Research Article Open Access
Neurofibrillary Tangle Predominant Dementia: Clinical and Pathological Description in a Case Series
Morgan Schwartz1, Thomas G Beach2, Andrew Tsai1, Michael Malek-Ahmadi3, Sandra Jacobson1, Lucia I Sue2, Kathryn Davis1, Marwan N Sabbagh1 and Geidy Serrano2*,
1The Cleo Roberts Center for Clinical Research, Banner Sun Health Research Institute, AZ, USA
2The Civin Laboratory for Neuropathology, Banner Sun Health Research Institute, Sun City, AZ, USA
3Banner Alzheimer’s Institute, Phoenix, AZ, USA
- Corresponding Author:
- Geidy E Serrano
Banner Sun Health Research Institute 10515 W Santa Fe Drive
Sun City, AZ 85351, USA
Tel: 623-832-5608
Fax: 623-832-5681
E-mail: geidy.serrano@bannerhealth.com
Received date: September 30, 2015; Accepted date: January 05, 2016; Published date: January 12, 2016
Citation: Schwartz M, Serrano G, Beach TG, Tsai A, Malek-Ahmadi M, et al. (2016) Neurofibrillary Tangle Predominant Dementia: Clinical and Pathological Description in a Case Series. J Alzheimers Dis Parkinsonism 6:204. doi:10.4172/2161-0460.1000204
Copyright: © 2016 Schwartz M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Objective: The aim of this study is to contribute to an understanding of the clinical presentation and pathological features of neurofibrillary tangle predominant dementia (NFTPD) that will assist with the eventual development of methods for its ante-mortem identification. Method: We contrast eight NFTPD cases identified in the Banner Sun Health Research Institute Brain and Body Donation Program (SHRI-BBDP) database to 114 Alzheimer’s disease (AD) subjects, in terms of their demographics, clinical features, and pathological features. Results: When NFTPD subjects were compared to AD subjects, they were found to have a later onset of symptoms, an older age at death, less impairment prior to death, and less frequent appearance of the Apolipoprotein E ε4 variant. None of the eight NFTPD subjects met the clinical criteria for probable AD. They possessed a diverse range of diagnoses including possible AD, mixed vascular dementia (VAD), dementia NOS, and dementia with Lewy bodies (DLB). AD-related pathology, for both amyloid plaques and neurofibrillary tangles, was less severe in NFTPD subjects than in AD subjects. All eight NFTPD subjects were classified as neurofibrillary tangle Braak stage IV and therefore had fewer tangles in the neocortex when compared to AD subjects with mean Braak stage V (range II–VI). Conclusion: NFTPD subjects have dementia despite a lower pathological burden when compared to AD subjects. In this small sample, the ante-mortem presentation is such that NFTPD subjects are not diagnosed with probable AD. The cognitive and non-cognitive clinical features (delusions, depression, parkinsonism, and hallucinations) of NFTPD and AD are very similar and do not serve as indicators for a diagnosis, but older age (>80), lack of an ApoE ε4 allele and less severe cognitive impairment should further inform the differential diagnosis of NFTPD from AD.
