Neurohistopathological Findings of the Effects of Amprolium on the Brain and Spinal Cord Changes in the a Animal Model
Received: 14-May-2014 / Accepted Date: 03-Jul-2014 / Published Date: 08-Jul-2014 DOI: 10.4172/2161-0681.1000180
Abstract
The aim of the study was to evaluate the polioencephalomalacia induced with amprolium in goats: pathologic changes of the central nervous system. Eight apparently healthy goats of 6 to 9 weeks of age were drenched with amprolium (200 mg/kg body weight) till the development of clinical signs. Three goats of the same age group were drenched with tap water only and these served as controls. Amprolium drenched goats were allowed to die after the onset of clinical signs and control goats were euthanised after the death of amprolium fed goats. At the time of development of typical clinical signs of Cerebrocortical Necrosis (CCN) the goats were killed and necropsied and a complete histopathologic examination was performed. At necropsy of the four goats, large necrotic lesion was found in the cerebral cortex, and tissue thiamine levels decreased significantly, especially in cerebrum and cerebellum. However, the brain as a whole in all experimental animals, exhibited moderate to severe congestion, oedema and numerous small yellowish foci of varying sizes scattered on the surface of the cerebral hemispheres. Microscopic changes in the brain were limited to gray matter structures of cerebral and cerebellar cortex, caudal colliculi of mid brain, thalamus, cerebellum and spinal cord. There was shrinkage of neurons, perivascular and pericellular edema, necrosis of neurons, satellitosis, glial nodule, gliosis, middle laminar necrosis and deep laminar edema. Blood vessel walls were thickened due to hypertrophy and hyperplasia of endothelial and adventitial cells. Swollen and prominent capillary epithelium, satellitosis, presence of ghost cells, gliosis and perivascular cuffing were also observed but only in some of the animals. In the cerebellar cortex, there was degeneration of Purkinje cells. The caudal colliculi of mid brain showed bilateral malacia.
To the best of our knowledge, these researchers questioned the hypothesis that the amprolium could be the major factor causing polioencephalomalacia. This is the first documentation of Amprolium-Induced cerebrocortical necrosis on goat in Iran.
Keywords: Polioencephalomalacia; Amprolium; Goat; Histopathology; Brain
314846Introduction
Although mature ruminant species such as sheep and cattle have not been considered to require thiamin in their diet, animals with clinical signs of Polioencephalomalacia (PEM) were reported to be in a state of thiamin inadequacy. The disease is characterized by necrosis in the cerebral cortex, and since its first description it has been diagnosed throughout the world. In certain regions of the world, the disease is known as Cerebrocortical Necrosis [1,2].
Polioencephalomalacia (PEM), a common neurologic disease of ruminants, is characterized by laminar necrosis of cerebral cortical gray matter that manifests grossly as softening and malacia to complete loss of gray matter. Clinical signs include seizures, ataxia, recumbency, and blindness. Microscopic lesions in ruminants consist of laminar necrosis of neurons, pericellular edema and small hemorrhages in the neuropil, and gliosis. Animals of all ages can be affected but young animals appear to be more vulnerable. It has been described in cattle, sheep, goats, deer and camels. Several risk factors such as thiamine deficiency, toxicity, lead toxicity, and water deprivation-sodium ion toxicity have been implicated in the development of PEM. All these factors produce similar brain lesions [3-8].
Amprolium, a potent coccidiostat and thiamine analogue, is believed to be another major factor associated with PEM. It inhibits the conversion of free-base thiamine to Thiamine Pyrophosphate (TPP), thereby depriving tissues (especially brain) of TPP. However, clinical and histopathological lesions indicative of thiamine deficiency have been produced in preruminant lambs by feeding a thiamine free artificial milk diet. These researchers questioned the hypothesis that the amprolium could be the major factor causing PEM [9,10].The aim of the study was to evaluate the polioencephalomalacia induced with amprolium in goats: pathologic changes of the central nervous system.
