Neurodevelopmental Screening in High risk Infants Using Denver Developmental Screening Test II
Received: 01-Jun-2023 / Manuscript No. nnp-23-97120 / Editor assigned: 07-Jun-2023 / PreQC No. nnp-23-97120 / Reviewed: 21-Jun-2023 / QC No. nnp-23-97120 / Revised: 23-Jun-2023 / Manuscript No. nnp-23-97120 / Published Date: 30-Jun-2023 DOI: 10.4172/2572-4983.1000323
Introduction
Advances in Perinatal care and establishment of improved neonatal services have increased the survival rates of many high-risk newborns in developing countries. Developmental delay is anticipated in these babies [1]. Delay in normal development is a predictor of neurodevelopmental disorders like cerebral palsy, mental retardation and various learning disabilities. Various causes attributed to developmental delay and there are different clinical presentations, depending on nature of insult [2]. There may be associated problems such as different forms of learning disabilities, seizures, difficulties in feeding, growth, vision and hearing. Numerous studies have shown that there is a high incidence of chronic morbidities and adverse neurodevelopmental outcomes among high-risk infants. This highlights the need for a follow up care service that would ensure systematic monitoring of the general health and neurodevelopmental after discharge from the hospital [3]. Early identification of developmental delay is very important to start early interventional services there by improving quality of life. Ideal developmental assessment tools are elaborate and require expertise in the field. Simplified tools need to be devised to help the pediatrician working under constraints. Denver Developmental Screening Test II (DDST II) is a simple, easy to perform, open test which can be performed in age group 0-6 years [4].
Objectives
Primary
To determine the incidence of developmental delay in high risk infants.
Secondary
To determine the demographic and clinical factors for their risk of leading to developmental delay
To estimate the proportion of high-risk infants with abnormal hearing screen
To estimate the proportion of high-risk neonates with ROP developing adverse visual
Methodology
Study population and study design
This prospective observational study of high-risk infants was conducted in the department of Pediatrics at Armed Forces Medical College and Command Hospital (Southern Command), Pune, a tertiary care, referral and teaching hospital for a period of eighteen months from January 2020 to July 2022 [5]. All the infants fulfilling the eligibility criteria and under follow up in high risk follow up clinic or admitted in the Command Hospital during this period were recruited in the study after written informed consent of parents/ guardian. Permission for the study was granted from the Institutional Ethics Committee [6] (Table 1).
| Objective | Outcome Variable | Definition of Outcome Variable |
Method of measurement |
|---|---|---|---|
| Incidence of Developmental Delay | Gross motor, Fine motor, Language and Social domain test items scored as Passed- P, Failed-F, Refused-R, Caution-C or Delayed-D |
Normal or Suspect | DDST II |
Inclusion criteria
All infants attending for follow-up in high-risk infant clinic will be enrolled for study after parents‘consent:
Term low birth weight (LBW) neonates ≤ 1800 grams
Preterm neonates ≤34 week, irrespective of their weights
Neonatal seizures
Severe neonatal jaundice
Ventilation including CPAP ≥ 48 hours
Neonatal sepsis and Meningitis
Twins: MCDA discordant twin
Prolonged or refractory hypoglycemia
Preterm neonate with IVH / NEC/ PDA/ BPD
For determining incidence sample size calculation is as enumerated below:-
Sample size calculation
P1= Probability of the disease in the exposed = 30% i.e. 0.3
P2= Probability of the disease in non-exposed = 5% i.e. 0.05
Confidence = 95% 4. RR=P1/P2 i.e. 6
E (relative precision) = 30% i.e. 0.3
Alpha = 0.05 7. Beta = 0.80
Total Sample size required is 36 infants.
Data analysis
All the data generated was noted on a proforma as per Annexure – A. A excel data sheet (Annexure – B) was generated to statistically analyze the complete data collected which was analyzed using the SPSS Ver. 22 software. Descriptive statistics would be used to analyze the baseline variables. The numerical variables such as biochemical investigation parameters and age of patients were expressed as mean + standard deviation (SD) or Median (range) as per data distribution pattern. The categorical variables were presented as absolute values or percentage/ proportions [7].
The two-sample t-test (Student‘s t) was used for analyzing the quantitative variables with normal distribution. The Chi (χ2) square test was used where distribution was skewed and for categorical variables [8] (Table 2).
| Objective | Outcome Variable | Definition of Outcome Variable |
Method of Measurement |
|---|---|---|---|
| Demographic and Clinical risk factor assessment | Gestation; Weight; Gender; Growth status; Growth restricted twin; Admission diagnosis; Postnatal morbidities | Standard definitions as given in literature | Data analysis |
| To estimate the proportion of high-risk neonates with ROP developing adverse visual outcomes |
ROP screening result | Type 1 ROP or Type 2 ROP | Indirect Ophthalmosco py (IDO) |
| Proportion of high-risk infants with abnormal hearing screen | Hearing screening result | Pass or Retest | OAE & BERA |
Descriptive statistics: Frequency of demographic and clinical characteristics of the enrolled population [9].