Keywords
Neurofibrillary tangle predominant dementia; Alzheimer’s disease; ApoE ε4; Neuritic plaques; B-amyloid; Tau; Cliniconeuropathological diagnosis
Introduction
Neurofibrillary tangle predominant dementia (NFTPD) is a sporadic subtype of progressive dementia, affecting elderly patients, as defined by a clinical diagnosis of dementia, the presence of 3R and 4R neurofibrillary tangles (NFT) isoforms in limbic areas (usually Braak Stage IV), and the absence or relative scarcity of amyloid (Aβ) plaques in the brain [1-4]. In contrast, Alzheimer’s Dementia (AD) is a progressive dementia characterized by the presence of 3R and 4R neurofibrillary tangles and abundant amyloid plaques [5]. In AD, neurofibrillary tangles are understood to be closely linked with the severity and duration of dementia [6-10]. Approximately 15-30% of those clinically diagnosed with mild to moderate probable AD lack sufficient plaques and/or neurofibrillary tangles to meet AD neuropathological criteria [11,12]. Some of these [12,13] are neuropathologically demonstrated NFTPD but are not clinically distinguishable from AD or other dementias ante-mortem. In a set of individuals clinically diagnosed with mild to moderate AD derived from US National Institute on Aging AD centers, Monsell et al. found that 45% had extensive neurofibrillary degeneration and minimal Aβ plaques [13]. In a group of Apolipoprotein E ε4 (APOE4) non-carriers with mild to moderate AD, 37% had low amyloid levels [13]. In contrast, only 13% of APOE4 carriers with the same diagnosis had low amyloid levels [13]. Identifying individuals who are diagnosed with AD, but have low amyloid levels will be critical as more amyloid-targeted treatments for AD are developed [14]. Clinical characterization of NFTPD has been limited by the dementia’s relatively low prevalence: only 0.7% to 5.8% of clinically diagnosed dementia cases are pathologically diagnosed as NFTPD [4].
Previous research on NFTPD has not established differences between the clinical presentation of NFTPD and AD. The aim of this study is to contribute to an understanding of the clinical and pathological presentation of NFTPD that might eventually enable ante-mortem diagnosis. We contrast eight NFTPD cases identified in the Brain and Body Donation Program (BBDP) database to 114 AD subjects [15,16].
Method
Human subjects
Complete clinical and neuropathological examinations were performed on deceased subjects who had enrolled in the Banner Sun Health Research Institute Brain and Body Donation Program (BSHRIBBDP) [15,16]. All enrolled subjects or their legal representatives signed an Institutional Review Board-approved informed consent form before the time of death. All subjects received annual standardized test batteries that include general neurological, cognitive, and movement disorder components.
From 1218 cases autopsied between January 1997 and December 2014, 848 individuals received a clinical diagnosis of dementia during their lifetime. Of these individuals, those with a neuropathological diagnosis of AD (n=114) and NFTPD (n=8) were included in this study. We excluded AD subjects with secondary pathological diagnoses and AD subjects who never received a complete cognitive assessment at BSHRI (n=575). Any individual with a diagnosis other than AD or NFTPD (n=151) was excluded.
Pathology
For neuropathological examination, multiple brain regions were microscopically examined. The neuropathological examination slide set included staining of paraffin-embedded sections (6 μm) with hematoxylin and eosin (H&E). Amyloid plaques, neurofibrillary tangles, and glial tauopathies, and white matter rarefaction were identified on 80 μm-thick, large-format (3 x 5 cm) formalin-fixed sections using two enhanced silver stains, the Gallyas and Campbell-Switzer methods, for neurofibrillary tangles (NFT) and plaques, respectively. Thioflavin S fluorescent stain was used for amyloid and NFT and H&E were used for white matter rarefaction. Neuritic plaques and NFT were graded as recommended by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) with separate semi-quantitative density estimates of none, sparse, moderate, or frequent (converted to a 0–3 scale for statistical purposes) using standardized published templates [17-19]. Neurofibrillary degeneration was staged by the original method of Braak, in thick sections using the Gallyas method. Regions scored included cortical gray matter from frontal (F), temporal (T), parietal (P), hippocampal CA1 (H), and entorhinal (E) regions. The individual carrying out the scoring (TB) was blinded to demographics and clinical diagnosis. Neuropathological AD diagnoses were made according to the National Institute on Aging/Reagan Institute criteria [18-20].
In addition, paraffin-embedded sections from multiple brain regions including the olfactory bulb, amygdala, brainstem and cerebral cortex were used to document α-synucleinopathies using an immunohistochemical method for α-synuclein phosphorylated at serine 129 [16,21-24].Densities of Lewy-type α-synucleinopathy (LTS) were graded by reference to the DLB Consortium III template.