Materials and Methods
This study was approved by the Animals Ethics Committee at Tehran University (Ethics code permit no.TU2013-1-11-007Y). The animals were placed in shade, in standard conditions, water ad libitum, and without restriction of movement according to the guidelines of the Institutional Animal Ethical Committee of the Tehran University of Animal Science, Iran. Eight apparently healthy goats (7-11 months of age) of both sex and nondescript breed were maintained under semi-intensive management system. They were offered concentrate at 150 g/d containing 12% DCP and 65% TDN along with 3-4 h grazing and adlib. Greens. Routine health cover included spraying/ dipping for ectoparasites and initial drenching with anti-helmintics. The animals were divided in to two groups (A and B) of four animals each. Each group was subdivided in to experimental (n=5) and control (n=3). Body weights of the animals ranged between 10 to 18 kg in groups A and B. The experimental animals were drenched with amprolium in increasing doses by stomach tube in three equally divided doses daily. The initial dose was 200 mg/kg BW per day. It was increased every week at 100 mg/kg BW per day for group A and group B until the 9th and 6th week, respectively. The drenching was continued until the animals exhibited clinical symptoms of CCN, and discontinued immediately after the initial signs were observed. After death, experimental animals were necropsied, and the controls were sacrificed along with the last animal of the group. Representative tissue pieces from cerebrum (in the vicinity of the rostral sylvian gyms, sylvian fissure, ectosylvian sulcus, precruciate gyrus, middle suprasylvian sulcus, marginal sulcus and ectomarginal sulcus), declivo of cerebellum, pons, medulla oblongata and spinal cord were collected in 10% neutral formalin and the microsections were stained by routine haematoxylin and eosin to study the general histopathological alterations.
Results
Clinical Symptoms
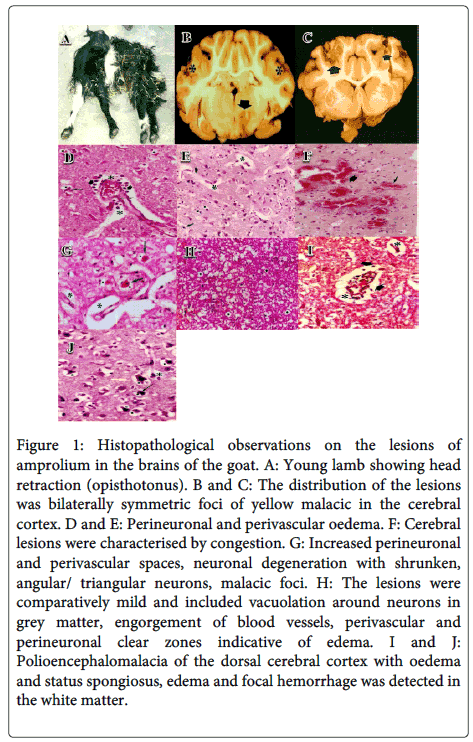
Initial stages contained anorexia, stupor, depression, ataxia and staggering gait. In later stages, incoordination, bilateral and vertical nystagmus, blindness, pressing of head against hard objects and subsequently turning the upwards head, twitching of facial muscles, clono-tonic convulsions, absence of menace response and terminally opisthotonous, trismus, paddling, dyspnoea, opisthotonos (Figure 1A), tetanus hand, the local tetanus in the extensor muscles, coma and death were recorded.
Macroscopic Findings
The carcass of these goats was in average nutritional status. Small amounts of fat and atrophy of fatty tissue were seen in sub-cutaneous/omental region and around heart and kidneys. Vital organs did not reveal any gross pathological lesion of significance except congestion and hemorrhage. The most common small intestinal lesions were intense mucosal and serosal hyperemia and hemorrhage; moderate transmural thickening; dull and dark red ulcerated mucosa with or without multifocal, tan to yellowish pseudomembranes. Lesions in the colon and cecum resembled those in the small intestine; the distribution was multifocal in three goats, locally extensive to diffuse in one goat. However, the brain as a whole in all experimental animals, exhibited moderate to severe congestion, oedema and numerous small yellowish foci of varying sizes scattered on the surface of the cerebral hemispheres. The demarcation between the sulcus and gyri around these yellowish foci was practically lost. The distribution of the lesions was bilaterally symmetric foci of yellow malacic in the cerebral cortex (Figure 1B and C). The affected foci were soft and friable in comparison to the surrounding unaffected areas.
