Neuroimmune Regulation in Health: Acute Febrile Illness and Healing
Received: 05-May-2016 / Accepted Date: 22-Jul-2016 / Published Date: 27-Jul-2016
Abstract
Adaptive Immunity (ADIM) is maintained by Growth hormone (GH), Prolactin (PRL), and during fetal life Placental lactones (PL) fulfill this role. Vasopressin (VP) is also an ADIM regulator. ADIM is also regulated by antigens and by cytokines and chemokines. Innate immunity (INIM) is the second part of our Immune System. This system is with us for life, capable of responding instantaneously and it is with us in acute febrile illness and in other pathological situations. It protects us till the last second of life. The Hypothalamic-Pituitary –Adrenal (HPA) axis and catecholamine’s regulate INIM.The acute phase response (APR), or acute febrile illness, is an emergency defence reaction against infectious disease and towards other pathogens. Here the ADIM system is suppressed and INIM function is significantly amplified. Cytokines, I l -1 beta, Tumor necrosis factor (TNF)-alpha and IL-6 stimulate corticotrophin releasing hormone (CRH), VP secretion and cause “sympathetic outflow”. Colony stimulating factors activate leukocytes. CRH is a powerful activator of the HPA-axis, and elevates glucocorticoid (GC) levels. Cytokines, GC and catecholamine’s (CAT) play fundamental role of INIM amplification. VP supports the APR at this stage, however when the disease turns to chronic, it is VP that will regulate, and not CRH, the chronic disease and proceed to recovery and healing. VP is able to cause recovery as it stimulates the HPA axis and also Prolactin. The ACTHadrenal axis stimulates NATIM and suppressor regulatory (T sr), which suppresses ADIM. It is concluded that VP regulates healing and recovery from disease.
Keywords: Adaptive immunity; Vasopressin; Neuroimmune regulation; Glucocorticoid
5441Introduction
Growth and lactogenic hormones (GLH), e.g. growth hormone (GH), prolactin (PRL) and placental lactogens (PL) are important immunoregulators. Several reviews are available on the subject [1-4]. Recent findings are that B cell differentiation into antibody forming cells is regulated by estradiol (for innate) and by prolactin (for adaptive immune) B cells [5].
The immune system functions as natural immunity (NATIM), synonym innate immunity (INIM). We are born with this immune system and never lose it. Monocyte-macrophages, granulocytes, a subset of B cells producing natural antibodies, Natural killer (NK), T cells, gamma-delta T cells and cells of the “reticulo - endothelial system” (RES) play major roles in INIM. Innate immune cells are fully mature and are capable of instantaneous responses. Such cells also work under catabolic conditions [6,7].
The adaptive or acquired immune response (ADIM) is based on cell proliferation and maturation, thymus derived T cells mediate cellular immunity and bone marrow derived B-cells form antibodies. Mature antigen specific cells enter the blood stream. Recirculate and populate the secondary lymphoid organs, which are the spleen, lymph nodes, and mucosal lymphoid tissue [8].
In Adaptive Immune Cell growth requires anabolic conditions and time is necessary to develop from a few cell clones a whole army of immune cells. So the primary immune response takes 7-10 days, the secondary response is 3-5 days. The ADIM system is regulated by antigen and regulatory T cells which may act by regulatory contact or by cytokines [9].
We distinguish cell mediated and humoral immunity in the ADIM system. Humoral immunity consists of antibodies, serum complement and acute phase proteins. Thymus derived, T cells may be effector (T-E e.g. Helper, killer) (gamma-delta T, and NK-T). The adaptive immune system is regulated by suppressor regulatory T cells (Tsr) and antigen, innate immunity is regulated independently of regulatory T cells [6,9].
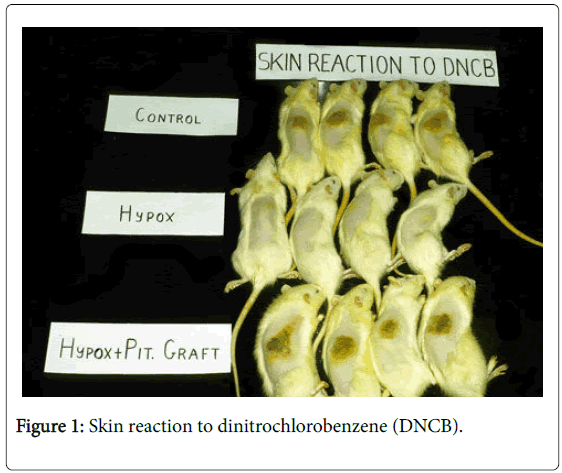
All the systems are regulated by cytokines, hormones, neurotransmitters and neuropeptides. The hypothalamus is the ultimate immunoregulator (Figure 1).
Normal control animals responded with skin inflammation 5 days after skin painting with DNCB. Hypophysectomized (Hypox) animals did not respond to DNCB challenge. Syngeneic pituitary grafts transplanted under the kidney capsule fully restored immune reactivity of Hypox rats. Note: Unlike in other species, which need to be sensitized first in order to react to DNCB, rats show natural immunity to DNCB, so they react to the first challenge.
Hormonal Regulation of Immunocompetence
We discovered immunoregulation by the pituitary gland in 1978 [10]. Cell mediated immunity to dinitrchlorobenzene was pituitary dependent. Antibody responses were also depended on pituitary hormones. The secondary antibody response was only partially dependent on pituitary function. The rejection of skin grafts and the development of adjuvant arthritis were also depended on growth and lactogenic hormones [11]. The dopamine antagonist, bromocriptine (BRC), which inhibits pituitary hormone secretion, was as effective in immunosuppression as were hyphophysectomized (Hypox) rats [12]. Adrenocorticotropic hormone (ACTH) and glucocorticoids (GC) antagonized the restoration of adaptive immune function by GLH in Hypox rats. (Figure 1) [13,14]. GLH regulates bone marrow function [15].
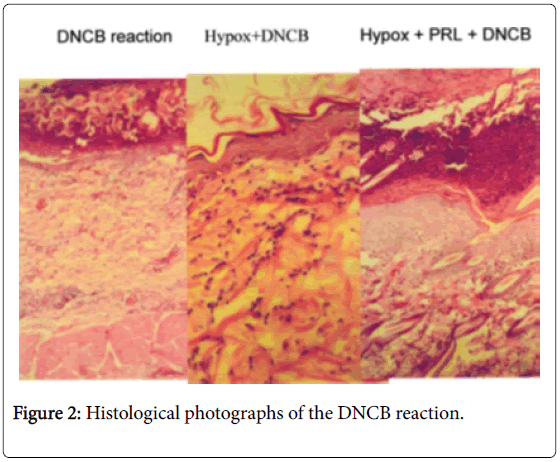
Passive cutaneous hypersensitivity was not affected by Hypox (unpublished). Experimental allergic encephalomyelitis (EAE) could not be induced 10 days after Hypox, but after 21 days the animals responded with EAE [16]. Hypox rats restore their PRL levels (up to 50% by day 63) thus become immunocompetent (Figure 2).