Result
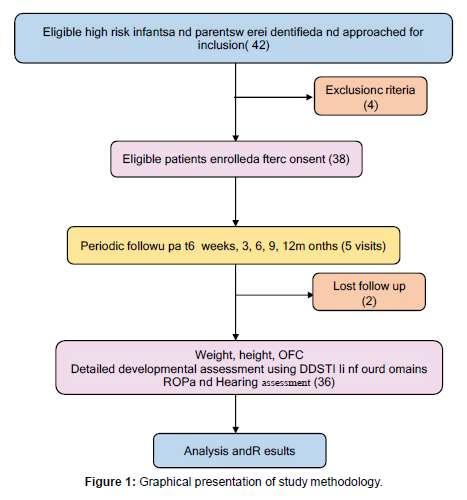
A total of 42 patients were evaluated for the study. Of these total 42 patients, 4 participants fulfilled the exclusion criteria whereas consent could not be obtained for 2 patients [10]. 36 high risk infants under follow up were finally recruited in the study after written informed consent from parents/ guardians. The flow of enrolment of participants in the study is depicted in the flow diagram below: (Figure 1)
Maternal factors vs abnormal DDST II:
When maternal factors were plotted against suspect status on DDST II, the maternal B+ blood group, gestational diabetes and gestational age less than 28 weeks were noted to be significantly correlated with p values of 0.005, 0.003 and 0.029 respectively (Table 3).
| Maternal Factors | DDST II | p-value | |
|---|---|---|---|
| Suspect | Normal | ||
| Maternal age>30 | 4 | 15 | 0.79 |
| Gravida ≥ 3 | 2 | 5 | 0.49 |
| Mother blood gp B+ | 4 | 3 | 0.005 |
| GA ≤ 28 wks | 4 | 5 | 0.029 |
| No AN steroids | 3 | 9 | 0.55 |
| LSCS delivery | 6 | 18 | 0.23 |
| GDM | 3 | 1 | 0.003 |
Conclusions
Out of a total 42 patients, 4 participants fulfilled the exclusion criteria whereas consent could not be obtained for 2 patients. 36 high risk infants under follow up were finally recruited in the study after written informed consent from parents/ guardians.
The mean maternal age was 30.3 ± 4.6 years. The commonest gestational age of mothers was 32 - 35 weeks with 15 participants followed by five mothers with gestational age of 23 - 26 weeks.
Gravidity of G1 and G2 were present in 15 and 13 participants respectively.
The common blood groups were O+ in 16 mothers, B+ in seven mothers and A+ in 6 mothers.
The common maternal comorbidities were hypertension including preeclampsia in seven mothers followed by gestational diabetes in four mothers and PPROM in two mothers. The commonest AFI was noted to be 11 - 15 in 19 mothers.
24 mothers in the cohort delivered baby by LSCS as compared to 12 mothers delivering baby by normal vaginal delivery. 20 babies were males and 16 babies were females. Delayed cord clamping was performed for 28 children in the cohort.
Children had APGAR of 5-7 followed by 10 children with APGAR of 3-4 at 1 minute. At 5 minutes 26 children had APGAR between 7-9. The commonest perinatal morbidity noted in children was respiratory distress in 23 cases followed by sepsis/ meningitis in 7 cases and HIE in 4 cases. even children each were born ELBW (weight of 0.5 to. 1 kg) and VLBW (weight of 1 to 1.5 kgs). 16 children were born low birth weight between 1.5 to 2.5 kg.
Delay in gross and fine motor milestones was noted in three children each.Five children had language delay and four had delaying social and adaptive milestones on performing DDST. Six children were noted to manifest ROP in one or both eyes. Only three children with ROP required laser treatment (*). None in the cohort had any hearing loss to have suspect status.
When maternal factors were plotted against suspect status on DDST II, the maternal B+ blood group, gestational diabetes and gestational age less than 28 weeks were noted to be significantly correlated with p values of 0.005, 0.003 and 0.029 respectively.
The neonatal factors of APGAR < 2 at 1’ (p-value 0.013), severe respiratory distress (p-value 0.001), sepsis/ meningitis (p-value 0.005), severe jaundice (p-value 0.004) and hypoglycemia (p-value 0.004) were noted to be significantly correlated with suspect status.
The incidence of developmental delay in high-risk infants was 19.4% or 194 per 1000 high risk cases.
DDST II test consistently picked up suspect cases with developmental delay since early infancy within 3 months of age. Hence is proved to be excellent evaluation to pick up developmental delay in high-risk cases.
References
- Branson RD (2013) Asynchrony and Dyspnea. Res Care 58:973–989.
- Van Kaam, Anton (2011) Lung-Protective Ventilation in Neonatology. Neonat 99:338–341.
- Howard Stein, Kimberly Firestone, BS, Peter C, Rimensberger (2018) Synchronized Mechanical Ventilation Using Electrical Activity of the Diaphragm in Neonates.
- Mallik M, Watson AR (2008) Antenatally detected urinary tract abnormalities more detection but less action. Pediatr Nephrol. Neonat 23: 897-904.
- Woodward M, Frank D (2002) Postnatal management of antenatal hydronephrosis. BJU Int 89: 149-156.
- Ocheke IE, Antwi S, Gajjar P, McCulloch MI, Nourse P (2014) Pelvi-ureteric junction obstruction at Red Cross Children’s Hospital, Cape Town:a six year review. Arab J Nephrol Trans 7: 33-36.
- Capello SA, Kogan BA, Giorgi LJ (2005) Kaufman RP. Prenatal ultrasound has led to earlier detection and repair of ureteropelvic junction obstruction. J Urol 174: 1425-1428.
- Johnston JH, Evans JP, Glassberg KI, Shapiro SR (1977) Pelvic hydronephrosis in children: a review of 219 personal cases. J Urol 117: 97-101.
- Williams DI, Kenawi MM (1976) The prognosis of pelviureteric obstruction in childhood: a review of 190 cases. Eur Urol 2: 57-63.
- Lebowitz RL, Griscom NT (1977) Neonatal hydronephrosis: 146 cases. Radiol Clin North Am 15: 49-59.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Kothapally KR (2023) Neurodevelopmental Screening in High risk Infants Using Denver Developmental Screening Test II. Neonat Pediatr Med 9: 323. DOI: 10.4172/2572-4983.1000323
Copyright: © 2023 Kothapally KR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 935
- [From(publication date): 0-2023 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 697
- PDF downloads: 238