Microscopic Findings
Spinal cord Lesions
The changes in the spinal cord and four cross sections from cervical, thoracic, lumbar and sacral levels were observed only in seven animals and characterised by mild to moderate perineuronal oedema, congestion, haemorrhage, endothelial cell hypertrophy or hyperplasia, perivascular and perineuronal clear zones indicative of edema, extravasation of erythrocytes, a few malacic foci and neuronal degeneration of varying degree in grey matter. The changes were more common in the cervical and lumbar segments and posterior horns.
Cerebrum Lesions
The basic pattern of lesions detected in experimental goats of multifarious groups was comparable, in all sites from which sections were studied. Moreover, polioencephalomalacia was mostly observed in parietal and occipital telecephalic lobes and also, pathohistological investigations revealed a polioencephalomalacia of the dorsal cerebral cortex with oedema and status spongiosus (Figure 1I and J). However, variation was observed in the intensity of the reaction in different experimental animals and at different sites but without any definite correlation with the duration of drenching of amprolium or of clinical signs of the individual animal. The lesions were characterised by a wide gap (oedema) between the meninges and rain substance proper with moderate to marked engorgement of meningeal blood vessels. Cerebral lesions were characterised by congestion(Figure 1F), Pyramidal cell layer revealed perineuronal and perivascular oedema (Figure 1D, E and H), pial congestion and neuronal degeneration, microcavitation, laminar necrosis, increased perineuronal and perivascular spaces, neuronal degeneration with shrunken, angular/triangular neurons, malacic foci (Figure 1G), extravasation of erythrocytes, gliosis, satellitosis, perivascular cuffing and prominemce of capillary epithelium confined to cerebral cortex. The degenerating neurons in the affected areas had shrunken, angular and sometimes triangular appearance, with basophilic cytoplasm and pyknotic nuclei.
Figure 1: Histopathological observations on the lesions of amprolium in the brains of the goat. A: Young lamb showing head retraction ( opisthotonus ). B and C: The distribution of the lesions was bilaterally symmetric foci of yellow malacic in the cerebral cortex. D and E: Perineuronal and perivascular oedema. F: Cerebral lesions were characterised by congestion. G: Increased perineuronal and perivascular spaces, neuronal degeneration with shrunken,angular/ triangular neurons, malacic foci. H: The lesions were comparatively mild and included vacuolation around neurons in grey matter, engorgement of blood vessels, perivascular and perineuronal clear zones indicative of edema . I and J:Polioencephalomalacia of the dorsal cerebral cortex with oedema and status spongiosus, edema and focal hemorrhage was detected in the white matter
In some of the sections malacic foci were seen having remnants of degenerating cells surrounded by apparently viable cells. Similarly in many of the sections, wide empty spaces with a definite boundary could also be noted. Swollen and prominent capillary epithelium, satellitosis, presence of ghost cells, gliosis and perivascular cuffing were also observed but only in some of the animals.
The lesions were comparatively mild and included vacuolation around neurons in grey matter, engorgement of blood vessels, perivascular and perineuronal clear zones indicative of edema, vacuoles in degenerating Purkinje cells and their disappearance. In a few sections, mild to moderate rarefaction was seen in the granular layer and also, edema of the folial white matter with focal hemorrhage was observed in the molecular layer. Furthermore, edema and focal hemorrhage was detected in the white matter.