Treatment of Hypox rats with prolactin (PRL) restores immunocompetence. Normal animals react with mononuclear cell infiltration of the challenge site; Hypox animals have no such infiltration. In Hypox animals are treated with PRL, the inflammatory response is fully restored.
Cell growth and DNA, RNA synthesis in the thymus spleen and bone marrow are dependent on GLH. Nucleic acid synthesis in lymphoid tissue showed a direct correlation with immunocompetence. Other experiments showed that the magnitude of tumor necrosis factor (TNF) response to bacterial lypopolysacharide (LPS) endotoxin was controlled by the HPA axis. In adrenalectomized (ADR-X) mice TNF levels rose 60 times over normal control levels within 30 minutes of lethal LPS injection. The lethal dose was reduced 500 times in (ADR-X) mice. Treatment with dexamethasone normalised ADR-X mice [17,18].
Mice with knockout genes for prolactin or its receptor are immunocompetent [19-22]. This could be expected as such mice do have GH which can maintain immune function [19-22].
The Regulation of Adaptive Immunity by Regulatory/ suppressor T (Tsr) Cells
During our original studies replacement doses of GLH stimulated and similar doses of ACTH suppressed adaptive immunity in Hypoxed rats. These hormones were designated as immunoregulatory.
Other hormones (e,g. steroid, thyroid hormones) were classified as immunomodulatory hormones. Initially it was assumed that the HPA axis supresses the thymus and T cell function via GC-induced apoptosis. However, the idea of having suppressor cells was much more compelling than having hormones as immunoregulators. So the idea of suppressor/regulatory (Tsr) cells and of other suppressor cells (B cess etc.) was favored. Some suppressor cells work by cell-to cell contact others secrete cytokines, (interleukin 10, FGF-beta) which does the job [23].
It is increasingly apparent that GC and KAT stimulate the growth of Tsr and of other suppressor cells. GC and KAT enhance the production of acute phase proteins and thus support the acute phase response (APR).
Acute Illness
The first review of acute illness was published by Hans Selye. He proposed that “nocuous” stimuli (stressors) induce a stress response. Selye recognized that stressed animal exert a defence reaction, which he named the general adaptation syndrome [23]. Today the response to various noxious insults is called the Acute Phase Response (APR) [24,25].
Clinically APR is characterized by fever, loss of appetite, inactivity and sleepiness. Changes in sleep are hallmark of APR to infectious challenge. The regulation of these responses involves a cytokine cascade within the brain including IL-1 and TNF, and several other substances such as growth hormone releasing hormone, PRL. Nitric oxide (NO) and nuclear factor NF-kappa B. These substances also regulate normal sleep [26,27].
Endotoxin, infectious disease and various forms of injury elicit a systemic elevation of IL-1-beta, TNF-alpha and IL-6 which are secreted by cells of the innate immune system, primarily by monocytemacrophages [28]. Colony stimulating factors (CSF) are cytokines originally stimulating the bone marrow [29]. The bone marrow is recognized today as a fundamental organ in natural immunity as it provides the leukocytes that fulfill this function.
The bone marrow is activated during APR which results in the generation of “leucocytosis” [30]. Recent studies indicate that Granulocyte-macrophage cytokines, (GM-CSF); macrophage cytokine, (M-CSF); and granulocyte cytokine (G-CSF); are involved in the maintenance of host resistance to infectious disease [31,32] parasite infestation [33] and cancer [34,35].
GM-CSF has been proposed as a physiological regulator as it provided defence without much disturbance [36]. It stimulates adaptive immunity and immunological tolerance [37,38]. GM-CSF is used today for immunotherapy and for the production of recombinant vaccines [39,40].
In turn the cytokines stimulate the hypothalamus, the bone marrow, liver, and leukocytes directly or indirectly thus eliciting an APR [24,30,41]. Profound changes occur in serum hormone levels, the HPA axis is activated and there is also sympathetic outflow, which raises serum CAT levels. GC exerts a powerful suppressive effect on the adaptive immune system and also controls the level of inflammatory cytokines. However, the idea of having suppressor cells was much more compelling than was hormonal suppression of immunity. Suppressor/regulatory (Tsr) cells and of other suppressor cells (B cell and so on) was confirmed. Some suppressor cells regulate by cell-to cell contact, others secrete cytokines, (interleukin 10, FGF-beta) which does the job [42].
Through the activation of the HPA axis and of the sympathetic nervous system, adaptive immune reactions are profoundly suppressed. Acute phase proteins are produced by the liver and natural antibody production is dramatically increased by a specific subset of B lymphocytes. Therefore the conversion of the immune system from the adaptive mode of reactivity to the amplification of natural immunity takes place [30,43-46].
PRL and GH stimulate the adaptive immune system and usually elevated within the first hour of endotoxin shock, which is followed by a decline and the level may become low normal to sub-normal in serious cases of endotoxin shock. Luteinizing hormone (LH), folliclestimulating hormone (FSH), estrogens, androgens, progesterone, decline during infection and endotoxin shock, as a rule. Insulin, glucagon, alpha-melanocyte stimulating hormone, endorphin, leptin, corticotrophin releasing hormone (CRH) and arginine vasopressin are increase during endotoxemia [2,30,41]. It is clear that dynamic and diurnal changes of hormones should be taken into consideration when hormonal changes are considered in APR. Much remains to be done in this area.
A subset of marrow-derived macrophages in the brain, termed perivascular cells, synthetize prostaglandins after systemic cytokine or endotoxin challenges and play a critical role in the IL-1 induced HPA activation [47]. It has been also observed that peptidergic sensory nerves present in the vagus and elsewhere provide feedback signals to the brain about the sites of local inflammation [48].
Acute-phase Proteins
Protein synthesis in the liver is altered in a major way during APR. The synthesis of acute phase proteins (APP) is dramatically enhanced, whereas some major normal proteins such as albumin and transferrin are significantly decreased. In patients with acute illness, C-reactive protein (CRP) and serum amyloid A (SAA) may increase over 1000- fold within 24-48 h. “fibrinogen-1”- antitrypsin and certain complement and properdin components (e.g. factor B and C3) show a more moderate increase [41,49].