Statistical analysis
All eight NFTPD subjects found in the BBDP database met the criteria of NFT Braak stage IV and a clinicopathological diagnosis of NFTPD [15,16]. The AD group was compared to the NFTPD group based on the following criteria: age at death, gender, years of education, ApoE genotype, post-mortem interval (PMI), time since last clinical assessment, age at onset, Mini Mental State Exam (MMSE), Global Deterioration Scale (GDS), Functional Assessment Staging Test (FAST), Braak stage, NFT density, total plaque density, cerebral white matter rarefaction, clinical diagnosis, CERAD diagnosis, argyrophilic grains, cerebral amyloid angiopathy, hippocampal sclerosis, and Unified Lewy Body stage.
The Mann-Whitney test was used to contrast clinical and neuropathological variables between the AD group and the NFTPD group. Categorical frequency values were compared using a Fisher test. Analysis of FAST scores was performed by replacing letter scores with number values according to the following transformation: 6a=6.0, 6b=6.2, 6c=6.4, 6d=6.6, 6e=6.8, 7a=7.0, 7b=7.16, 7c=7.32, 7d=7.48, 7e=7.64, 7f=7.8.
Results
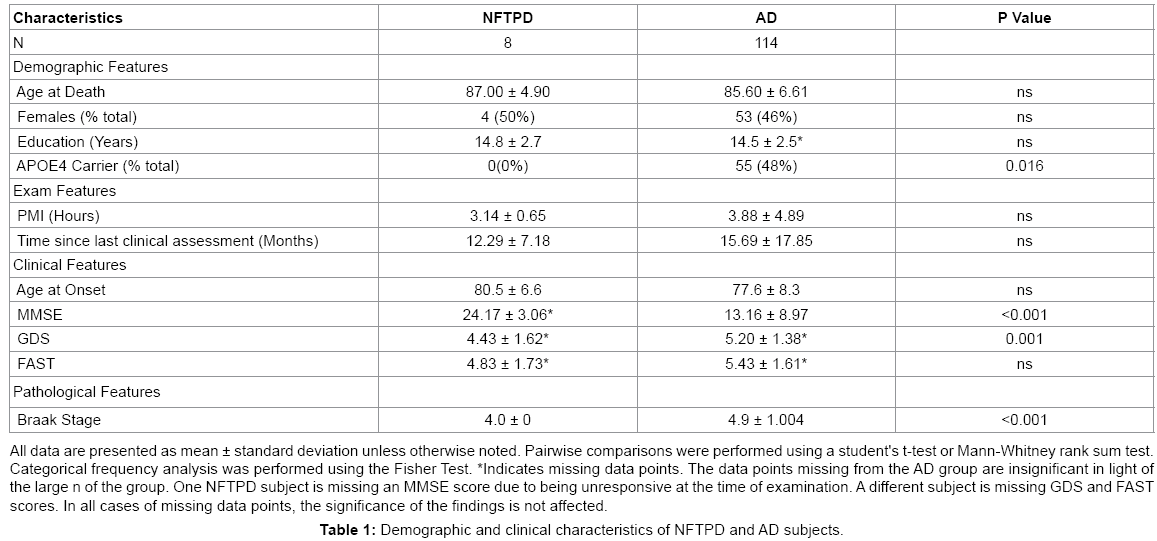
A summary of demographics and clinical characteristics of the NFTPD subjects and AD subjects is presented in Table 1. Compared to AD patients, the NFTPD subjects had a similarly equal gender distribution (NFTPD 50% female vs. AD 46% female) and none of the NFTPD subjects were APOE4 carriers (0% vs. 48%; P=0.016). The NFTPD group had a slightly higher age of symptom onset (69-89, mean 80.5 vs. 58-96, mean 77.6) and age at death (78-94, mean 87.00 vs. 61- 103, mean 85.60) than the AD group; however, these differences were not statistically significant. The NFTPD group was not significantly different from the AD group in terms of PMI (mean 3.14 vs. 3.88; P=0.67), time since last assessment (mean 12.29 vs. 15.69; P=0.62), and years of education (mean 14.8 vs. 14.5; P=0.78).