Discussion
Amprolium, one of the thiamine antagonists which can induce the thiamin deficiency, was used in our experiment, the reason being that according to Mark-son the histopathological changes induced by the administration of this antimetabolite in central nervous system of young calves resemble very much the changes developed in spontaneous cerebrocortical necrosis. The origin of these changes could be explained by the action of Amprolium that blocks the active transport of thiamine, probably by competition on membranes. Clinical signs experimentally produced in calves by the administration of Amprolium were very similar to those of spontaneously diseased calves with CCN [9,11,12].
It follows from our studies of cerebtocortical necrosis and of experimentally induced CCN syndrom that for establishing an objective diagnosis of CCN the knowledge of reliable' history together with a repeated clinical examination are essential. The pathologico-morphological findings in the cerebral cortex [1,13,14].
Changes in the cerebral hemisphere comprised mild neuronal degeneration with the surrounding glial cells, satellitosis and vacuolation. These findings are in agreement with Krishnamoorthy et al., Malik et al. and Yadav et al. who reported perivascular and perineuronal oedema, gliosis and degeneration of a few neurons and Purkinje cells in broilers fed with chlorpyriphos. But whether these changes are due to thiamine deficiency or as a result of high dose of amprolium used during the study needs further elucidation. Haemorrhagic foci observed in the cerebrum of experimental goats might be the result of hypoxia and increased intracranial pressure [15-17]. Morgan mentioned that in amprolium-induced CCN the intracranial pressure is increased. Comparable changes also occurred in the cerebellum, but the intensity of the reaction was low. Confinement of lesions invariably in the grey matter either in brain or spinal cord might be taken as an indication that lesions of CCN have an affinity for grey matter in comparison to white matter. Further, hardly any difference could be noted in the pattern of lesions in different parts of the cerebral cortex in agreement with the findings of Goyal [18].
In present study,gross and histopathological changes were mainly confined to the brain in amprolium fed calves. Gross lesions included congestion and haemorrhages in the meninges. The cerebral gyri were swollen with yellowish discolouration of cerebral cortex. Microscopic changes in the brain were limited to gray matter structures of cerebral and cerebellar cortex, caudal colliculi of mid brain and thalamus. There was shrinkage of neurons, perivascular and pericellular edema, necrosis of neurons, satellitosis, glial nodule and gliosis. Blood vessel walls were thickened due to hypertrophy and hyperplasia of endothelial and adventitial cells.
In the cerebellar cortex, there was degeneration of Purkinje cells. The caudal colliculi of mid brain showed bilateral malacia. In the necrotic areas, neuropils were fragmented, edematous and hypercellular due to increased number of microglial cells and there was neocapillary formation. Subcortical gray matter of the thalamus showed necrosis of neurons, gliosis with formation of glial nodule.
Conclusion
Amprolium-induced CCN was found to be indistinguishable from spontaneous CCN. Lesions were mostly confined to the brain. Furthermore, findings at necropsy and upon microscopic examination were bilaterally symmetrical areas of necrosis of the cerebral hemispheres in the area of the neostriatum that were well demarcated from the surrounding normal neuropil. Finally, treatment of PEM affected goat with thiamine rapidly brought the histopathology status of the animals to normal.
References
- Matayoshi M, Tsuha O, Shimoji S, Araki M, UchiharaT,et al. (2012) Occurrence of cerebrocortical necrosis in a goat in Okinawa prefecture. J Vet Med Sci 74: 1199-1201.
- Ungerfeld EM, Rust SR, Burnett R (2009) The effects of thiamine inhibition on ruminal fermentation: a preliminary study. Folia Microbiol (Praha) 54: 521-526.
- Allen AL, Goupil BA, Valentine BA (2013) A retrospective study of brain lesions in goats submitted to three veterinary diagnostic laboratories. J Vet Diagn Invest 25: 482-489.
- Drewnoski ME, Ensley SM, Beitz DC, Schoonmaker JP, Loy DD (2012) Assessment of ruminal hydrogen sulfide or urine thiosulfate as diagnostic tools for sulfur induced polioencephalomalacia in cattle. J Vet Diagn Invest. 24: 702-709.