CRP binds to C-type pneumococcal cell walls in presence of Ca2+. It is present in multiple species of mammals, in fish and crab. CRP consists of five identical subunits which form a ring shaped molecule named pentraxin, which also stands for a protein family [47]. CRP recognizes homotopes, which are frequently present on the surface of bacteria, fungi, parasites and in damaged cells and tissues. After combination with specific homotope, CRP activates complement with the classical pathway, which leads to enhanced chemotaxis, activation of phagocytosis by neutrophil leukocytes and macrophages. CRP stimulates the synthesis of IL-1, and TNF-alpha, and potentiates the cytotoxic activity of T-cells, NK cells and platelets. CRP localizes in sites of inflammation it binds to platelet derived growth factor (PAF) and blocks it’s activity. In the Clinics the presence of CRP in a patient means infectious, inflammatory disease [48,49]. Human CRP protects mice from otherwise lethal Streptococcus pneumoniae infection [50].
Lipopolysaccharide binding protein (LBP) shows 100 fold increase in the serum during APR. LBP can opsonize LPS binding particles. LPS activates complement by the alternative pathway. LBP-LPS binds complement and stimulate cytokines from monocyte-macrophages that bind CD 14 and toll-like receptor (TLR)-4. In humans high dose LBP suppressed the binding of both R-type and S-type LPS to CD14 and inhibited nuclear translocation of nFkappaB. LBP mediated the transfer of S-type LPS, to HDL.
Haptoglobin (HG) is an APP that binds hemoglobin, prevents iron loss and alleviates kidney damage. It is an antioxidant, anti-micobial and modulates the APR [51]. HG suppressed in vitro the production of TNF-alpha, IL-10, by LPS-stimulated monocytes. Did not inhibit IL-6, IL-8 and IL-1-receptor antagonist. HG knockout mice were sensitised to LPS [52].
The APP, alpha- (1)-acid glycoprotein and alpha(1)-antitrypsin exert anti-apoptotic anti-inflammatory effects and protect from ischemic kidney disease and from similar conditions [52].
Mannose-binding lectin (MBL) is an APP. It binds to sugar arrays present on many microorganisms. After binding MBL is activates the complement system via the serum protease, MASP-2. MBL mutations lead to frequent autoimmune disease such as systemic lupus erythematosus and rheumatoid arthritis [53]. GH regulates MBL levels [54].
Other APP-s proteinase inhibitors are alpha-2 macroglobulin, alpha-1 acid glycoprotein, antithrombin III, alpha-1 acute phase globulin, and alpha-1 proteinase inhibitor.
Kuppfer cells stimulate alpha-2 macroglobulin synthesis by hepatocytes in nitro in the presence of 10-9 M Dexamethasone.
Fibrinogen is also an APP, important with blood coagulation and healing.
Alpha-macrofetoprotein (alpha-MFP) is a strong inhibitor of inflammatory mediators, such as histamine, bradykinin, serotonin, and prostaglandin E2. It also inhibits polymorphonuclear chemotaxis [40].
Metabolic Effect of APR
Hyperglycemia, and lipolysis are characteristic of APR and catabolism prevails [55,56].
A clinical trial with GH on patients with acute illness did not reveal any advantage of GH. If anything significant, the mortality of treated patients was higher [57]. Apparently it is a mistake to use an anabolic hormone on a catabolic pathologic process. The disease may be aggravated.
Recently it was shown that GH treatment inhibited the production of APP in rats, and with burn injury of humans. This was also observed on human hepatocytes [58,59]. The above hypothesis is confirmed here to be true.
The Regulation Of APP
GC and KAT induce alpha-MFP, synergistically in normal rats [60]. GC stimulated hepatic APR by pro-inflammatory macrophage, migration inhibitory factor and secretion and the expression of cytokine, chemokine receptors [61]. Adrenaline induces a high level of IL-6, which may be inhibited by propranolol. When IL-6 is blocked the fast reacting APP, alpha-2 macroglobulin and cystein proteinase inhibitors are strongly depressed. Isoprenalin a beta-2 adrenergic receptor agonist also cause high levels of IL-6 [62].
IL-6 is a major inducer of APR. IFN-gamma, leukemia inhibitory factor, TGF beta, oncostatin M also induce APP from the liver. IL-6 activates the genes of APP trough the DNA binding protein NF-IL-6. NF-IL-6 is a pleiotropic mediator serving many genes. The inducible genes are Il-6, IL-8 and several acute phase genes [63].
In humans IL-6 exerted a hyperglycemic effect, whereas Il-2 acted the opposite [64].
Immunoconversion In APR
In APR the HPA axis and CAT are active. Glucocorticoids and CAT suppress the T-cell dependent adaptive immune system (ADIM). This suppression is further amplified by the suppression of hormones (PRL; GH; IGF-I), which support this system. APR induces apoptosis in the thymus with striking efficiency. The elevated levels of TNF-alpha, zinc deficiency, which develop during APR, also contribute to thymus involution and the suppression of ADIM [7,22].
Healing
Febrile illness occurs on numerous occasions during a lifetime. Most of these diseases will heal and followed by recovery. We understand a lot about the pathological process, but know little about the healing process. Recent observations revealed that in chronic inflammation it is VP, and not CRH, is elevated. The implication is that VP coordinates recovery and also helps the healing process. VP is well suited for this action as it stimulates the HPA axis and also PRL, so that immune function may develop (immunostimulation) [7,65,66].
Vasopressin and Stress
CRH and VP are released during stress by the elevated levels of epinephrine (EP) and norepinephrine. Seconds later the secretion of ACTH is induced, which elicits the secretion of GC by the adrenal cortex. CRH coordinates the endocrine, autonomic and behavioral and immune responses to stress and also acts as a neurotransmitters or neuromodulators in the amigdala, dorsal raphe nucleus, and hippocampus and locus coeruleus. VP, 5-hydroxitriptamine, CAT, substance P, vasoactive intestinal polypeptide, neuropeptide Y and cholecystokinin are produced in these loci. Cytokines, such as IL-1beta, TNF-alpha and IL-6, stimulate CRH and VP gene expression and are implicated in immune-neuroendocrine regulation. The expression profiles of CRH and VP genes are not uniform after stress exposure and the VP gene appears to be more sensitive to GC suppression [63,64].
Cytokine induced CRH and VP secretion is modulated by CAT, prostaglandins and NO [65].