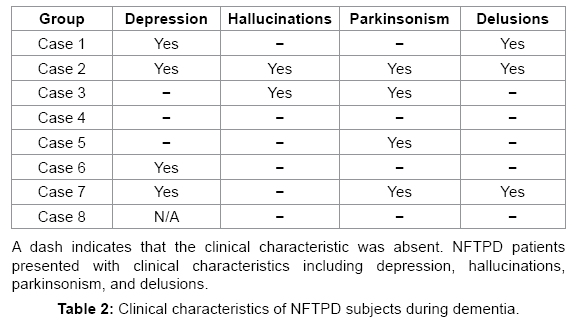
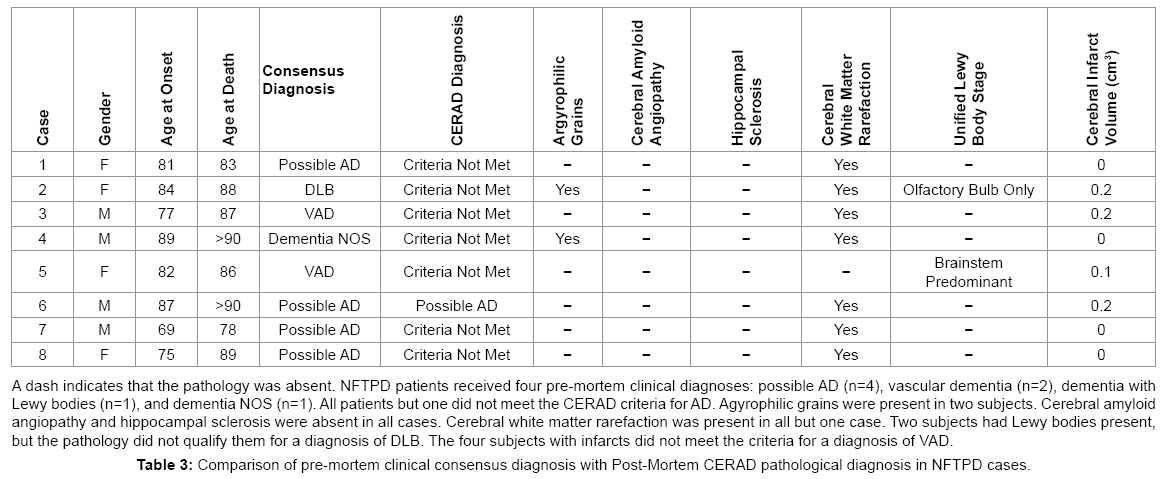
Before death, NFTPD patients had significantly higher MMSE scores than the AD group (20-28, mean 24.17 vs. 0-28, mean 13.16; P<0.001). GDS (2-7, mean 4.43 vs. 2-7, mean 5.20; P=0.001) and FAST (2-7, mean 4.83 vs. 1-7.48, mean 5.43; P=0.448) scores also indicated that the NFTPD group had significantly lower global impairment than the AD group. Table 2 summarizes the four major clinical findings in the NFTPD group during the course of their dementia: depression (4/7), hallucinations (2/8), delusions (3/8), and parkinsonism (4/8). NFTPD cases were heterogeneous in their clinical diagnoses of dementia (Table 3). None were classified as probable AD [5]. Four cases were clinically diagnosed as possible AD [5] with the rest divided between mixed vascular dementia (n=2) [25,26], dementia NOS (n=1), and dementia with Lewy bodies (n=1) [27]. Post mortem examination revealed that none of the cases had enough infarcts or vascular pathology to qualify for a final diagnosis of VAD [25,26] or enough Lewy body pathology to be classified as DLB [27]. Further examination of the aggregate pathology is presented below.
