasonal (sporadic) disease. We speculate that most of the neurological complications are immune mediated and calling for further studies to test for the potential benefits of early steroids use in the disease. We have clear limitations in this study due to the incomplete information in most of the case reports retrieved from the medical literature. However, we presented the best evidence available by reviewing published articles in details. We are calling for an international collaboration and registry for Influenza A (H1N1) pdm09 infection. |
References
- Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Frederick G, et al. (2010) Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 362:1708-1719.
- Dawood FS, Jain S, Finelli L, Massingale S, Pippin T, et al. (2009) Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360:2605-2615.
- Davis LE (2010) Neurologic and muscular complications of the 2009 influenza A (H1N1) pandemic. Curr Neurol Neurosci Rep10:476-483.
- Cardenas G, Soto-hernandez JL, Diaz-alba A,Ugalde Y, Merida-Puga J, et al. (2014) Neurological events related to influenza A (H1N1) pdm09. Influenza Other Respir Viruses 8:339-346.
- Reed C, Chaves SS, Perez A, D'Mello T, Daily Kirley P, et al. (2014) Complications among adults hospitalized with influenza: a comparison of seasonal influenza and the 2009 H1N1 pandemic. Clin Infect Dis 59:166-174.
- Prerna A, Lim JY, Tan NW, Isa MS, Oh HM, et al. (2015) Neurology of the H1N1 pandemic in Singapore: a nationwide case series of children and adults. J Neurovirol 21:491-499.
- Glaser CA, Winter K, Dubray K, Harriman K, Uyeki TM, et al. (2012) A population-based study of neurologic manifestations of severe influenza A(H1N1)pdm09 in California. Clin Infect Dis 55:514-520.
- Takkar A, Goyal M, Modi M, Kharbanda PS, Yaddanapudi L, et al. (2015) Status epilepticus as presenting manifestation of H1N1 infection. International Journal of Epilepsy 2:84-86.
- Hasegawa S, Matsushige T, Inoue H, Shirabe K, Fukano R, et al. (2011) Serum and cerebrospinal fluid cytokine profile of patients with 2009 pandemic H1N1 influenza virus-associated encephalopathy. Cytokine 54:167-72.
- Ito Y, Ichiyama T, Kimura H, Shibata M, Ishiwada N, et al. (1999) Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol 58:420-425.
- Kim KJ, Park ES, Chang HJ, Suh M, Rha DW, et al. (2013) Novel Influenza A (H1N1)-Associated Acute Necrotizing Encephalopathy: A Case Report. Ann Rehabil Med 37:286-290.
- Wu X, Wu W, Pan W, Wu L, Liu K, et al. (2015) Acute necrotizing encephalopathy: an underrecognizedclinicoradiologic disorder. Mediators Inflamm2015:792578.
- Lee YJ, Smith DS, Rao VA,Siegel RD, Kosek J, et al. (2011) Fatal H1N1-Related Acute Necrotizing Encephalopathy in an Adult. Case Rep Crit Care2011:562516.
- Ventura E, Summa A,Ormitti F, Picetti E, Crisi G (2012) Influenza A H1N1 Related Acute Necrotizing Encephalopathy: Radiological Findings in Adulthood. Neuroradiol J 25:397-401.
- Narra R, Mandapalli A, Kamaraju SK (2015) Acute necrotizing encephalopathy in an adult. J Clin Imaging Sci5:20.
- Kim YN, You SJ (2012) A case of acute necrotizing encephalopathy associated with parainfluenza virus infection. Korean J Pediatr 55:147-150.
- Akins PT, Belko J, Uyeki TM, Axelrod Y, Lee KK, et al. (2010) H1N1 encephalitis with malignant edema and review of neurologic complications from influenza. Neurocrit Care 13:396-406.
- Yildiz O, Pul R, Raab P, Hartmann C, Skripuletz T, et al. (2015) Acute hemorrhagic leukoencephalitis (Weston-Hurst syndrome) in a patient with relapse-remitting multiple sclerosis. J Neuroinflammation12:175.
- An SF, Groves M, Martinian L, Kuo LT, Scaravilli F (2002) Detection of infectious agents in brain of patients with acute hemorrhagic leukoencephalitis. J Neurovirol 8:439-446.
- Kuperan S, Ostrow P, Landi MK, Bakshi R (2003) Acute hemorrhagic leukoencephalitis vs ADEM: FLAIR MRI and neuropathology findings. Neurology 60:721-722.
- Pirko I, Suidan GL, Rodriguez M, Johnson AJ (2008) Acute hemorrhagic demyelination in a murine model of multiple sclerosis. J Neuroinflammation5:31.
- Lann MA, Lovell MA, Kleinschmidt-demasters BK (2010) Acute hemorrhagic leukoencephalitis: a critical entity for forensic pathologists to recognize. Am J Forensic Med Pathol 31:7-11.
- Payne ET, Rutka JT, Ho TK, Halliday WC, Banwell BL (2007) Treatment leading to dramatic recovery in acute hemorrhagic leukoencephalitis. J Child Neurol 22:109-113.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 11036
- [From(publication date):
March-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9991
- PDF downloads : 1045