Wistar and Lewis rats show differences in VP and CRH activity [66].
Elevated plasma VP levels were noted in patients with status asthmaticus during acute attack. The levels returned to normal with resolution of the acute phase [67]. MS patients were studied with normal controls. Patients with MS had higher cortisol levels but responded normally to ACTH stimulation with ovine CRH. The response to VP was blunted [68].
Vasopressin in Chronic Inflammation
During chronic inflammation VP takes over as the regulator of the HPA axis [69]. Adjuvant arthritis in rats, EAE, eosinophilia-mialgia syndrome, systemic lupus erythemathosus and leismaniasis are such conditions. Only CRH can stimulate proopiomelanocortin (POMC), VP does not do this.
VP and Cytokines
Il-1-beta, releases VP, this may be blocked by antibodies, atropine, or mecamylamine ACTH is also released [70].
Il-6 –induced ACTH was suppressed by both CRH and VP antisera [71].
TNF-alpha induced ACTH did not respond to anti-VP, but responded to anti-CRH antibodies [72].
IL-6 potentiated acetylcholine induced VP release [73].
Leukemia inhibitory factor administered centrally, significantly increased plasma VP, 5-60 min. after injection [74].
Vasopressin in APR
VP attenuates the febrile response of rabbits to bacterial pyrogen [74]. Endotoxin may stimulate NO, which inhibits CRH, and generates carbon monoxide, which modulates the release of VP. These are potential counter regulatory controls of HPA activation [75].
LPS acts first within the median eminence where it stimulates peptidergic nerve terminals [76].
LPS potently stimulated CRH and VP secretion in pituitary blood of alert ewes. VP response was 10 times over CRH. Gonadotropinreleasing hormone and LH pulsation were suppressed and fever developed [77].
In Holstein steers LPS induced fever, increased plasma ACTH and cortisol. Pituitary VP receptor V3 mRNA was decreased at 2, 4 and 12 h following LPS injection, returned to normal by 24 h. Pituitary POMC-RNA did not respond [78].
Regulation Of Pituitary Hormones By VP
The ACTH response to exogenous application of VP was impaired in V1bR-/- mice, while CRH stimulated ACTH release. The increase of ACTH after forced swim stress was significantly suppressed in V1bR-/- mice [79].
In conscious male rats ICV infusion of histamine (HA) induced PRL secretion. This could be inhibited by antibodies and by VP antagonist. This antagonist also inhibited the PRL response to restraint stress. An oxytocin (OT) antagonist had no effect [80].
VP antiserum suppressed the suckling induced rise in plasma prolactin [81]. Antisera to VP and vasoactive intestinal peptide completely abolished PRL release to suckling [82].
CRH or VP released ACTH and immunoreactive beta-endorfin response of HA and restraint stress. Pre-treatment with CRH antiserum abolished the ACTH response to 60%. Immunoneutralization by VP antiserum had half of the effect. CRH (100 pmol iv.) increased plasma ACTH and beta-endorfin. This effect was abolished by CRH antiserum, whereas VP antiserum abolished half of the effect. VP (2800 pmol iv.) stimulated ACTH and beta-END, in a dose dependent manner. CRH and VP antisera each prevented the VP-effect, whereas the beta-END response was 60% inhibited [83].
Endogenous VP and OT contribute to basal GH release, and play a significant stimulatory role in basal ACTH release [84].
The novel high-affinity nanopeptide, CRHR1antagonist, R121919, attenuates the stress induced release of corticosterone, PRL and OT. The decrease of testosterone after stress is abolished by R121919 [85].
In rats with paraventricular nucleus lesions LPS was able to activate the hypophysis-adrenal system in absence of hypophysis stimulating neuropeptides of paraventricular origin [86].
Vasopressin and the Immune Response
Brattlebero diabetes insipidus (DI) rats derived from Long Ewans rats (LE). DI rats lack VP, exhibit permanent decrease of blood lymphocytes, neturophils are increased, macrophage activity is reduced, the thymus and spleen involuted early and antibody production was suppressed as well [87]. NK cell activity was higher than LE rats. VP replacement normalized water intake in DI rats but this did not affect NK cell activity. DI rats exhibited lower plasma corticosterone, which was not affected by VP replacement [88].
In mice with the disruption of VP receptor (VPR1a) gene a shift was observed from IGM(high)/IGD(high) to the more mature IGM(low)/IGD(high) in B cell status. Splenic B cell proliferation was significantly greater with anti-IgM stimulation. IGG1 and IGG2b productions were enhanced in response to challenge with T-dependent antigen. T-cell differentiation and activation were normal in VPR (-/-) mice [89].
The VP-binding nonapeptide sequence is: Thr-Met-Lys-Val-Leu- Thr-Gly-Ser-Pro. VP and its 6 amino acid, N terminal cyclic ring, pressinoic acid (PA) are both capable of replacing the IL-2 requirement of IFN-gamma production in mouse splenic lymphocytes. The VP binding peptide specifically and reversibly blocked VP help in IFNgamma production but failed to block the helper signal of PA. Thus the intact VP molecule and not just the N-terminal cyclic ring are important for interaction with the binding peptide [90,91].
VP enhanced the mixed lymphocyte reaction. Enhanced proliferation, which appeared to be specific for the arginine residues, is at position 8 of VP [92].
VR1 receptor antagonist induced a complex intracellular Ca2+ signaling cascade event in cortical astrocytes. It dramatically reduced the mRNA response to five cytokines including IL-1-beta and TNFalpha [93].
Our Observations
Membrane VP receptors are present in several immune cell type [89,94,95]. We investigated the effect of neurointermediate pituitary lobectomy (NIL) on the immune function of rats. NIL rats have low plasma level of VP and OT [96].
The adrenal glands are enlarged in some experiments but not in others [97,98]. If thyroidectomy was also done in NIL animals, the adrenal glands are enlarged in NIL rats the increase of adrenal size and decrease in thymus and spleen weights was observed. NIL inhibited Immune function including IgG and IgM responses too sheep red blood cells [99]. Plasma IgG and IgM and intestinal secretion of IgA antibodies to Salmonella tyhimurium were inhibited [100]. Delayed type hypersensitivity to dinitrochrorobenzene (DNCB) and the Arthus reaction could not be induced in NIL rats. The incidence and severity of EAE [17] was reduced. And there was failure to develop adjuvant arthritis [101].