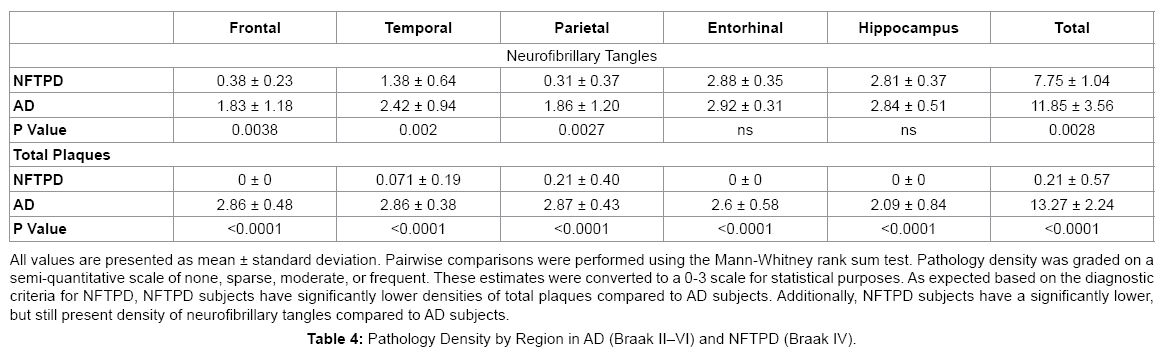
NFTPD subjects had significantly lower Braak scores (all Braak stage IV) than the AD subjects (range II-VI, mean V; P=0.002). As expected by diagnostic definition, the NFTPD group had significantly lower densities of total plaques (0-1.5, mean 0.21 vs. 3-15, mean 13.27; P<0.0001) and neuritic plaques (0-1, mean 0.14 vs. 1-3, mean 2.83). Total cerebral white matter rarefaction was comparable in both groups overall (mean 4.00 vs. 4.77; P=0.74). Table 4 summarizes the pathology present in NFTPD and AD by region. NFTPD patients had similar tangle densities in the entorhinal cortex (mean 2.88 vs. 2.92; P=0.62) and hippocampus (mean 2.81 vs. 2.84; P=0.27), but had significantly lower tangle densities in the frontal (mean 0.38 vs. 1.83; P=0.0038), temporal (mean 1.38 vs. 2.42; P=0.002), and parietal lobes (mean 0.31 vs. 1.86; P=0.0027).
Discussion
This paper compares the clinical and pathological presentation of autopsy-confirmed NFTPD to AD. We found that NFTPD cases had a later onset of symptoms, an older age at death, less impairment before death, and less frequent appearance of APOE4. We found that none of the NFTPD subjects met the clinical criteria for probable or definite AD. The overall AD-related pathology was less severe in NFTPD cases than AD cases. NFTPD subjects had lower neuritic plaque densities as well as total tangle density. All the NFTPD subjects were classified as NFT Braak stage IV and therefore had fewer tangles in the neocortex when compared to most AD subjects.
We found different rates of hallucinations, parkinsonism, and depression in our eight NFTPD cases than have been previously identified for NFTPD; however, our conclusions may be limited by our small sample size. We found that 37.5% of NFTPD cases presented with delusions during the course of the dementia. A study that found delusions in an NFTPD group attributed the symptom to the presence of argyrophilic grains in the nucleus accumbens [28]. However, only one case of delusions in our NFTPD group had argyrophilic grain pathology. We identified three clinical symptoms for which our prevalence was more frequent than what was reported by Jellinger and Attems: hallucinations (25% vs. 1.96%), parkinsonism (50% vs. 1.96%), and depression (50% vs. 17.5%) [4]. These differences suggest that hallucinations, parkinsonism, and depression may be present in NFTPD at a higher rate than has been previously identified.
When the cognitive assessment and detailed clinical presentation of our eight NFTPD cases is compared to the documented clinical presentation of AD cases, there is no distinguishable difference in the rates of delusions (NFTPD 37.5% vs. AD 30-55% [29-31]), hallucinations (25% vs. 3-53% [29-33]), and depression (57% vs. 20- 52% [29,34-37]). Further studies are needed in order to explore if the presentation of delusions underlies the pathological damage of the limbic area in both diseases.