- Scott PR (2012) Diagnosis and treatment of coenurosis in sheep.VetParasitol 189: 75-78
- Sakhaee E, Derakhshanfar A (2010) Polioencephalomalacia associated with closanteloverdosage in a goat. J S Afr Vet Assoc 81: 116-117.
- Javanbakht J, Hobbenaghi R, Hosseini E, Bahrami AM, Khadivar F, et al. (2013) Histopathological investigations of neuroprotective effects of Nigella sativa on motor neurons anterior horn spinal cord after sciatic nerve crush in rats. PatholBiol (Paris) 61:250-253.
- Hobbenaghi R, Javanbakht J, SadeghzadehSh, Kheradmand D, Abdi FS, et al. (2014) Neuroprotective effects of Nigella sativa extract on cell death in hippocampal neurons following experimental global cerebral ischemia-reperfusion injury in rats.JNeurolSci 337: 74-79.
- Bizon-Zygmanska D, Jankowska-Kulawy A, Bielarczyk H, Pawelczyk T, Ronowska A, et al. (2011)Acetyl-CoA metabolism in amprolium-evoked thiamine pyrophosphate deficits in cholinergic SN56 neuroblastoma cells. NeurochemInt 59: 208-216.
- Iqbal A, Tariq KA, Wazir VS, Singh R (2013) Antiparasitic efficacy of Artemisia absinthium, toltrazuril and amprolium against intestinal coccidiosis in goats. J Parasit Dis 37: 88-93.
- Ke ZJ, Wang X, Fan Z, Luo J (2009) Ethanol promotes thiamine deficiency-induced neuronal death: involvement of double-stranded RNA-activated protein kinase. Alcohol ClinExp Res 33: 1097-1103.
- ParkhomenkoIuM, Strokina AA, PilipchukSIu, Stepanenko SP, Chekhovskaia LI, et al. (2010) Existence of two different active sites on thiamine binding protein in plasma membranes of synaptosomes. UkrBiokhimZh 82: 34-41.
- Amat S, McKinnon JJ, Olkowski AA, Penner GB, Simko E, et al. (2013) Understanding the role of sulfur-thiamine interaction in the pathogenesis of sulfur-induced polioencephalomalacia in beef cattle. Res Vet Sci 95: 1081-1087.
- Cooper JJ, Schatzberg SJ, Vernau KM, Summers BA, Porter BF, et al. (2014) Necrotizing meningoencephalitis in atypical dog breeds: a case series and literature review. J Vet Intern Med 28: 198-203.
- Krishnamoorthy P, Vairamuthu S, Balchandran C, Muralimanoha B (2007) Pathology of chlorpyriphos and T-2 toxin on broiler chicken. Vet Arhiv 77: 47-57.
- Malik G, Dhahiya JP, Sandeep G, Mishra SK (2008) Clinicopathological studies on chlorpyriphos intoxication in broiler chicken. Proceedings of 19th Annual Conference of Indian Association of Veterinary Pathologists, India.
- Yadav SS, Mukhopadhayay SK, Purohit K (2011) Experimentally induced chlorpyriphos toxicity in broilers: Haematobiochemical and pathomorphological studies. Proceedings of 20th Annual Conference of Indian Association of Veterinary Pathologists, India.
- Goyal S, Rajinder HS, Sandhu SB (2010) Histopathological alterations induced after oral sub-acute thiacloprid toxicity in Gallus domesticus. VeterinarskiArhiv 80: 673-682.
Citation: Sedaghat R, Javanbakht J (2014) Neurohistopathological Findings of the Effects of Amprolium on the Brain and Spinal Cord Changes in the a Animal Model. J Clin Exp Pathol 4:180. DOI: 10.4172/2161-0681.1000180
Copyright: © 2014 Sedaghat R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15258
- [From(publication date): 9-2014 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 10717
- PDF downloads: 4541