Using EAE in NIL animals we studied desmopressin (DP), a synthetic analog of VP. Immune-inflammatory responses and HPA axis were studied. DP antagonized the NIL effect. It restored EAE reactivity in NIL animals which was accompanied with high ACTH and Cortisol levels (Tables 1 and 2 and Figure 3).
| Control | Sham | Sham+DP | Hypox | Hypox+DP | NILc | NIL+DP | NIL+TX | |
|---|---|---|---|---|---|---|---|---|
| Adrenal weight | ≠ | ↑↑↑ | ↓↓↓ | ↓ | ↑ | ↑↑ | ↑↑ | |
| Thymus weight | ≠ | ≠ | ↓ | ↑Compared to Hypox | ↓ | ≠ | ↓↓↓ | |
| Spleen weight | ≠ | ↑↓ | ↓ | ↑Compared to Hypox | ↑↓ | ≠ ↓ | ||
| Serum SRBC antibody | ≠ | ↓↓ | ↓↓ | |||||
| Serum SRBC C'Fixing antibody | ↑↑ | ↑↑ | ↓↓ | ↓↓ | ||||
| Serum IgG, IgM, anti-SRBC | ↑↑ | ↑↑ | ↓ | ↓ | ||||
| Arthus reaction (SRBC) | ↑↑ | ↑↑ | ↓ | ↓ | ||||
| Serum IgG, IgM; intestinal IgA, anti-salmonella typhi* | ↑↑ | ↑↑ | ↓↓ | ↓ | ||||
| Contact sensitivity to DNCB | ↑↑ | ↑↑ | ↓ | ↓ | ||||
| EAE: clinical score | ↑↑ | ↑↑ | ↓ | ↑ | ↑↑↑ | ↓↓ | ↑↑ | |
| EAE: plasma ACTH and corticosterone | ↑ | ↑ | ↓↓↓↓ | ↓↓↓↓ | ≠ | |||
| Adenohypophyseal cell counts | ≠ | GHb | TSH | |||||
| ↓ | ↑↑↑ | |||||||
| LH | GH | |||||||
| ↓ | ↓↓↓↓ | |||||||
| PRL | ||||||||
| ↓↓↓ |
Table 1: In long surviving rats (8 months), adrenal weight increased 30%, whereas thymus weight decreased 50% when compared to the intact control. Oral infection was used in the experiments with Salmonella typhimurium [7].
| Parameter | CRH | VP |
|---|---|---|
| Pituitary adrenal axis | ↑↑↑ | ↑↑ |
| POMC | ↑↑↑ | 0 |
| Prolactin | ↓↓ | ↑↑ |
| Cytokines | IL-1,6 TNF-? | Il-1,2,6 LIF |
| Adaptive Im. | ↓↓↓ | ↑↑↑ |
| Autoimmunity | ↓↓↓ | ↑permissive |
| Chronic inflam. | ↓↓↓ | ↑↑ |
| Nat. Imm., APR | ↑↑↑ | ↑↑↑ |
| LPS response | ↑↑↑ | ↑↑↑ |
Table 2: Hypothalamic Immunoregulation.
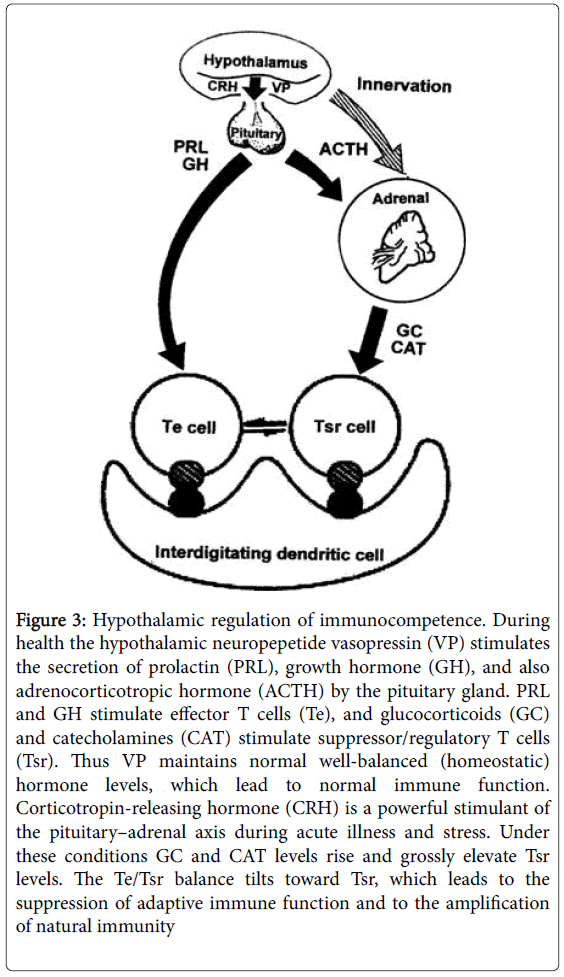
Figure 3: Hypothalamic regulation of immunocompetence. During health the hypothalamic neuropepetide vasopressin (VP) stimulates the secretion of prolactin (PRL), growth hormone (GH), and also adrenocorticotropic hormone (ACTH) by the pituitary gland. PRL and GH stimulate effector T cells (Te), and glucocorticoids (GC) and catecholamines (CAT) stimulate suppressor/regulatory T cells (Tsr). Thus VP maintains normal well-balanced (homeostatic) hormone levels, which lead to normal immune function. Corticotropin-releasing hormone (CRH) is a powerful stimulant of the pituitary–adrenal axis during acute illness and stress. Under these conditions GC and CAT levels rise and grossly elevate Tsr levels. The Te/Tsr balance tilts toward Tsr, which leads to the suppression of adaptive immune function and to the amplification of natural immunity
Conclusions
This overview indicates that VP is a major immunoregulator, more versatile than CRH. CRH is specific for APR and regulates proopiomelanocotin. VP supports the APR but its major role is to take care of healing and also regulates immune function during healthy life. VP is the number-1 hypothalamic hormone we need every day of our life. These observations promise a better understanding of illness and recovery to significantly aid patient management.
More studies are needed for better understanding of this subject.
Acknowledgements
Supported by UAAPIFF08-1 and Consejo Nacional the Ciencia y Tecnologia (CONACYT)-62317, Mexico.
This paper is a shortened version of paper no 101. Because the original wok is a highly concentrated text, it was not possible to reword definitions and such portions of the text that are irreplaceable. So here we would like to give credit to the original paper. I am satisfied that this story will be presented for the second time.
References
- Berczi I, A Szentivanyi (2003) Growth and lactogenic hormones, insulin-like growth factor and insulin. In Neuroimmune Biology, Volume 3: The Immune Neuroendocrine Circuitry. History and Progress, Newyork.