There may be a slightly higher rate of parkinsonism in NFTPD than AD (50% vs. 9-36% [32,38]). The four non-cognitive clinical features (delusions, hallucinations, depression, and parkinsonism) examined here do not clinically distinguish NFTPD patients from AD patients, suggesting that, as documented by autopsy studies, the dominant underlying cause of dementia in both diseases, accumulation of neurofibrillary tangles, results in a very similar clinical profile.
Our group of NFTPD patients received a mixed set of clinical diagnoses: possible AD (50%), VAD (25%), dementia NOS (12.5%) and DLB (12.5%). Post-mortem, none of the cases had enough pathology to meet criteria for clinical-pathological diagnosis of AD, VAD, or DLB. These clinical diagnoses differ in frequency from those reported by Jellinger and Attems. The three most frequent diagnoses in Jellinger and Attems’ results were probable AD (47%), possible AD (17.6%), and nonspecific dementia (17.6%) [4]. Differences in the rate of cases that meet a clinical diagnosis of probable AD might reflect how rigorously the clinical criteria for diagnosing AD were used in each study. In this study we analyzed a small group comprised of eight subjects while Jellinger and Attems presented data for fifty-one subjects [4]. The wide variety of clinical diagnoses received by NFTPD patients might indicate that clinicians often recognize that these subjects have something other than the common presentation of AD.
Recently, the diagnosis, primarily age-related tauopathy (PART), has been introduced as a contrasting diagnosis to NFTPD [2].The PART diagnosis is based on the presence of neurofibrillary tangles and the absence of amyloid plaques in the brain of an individual with or without dementia [2]. PART is distinct from NFTPD because it is a purely pathological diagnosis that does not consider the clinical presentation of the individual [2]. While PART may be advantageous in a pathology setting by increasing the diversity of vocabulary available to pathologists to accurately describe patients, it does not contribute to this study’s consideration of NFTPD. The PART diagnosis has also been challenged in its distinction from AD. An examination of the pathology present in age groups ranging from 0 to 100 years old indicates that tauopathies may be part of the pathological process that produces AD [39]. The aim of this study is to identify clinical features that may distinguish NFTPD patients from AD patients. The usage of the PART diagnosis would include individuals who do not have dementia and who are not at risk for an AD misdiagnosis.
The APOE4 allele is known to be associated with an increased risk of developing AD and can contribute to an earlier onset [40-46]. Along with other researchers, we found that, compared to AD, NFTPD subjects have a lower carriage rate of the ε4 allele [4,47-51], a later onset of disease and death [4,48,50] and a lesser degree of cognitive impairment [4,48,50,52]. Both plaques and tangles are correlated with the degree of cognitive impairment, but this correlation is stronger for tangles [53]. This may explain why cognitive impairment is less severe in NFTPD with few or no plaques and a more restricted distribution of tangles. Overall NFTPD clinical syndrome is very similar to AD probably due to the dominance of tangles causing cognitive impairment.
In over 15 years of data collection including 1,218 cases, the BBDP diagnosed only eight cases of NFTPD, which represents only one percent of dementia cases. This may be partially reflective of a decreased tendency to diagnose NFTPD in earlier years, prior to its widespread recognition as a diagnostic entity. The BBDP performs detailed brain examinations on every subject who comes to autopsy and several staining’s are performed to unmask a myriad of possible pathologies [15,16]. The strength of our eight cases lies in the detailed clinical data collected and the thorough pathological analysis.
Conclusion
The findings of our study and others on the clinical diagnoses of NFTPD patients indicate that this dementia is often recognized by clinicians as something different from classical AD, but there is no clear set of differentiating features. The cognitive and non-cognitive clinical features of NFTPD and AD are very similar and do not serve as indicators for a diagnosis, but older age (>80), lack of an ApoE ε4 allele and less severe cognitive impairment should bring NFTPD into the differential diagnosis. The details that will clinically distinguish NFTPD from AD most likely lie in the nuances of neuropsychological testing. An in-depth review of testing results for large cohorts of NFTPD and AD has the potential to reveal the distinguishing factors that will allow for the pre-mortem diagnosis of NFTPD.