- O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, et al. (2008) Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell. Immunol. 252: 91-110.
- Savino W (2007) Neuroendocrine control of T cell development in mammals: role of growth hormone in modulating thymocyte migration. Exp Physiol 92: 813-817.
- Kelley KW, Douglas AW, Kooijmand R (2007) Protein hormones and immunity. Brain BehavImmun 21: 384-392.
- Venkatesh J, Peeva E, Xu X, Diamond B (2006) Cutting edge: hormonal milieu, not antigenic specificity, determines the mature fenotype of autoreactive B cells. J Immun 176: 3311-3314.
- Bertok L, DA Chow (2005) Natural Immunity. I. Berczi& A. Szentivanyi, Series Eds. Elsevier. Amsterdam.
- Berczi I, Quintanar-Stephano A, Kovacs K (2006) Chapter 14. Immunoconversion in the acute phase response. In Cytokines, Stress and Immunity. CRC Press, Taylor & Francis Group. Boca Raton, FL.
- Straub RH, Mocchegiani E (2004) Neuroimmune Biology, Volume 4. The Neuroendocrine Immune Network in Ageing. Elsevier, Amsterdam.
- Janeway CA, Travers P, Walport M (2005) Immunobiology, 6th ed. Garland Science Publishing. New York.
- Nagy E, BercziI (1978) Immunodeficiency in hypophysectomized rats. Acta Endocrinol 89: 530-537.
- Berczi I, Nagy E, Kovacs K, Horvath E (1981) Regulation of humoral immunity in rats by pituitary hormones. Acta Endocrinol 98: 506-513.
- Nagy E, IstvanBerczi, Henry G. Friesen (1983) Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol 102: 351-357.
- Nagy E, Berczi I, Wren GE, Asa SL, Kovacs K (1983) Immunomodulation by bromocriptine. Immunopharmacology 6: 231-243.
- Berczi I, Nagy E, Asa SL, Kovacs K (1984) The influence of pituitary hormones on adjuvant arthritis. Arthritis Rheum 27: 682-688.
- Berczi I, Nagy E, Asa SL, Kovacs K (1983) Pituitary hormones and contact sensitivity in rats. Allergy 38: 325-330.
- Nagy E, I Berczi (1989) Pituitary dependence of bone marrow function. Br J Haematol 71: 457-462.
- Quintanar-Stephano A, Chavira-RamÃrez R, Kovacs K, Berczi I (2005) Neurointermediate pituitary lobectomy decreases the incidence and severity of experimental autoimmune encephalomyelitis in Lewis rats. J Endocrinol 184: 51-58.
- Ramachandra RN, Sehon AH, Berczi I (1992) Neuro-hormonal host defence in endotoxin shock. Brain BehavImmun 6: 157-169.
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, et al. (1997) Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16: 6926-6935.
- Bouchard B, Ormandy CJ, Di Santo JP, Kelly PA (1999) Immune system development and function in prolactin receptor-deficient mice. J Immunol 163: 576-582.
- Berczi I (1986) Immunoregulation by the pituitary gland. In Pituitary Function and Immunity CRC Press, Inc. Boca Raton, FL.
- Del Rey,Chrousos, Besedovsky(2008) Neuroimmune Biology Volume 7: The Hypothalamus- Pituitary-Adrenal Axis. Elsevier. Amsterdam.
- Eiza N, Toubi E, Vadasz Z (2016) Increased T and B Regulatory Cell Function Contributes to the Persistence of HCV and Other Viral Infections. Isr Med Assoc J 18: 159-61.
- Selye H (1946) The general adaptation syndrome and the diseases of adaptation. J ClinEndocrinolMetab 6: 117-230.
- Berczi I (1998) Neuroendocrine response to endotoxin. Ann N Y AcadSci 851: 411-415.
- Paltrinieri S (2008) The feline acute phase reaction. Vet J 177: 26-35.
- Krueger JM, Majde JA, Obál F (2003) Sleep in host defense. Brain BehavImmun 17 (Suppl 1): S41-47.
- Krueger JM (2008) Cytokines and sleep. In Neuroimmune Biology, Volume 6: Cytokines and the Brain. Elsevier Amsterdam.
- Berczi I, Szentivanyi A (2003) The acute phase response. In Neuroimmmune Biology, Volume 3: The Immune-Neuroendocrine Circuitry. History and Progress. Elsevier Amsterdam.
- O’Mahony D S, Pham U, Iyer R, Hawn TR, Liles WC (2008) Differential constitutive and cytokine-modulated expression of human Toll-like receptors in primary neutrophils, monocytes, and macrophages. Int J Med Sci. 5: 1-8.
- Spight D, Trapnell B, Zhao B, Berclaz P, Shanley TP (2008) Granulocyte-macrophage colony-stimulating factor dependent peritoneal macrophage responses determined by survival in experimentally induced peritonitis and sepsis in mice. Shock 30: 434-442.
- Kaur A (2004) Bioimmunotherapy of rodent malaria: co-treatment with recombinant mouse granulocyte-macrophage colony-stimulating factor and an enkephalin fragment peptide Tyr-Gly-Gly. Acta Trop 91: 27-41.
- Everly JJ, Lonial S (2005) Immunomodulatory effects of human recombinant granulocyte-macrophage colony-stimulating factor (rhuGMCSF): evidence of anti - tumour activity. Expert OpinBiolTher 5: 293-311.
- Ezaki K, Tsuzuki M (1997) Cytokine therapy for hematological malignancies. Gan to Kagaku RyohoSuppl 1: 182-194.
- Selig C, Nothdurft W (1995) Cytokines and progenitor cells of granulocytopoiesis in peripheral blood of patients with bacterial infections. Infect Immun 63: 104-109.
- Mels AK, Statius Muller MG, van Leeuwen PA, von Blomberg BM, Scheper RJ (2001) Immune-stimulating effects of low-dose perioperative recombinant granulocyte-macrophage colony-stimulating factor in patients operated on for primary colorectal carcinoma. Br J Surg 88: 539-544.
- Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M (2007) MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and anti - inflammatory activities of GM-CSF. J Clin Invest 117: 1902-1913.
- Han S, Song Y, Lee YH, Lee YR, Lee CK (2005) Macrophage-colony stimulating factor enhances MHC-restricted presentation of exogenous antigen in dendritic cells. Cytokine 32: 187-193.