References
- Ulrich J, Spillantini MG, Goedert M, Dukas I, Staehelin IB, et al. (1992) Abundant neurofibrillary tangles without senile plaques in a subset of patients with senile dementia. Neurodegeneration 1: 257-285.
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, et al. (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. ActaNeuropathol 128: 755-766.
- Dickson DW (2009) Neuropathology of non-Alzheimer degenerative disorders. Int J ClinExpPathol 3: 1-23.
- Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. ActaNeuropathol 113: 107-117.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939-944.
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 42: 631-639.
- Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, et al. (1995) Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol 52: 81-88.
- Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, et al. (1997) Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol 41: 17-24.
- Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, et al. (2003) Tangle and neuron numbers, but not amyloid load, predicts cognitive status in Alzheimer's disease. Neurology 60: 1495-1500.
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, et al. (2004) Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 62: 925-931.
- Serrano-Pozo A, Qian J, Monsell SE, Blacker D, Gómez-Isla T, et al. (2014) Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann Neurol 75: 597-601.
- Beach TG, Monsell SE, Phillips LE, Kukull W (2012) Accuracy of the clinical diagnosis of Alzheimer’s disease at National Institute on Aging Alzheimer’s disease centers, 2005-2010. J NeuropatholExpNeurol 71: 266-273.
- Monsell S, Kukull W, Roher AE, Maarouf CL, Serrano G, et al. (2015) Characterizing Apolipoprotein E e4 carriers and noncarriers with the clinical diagnosis of mild to moderate Alzheimer Dementia and minimal ß-Amyloid peptide plaques. JAMA Neurol 72: 1124-1131.
- Salloway S, Sperling R (2015) Understanding conflicting neuropathological finding in patients clinically diagnosed as having Alzheimer’s dementia. JAMA Neurol 72: 1106-1108.
- Beach TG, Sue LI, Walker DG, Roher AE, Lue L, et al. (2008) The Sun Health Research Institute Brain Donation Program: description and experience, 1987-2007. Cell Tissue Bank 9: 229-245.
- Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, et al. (2015) Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology 35: 354-389.
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, et al. (1991) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 41: 479-486.
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging—Alzheimer’s Association guidelines for neuropathological assessment of Alzheimer’s disease. Alzheimers dement 8: 1-13.
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging—Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. ActaNeuropathol 123: 1-11.
- [No authors listed] (1997) Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging 18: S1-2.
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, et al. (2002) a-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol4: 160-164.
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, et al. (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. ActaNeuropathol 117: 613-634.
- Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, et al. (2008) Evaluation of alpha-synucleinimmunohistochemical methods used by invited experts.ActaNeuropathol 116: 277-288.
- Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, et al. (2008) Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. ExpNeurol 210: 409-420.
- Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, et al. (2004) Towards defining the neuropathological substrates of vascular dementia. J NeurolSci 226: 75-80.
- Jellinger KA (2008) The pathology of "vascular dementia": a critical update. J Alzheimers Dis 14: 107-123.
- McKeith I (2006) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 9: 417-423.
- Kawakami I, Hasegawa M, Arai T, Ikeda K, Oshima K, et al. (2014) Tau accumulation in the nucleus accumbens in tangle-predominant dementia. ActaNeuropatholCommun 2: 40.
- Ala TA, Yang KH, Sung JH, Frey WH 2nd (1997) Hallucinations and signs of parkinsonism help distinguish patients with dementia and cortical Lewy bodies from patients with Alzheimer's disease at presentation: a clinicopathological study. J NeurolNeurosurg Psychiatry 62: 16-21.
- Bassiony MM, Steinberg MS, Warren A, Rosenblatt A, Baker AS, et al. (2000) Delusions and hallucinations in Alzheimer's disease: prevalence and clinical correlates. Int J Geriatr Psychiatry 15: 99-107.
- Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA (2000) Hallucinations, delusions, and cognitive decline in Alzheimer's disease. J NeurolNeurosurg Psychiatry 69: 172-177.
- Chaudhury S (2010) Hallucinations: Clinical aspects and management. Ind Psychiatry J 19: 5-12.
- Wragg RE, Jeste DV (1989) Overview of depression and psychosis in Alzheimer's disease. Am J Psychiatry 146: 577-587.
- Van der Mussele S, Bekelaar K, Le Bastard N, Vermeiren Y, Saerens J, et al. (2013) Prevalence and associated behavioral symptoms of depression in mild cognitive impairment and dementia due to Alzheimer's disease. Int J Geriatr Psychiatry 28: 947-958.
- Payne JL, Lyketsos CG, Steele C, Baker L, Galik E, et al. (1998) Relationship of cognitive and functional impairment to depressive features in Alzheimer's disease and other dementias. J Neuropsychiatry ClinNeurosci 10: 440-447.
- Starkstein SE, Jorge R, Mizrahi R, Robinson RG (2005) The construct of minor and major depression in Alzheimer's disease. Am J Psychiatry 162: 2086-2093.
- Tschanz J, Corcoran C, Schwartz S, Treiber K, Green R, et al. (2011) Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County dementia progression study. Am J Geriatr Psychiatry 19: 532-542.
- Morris JC, Drazner M, Fulling K, Grant EA, Goldring J (1989) Clinical and pathological aspects of parkinsonism in Alzheimer's disease. A role for extranigral factors? Arch Neurol 46: 651-657.
- Braak H, Del Tredici K (2014) Are cases with tau pathology occurring in the absence of Aβ deposits part of the AD-related pathological process? ActaNeuropathol 128: 767-772.
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261: 921-923.
- Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, et al. (1995) Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer's disease.Neuroscience 69: 757-761.
- Ohm TG, Kirca M, Bohl J, Scharnagl H, Gross W, et al. (1995) Apolipoprotein E polymorphism influences not only cerebral senile plaque load but also Alzheimer-type neurofibrillary tangle formation. Neuroscience 66: 583-587.
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, et al. (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta AnalysisConsortium.JAMA 278: 1349-1356.
- DeMattos RB (2004) Apolipoprotein E dose-dependent modulation of beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease. J MolNeurosci 23: 255-262.
- Raber J, Huang Y, Ashford JW (2004) ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging 25: 641-650.
- Caselli RJ, Walker D, Sue L, Sabbagh M, Beach T (2010) Amyloid load in nondemented brains correlates with APOE e4. NeurosciLett 473: 168-171.
- Ikeda K, Akiyama H, Sahara N, Mori H, Usami M, et al. (1997) Senile dementia with abundant neurofibrillary tangles without accompanying senile plaques: a subset of senile dementia with high incidence of the APOE e2 allele. In: Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM (Eds.), Alzheimer’s disease: biology, diagnosis and therapeutics. Wiley, New York.
- Jellinger KA, Bancher C (1998) Senile dementia with tangles (tangle predominant form of senile dementia). Brain Pathol 8: 367-376.
- Iseki E, Yamamoto R, Murayama N, Minegishi M, Togo T, et al. (2006) Immunohistochemical investigation of neurofibrillary tangles and their tau isoforms in brains of limbic neurofibrillary tangle dementia. NeurosciLett 405: 29-33.
- Janocko NJ, Brodersen KA, Soto-Ortolaza AI, Ross OA, Liesinger AM, et al. (2012) Neuropathologically defined subtypes of Alzheimer's disease differ significantly from neurofibrillary tangle-predominant dementia. ActaNeuropathol 124: 681-692.
- Santa-Maria I1, Haggiagi A, Liu X, Wasserscheid J, Nelson PT, et al. (2012) The MAPT H1 haplotype is associated with tangle-predominant dementia. ActaNeuropathol 124: 693-704.
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, et al. (2009) Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer’s disease. J NeuropatholExpNeurol 68: 774-784.
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring HarbPerspect Med 1: a006189.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 12433
- [From(publication date):
March-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11234
- PDF downloads : 1199