- Wexler BC, Dolgin AE, Tryczynski EWÂ (1957) Effects of bacterial polysaccharide (Piromen) on the pituitary-adrenal axis: adrenal ascorbic acid, cholesterol and histological alterations. Endocrinology 61: 300-308.
- Wilder RL (1995) Neuroendocrine-immune system interactions and autoimmunity. Annu. Rev. Immunol 13: 307-338.
- Torpy DJ, Chrousos GP (1996) The three way interaction between the hypothalamic–pituitary– adrenal and gonadal axes and the immune system. Rheumatol London: BailliereTindall 10: 181-198.
- Berczi I, Chalmers IM, Nagy E, Warrington RJ (1996) The immune effects of neuropeptides. Rheumatol, London: BailliereTindall 10: 227–257.
- Schiltz JC, Sawchenko PE (2003) Signaling the brain in systemic inflammation: the role of perivascular cells. Front. Biosci 8: S1321-S1329.
- Jancso GE (2008) Neurogenic inflammation in health and disease. In Neuroimmune Biology, Volume 8, Elsevier, Amsterdam.
- Kolb-Bachofen V (1991) A review on the biological properties of C-reactive protein. Immunobiology 183: 133-445.
- Ballou SP, Kushner I (1992) C-reactive protein and the acute phase response. Adv Intern Med 37: 313-336.
- Raetz CH, Ulevitch RJ, Wright SD, Sibley CH, Ding A, et al. (1991) Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J 5: 2652-2660.
- Hamann L, Alexander C, Stamme C, Zähringer U, Schumann RR (2005) Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infect Immun 73: 193-200.
- Arredouani MS, Kasran A, Vanoirbeek JA, Berger FG, Baumann H (2005) Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology 114: 263-271.
- Liu H, Jensen L, Hansen S, Petersen SV, Takahashi K et al. (2001) Characterization and quantification of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand J Immunol 53: 489-497.
- Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G (2003) Intensive insulin therapy exerts anti - inflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab 88: 1082-1088.
- Bauer J, Birmelin M, Northoff GH, Northemann W, Tran-Thi TA (1984) Induction of rat alpha2-macroglobulin in vivo and in hepatocyte primary cultures: synergistic action of glucocorticoids and a Kupffer cell derived factor. FEBS Lett 177: 89-94.
- Collier B, Dossett LA, May AK, Diaz JJ (2008) Glucose control and the inflammatory response. NutrClinPract 23: 3-15.
- Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, et al. (1999) Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341: 785-792.
- Berczi I, Bertók L, Chow DA (2001) Natural immunity and neuroimmune host defence. Ann N Y AcadSci 917: 248-257.
- Derfalvi B, Igaz P, Fulop KA, Szalai C, Falus A (2000) Interleukin-6-induced production of type II acute phase proteins and expression of jun B gene are downregulated by human recombinant growth hormone in vitro. Cell BiolInt 24: 109-114.
- BercziI (1986) The influence of pituitary-adrenal axis on the immune system. In Pituitary Function and Immunity. Boca Raton, FL.
- Van Gool J, Boers W, Sala M, Ladiges NCÂ (1984) Glucocorticoids and catecholamines as mediators of acute-phase proteins, especially rat alpha-macrofoetoprotein. Biochem J 220: 125-152.
- Yeager MP, Guyre PM, Munck AU (2004) Glucocorticoid regulation of the inflammatory response to injury. ActaAnaesthesiolScand 48: 799-813.
- Van Gool J, Van Vugt H, Helle M, Aarden LA (1990) The relation among stress, adrenalin, interleukin 6, and acute phase proteins interact. ClinImmunolImmunopathol 57: 200-210.
- Akira S, Kishimoto T (1992) IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev 127: 26-50.
- Harnish MJ, Kevin SG, Maria GR (2005) Differential regulation of human blood glucose level by interleukin-2 and -6. Exp. Clin. Endocrinol. Diabetes 113: 43-48.
- Ekman R, Gobom J, Persson R, Mecocci P, Nilsson CL (2001) Arginine vasopressin in the cytoplasm and nuclear fraction of lymphocytes from healthy donors and patients with depression or schizophrenia. Peptides 22: 67-72.
- Itoi K, Jiang YQ, Iwasaki Y, Watson SJ (2004) Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol 16: 348-355.
- Carrasco GA, Van de Kar LD (2003) Neuroendocrine pharmacology of stress. Eur J Pharmacol 463: 235-272.
- Chowdrey HS, Larsen PJ, Harbuz MS, Jessop DS, Aguilera G, et al. (1995) Evidence for arginine vasopressin as the primary activator of the HPA axis during adjuvant-induced arthritis. Br J Pharmacol 116: 2417-2424.
- Michelson D, Stone L, Galliven E, Magiakou MA, Chrousos GP, et al. (1994) Multiple sclerosis is associated with alterations in hypothalamic-pituitary adrenal axis function. J Clin Endocrinol Metab 79: 848-853.
- Harbuz MS, Chover-Gonzalez AJ, Jessop DS (2003) Hypothalamo-pituitary adrenal axis and chronic immune activation. Ann N Y AcadSci 992: 9-106.
- DeGoeij D, Kvetnansky R, Whitnall MH, Jezova D, Berkenbosch F, et al. (1991) Repeated stress activation of corticotropin releasing factor neurons enhances vasopressin stores and colocalization with corticotropin-releasing factor in the median eminence of rats. Neuroendocrinology 53: 150-159.
- Wilkinson MF, Horn TF, Kasting NW, Pittman QJ (1994) Central interleukin-1 beta stimulation of vasopressin release into the rat brain: activation of an antipyretic pathway. J Physiol 481(Pt 3): 641-646.
- Raber J, Bloom FE (1994) IL-2 induces vasopressin release from the hypothalamus and the amygdala: role of nitric oxide-mediated signaling. J Neurosci 14: 6187-6195.
- Malkinson TJ, Bridges TE, Lederis K, Veale WL (1987) Perfusion of the septum of the rabbit with vasopressin antiserum enhances endotoxin fever. Peptides 8: 385-389.
- Kostoglou-Athanassiou I, Costa A, Navarra P, Nappi G, Forsling ML, et al. (1998) Endotoxin stimulates an endogenous pathway regulating corticotropin-releasing hormone and vasopressin release involving the generation of nitric oxide and carbon monoxide. J Neuroimmunol 86: 104-109.
- Giovambattista A, Chisari AN, Gaillard RC, Spinedi E (2000) Modulatory role of the epinergic system in the neuroendocrineimmune system function. Neuroimmunomodulation 8: 98-106.
- Lee S, Barbanel G, Rivier C (1995) Systemic endotoxin increases steady-state gene expression of hypothalamic nitric oxide synthase: comparison with corticotropin releasing factor and vasopressin gene transcripts. Brain Res 705: 136-148.
- Battaglia DF, Brown ME, Krasa HB, Thrun LA, Viguié C, et al. (1998) Systemic challenge with endotoxin stimulates corticotropin-releasing hormone and arginine vasopressin secretion into hypophyseal portal blood: coincidence with gonadotropin-releasing hormone suppression. Endocrinology 139: 4175-4181.
- Qahwash IM, Cassar CA, Radcliff RP, Smith GW (2002) Bacterial lipopolysaccharide-induced coordinate downregulation of arginine vasopressin receptor V3 and corticotropin-releasing factor receptor 1 messenger ribonucleic acids in the anterior pituitary of endotoxemic steers. Endocrine 18: 130-20.
- Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, et al. (2004) The vasopressin V1b receptor critically regulates hypothalamic-pituitary adrenal aids activity under both stress and resting conditions. J Clin Invest 113: 302-309.
- Kjaer A, Knigge U, Bach FW, Warberg J (1992) Histamine-and stress-induced secretion of ACTH and beta-endorphin: involvement of corticotropin-releasing hormone and vasopressin. Neuroendocrinology 56: 419-428.
- Nagy GM, Görcs TJ, Halász B (1991) Attenuation of the suckling induced prolactin release and the high afternoon oscillations of plasma prolactin secretion of lactating rats by antiserum to vasopressin. Neuroendocrinology 54: 566-570.
- ELHalawani ME, Silsby JL, Koike TI, Robinzon B (1992) Evidence of a role for the turkey posterior pituitary in prolactin release. Gen Comp Endocrinol 87: 436-442.
- Franci CR, Anselmo-Franci JA, Kozlowski GP, McCann SM (1993) Actions of endogenous vasopressin and oxytocin on anterior pituitary hormone secretion. Neuroendocrinology 57: 693-699.
- Keck ME, Welt T, Müller MB, LandgrafR, Holsboer F (2003) The high-affinity non peptide CRH1 Receptor antagonist R121919 attenuates stress-induced alterations in plasma oxytocin, prolactin, and testosterone secretion in rats. Pharmacopsychiatry 36: 27-31.
- Elenkov IJ, Kovács K, Kiss J, Bertók L, Vizi ES (1992) Lipopolysaccharide is able to bypass corticotrophin-releasing factor in affecting plasma ACTH and corticosterone levels: evidence from rats with lesions of the paraventricular nucleus. J Endocrinol 133: 231-236.
- Khegai II, Gulyaeva MA, Popova NA, Zakharova LA, Ivanova LN (2003) Immune system in vasopressin-deficient rats during ontogeny. Bull ExpBiol Med 136: 448-450.
- Yirmiya R, Shavit Y, Ben-Eliyahu S, Martin FC, Weiner H, et al. (1989) Natural killer cell activity in vasopressin-deficient rats (Brattleboro strain). Brain Res 479: 16-22.
- Hu SB, Zhao ZS, Yhap C, Grinberg A, Huang SP (2003) Vasopressin receptor 1a mediated negative regulation of B cell receptor signaling. J Neuroimmunol 135: 72-81.
- Johnson HM, Torres BA (1988) A novel arginine vasopressin-binding peptide that blocks arginine vasopressin modulation of immune function. J Immunol 141: 2420-2423.
- Torres BA, Johnson HM (1990) Arginine vasopressin binding peptides derived from the bovine and rat genomes differ in their abilities to block arginine vasopressin modulation of murine immune function. J Neuroimmunol 27: 191-199.
- Bell J, Adler MW, Greenstein JI, Liu-Chen LY (1993) Identification and characterization of [125I]-arginine vasopressin binding sites on human peripheral blood mononuclear cells. Life Sci 52: 95-105.
- Shibasaki T, Hotta M, Sugihara H, Wakabayashi I (1998) Brain vasopressin is involved in stress-induced suppression of immune function in the rat. Brain Res 808: 84-92.
- Elands J, Resink A, De Kloet ER (1990) Neurohypophyseal hormone receptors in the thymus, spleen, and lymphocytes. Endocrinology 126: 2703-2710.
- Bell J, Adler MW, Greenstein JI, Liu-Chen LY (1993) Identification and characterization of (125)-arginine vasopressin binding sites on human peripheral blood mononuclear cells. Life Sci 52: 95-105.
- Moll J, D De Wied (1962) Observations on the hypothalamo-posthypophyseal system of the posterior lobectomized rat. Gen Comp Endocrinol 2: 215-228.
- Miller RE (1974) Anterior hypophysial function in the posterior-hypophysectomised rat: normal regulation of the adrenal system. Neuroendocrinology 14: 233-250.
- Organista-Esparza A (2003) Efectos de la lobectom´?aneurointermediahipofisiariay la hipofisectom´?asobre la respuestainmunehumoral en la rata Wistar. In Proc. XLVI National Congress of Physiological Sciences, Aguascalientes, Mexico.
- Quintanar A (2007) Increased IgM and decreased IgG response to rabbit red blood cell immunization in hypophysectomised and neurointermediate pituitary lobectomized rats. FASEB J, Abstract No. 962.2.
- Campos-Rodriguez R, Quintanar-Stephano A, Jarillo-Luna RA, Oliver-Aguillón G, Ventura-Juárez J, et al. (2006) Hypophysectomy and neurointermediate pituitary lobectomy reduce serum immunoglobulin M (IgM) and IgG and intestinal IgA responses to Salmonella entericaserovarThyphimurium infection in rats. Infect Immun 74: 1883-1889.
- Quintanar-Stephano A (2004) Effects of neurointermedate pituitary lobectomy on humoral and cell-mediated immune responses in the rat. Neuro immunomodulation 11: 233.
- Quintanar-Stephano A (2004) Effects of neurointermediate pituitary lobectomy and desmopressin on experimental autoimmune encephalomyelitis in rats. FASEB J 19: 148-157.
- IstvanBerczi, Andres QuintanrStehano, Kalman Kovacs (2009) Neuroimmune Regulation in Immunocompetence, Acute Illness and Healing. Ann. N. Y. Acad, Sci. 1153: 220-239.
Citation: Berczi I (2016) Neuroimmune Regulation in Health: Acute Febrile Illness and Healing. J Clin Exp Neuroimmunol 1: 107.
Copyright: ©2016 Berczi I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 11383
- [From(publication date): 12-2016 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 10518
- PDF downloads: 865