Research Article Open Access
Neurocognitive Changes after Sustained Ketamine Administration in Children with Chronic Pain
Amy Lee Bredlau1*, Brian T Harel2, Michael P McDermott3, Robert H Dworkin4, David N Korones5, James G Dolan6 and Heather R Adam7
1Department of Pediatrics, University of Rochester, Rochester, New York, USA
2Cog State, Inc. Yale University School of Medicine, Child Study Center, New Haven, USA
3Department of Biostatistics and Computational Biology, University of Rochester, Rochester, New York, USA
4Departments of Anesthesiology and Neurology, University of Rochester, Rochester, New York, USA
5Departments of Pediatrics and Palliative Care, University of Rochester, Rochester, New York, USA
6Department of Public Health Sciences, University of Rochester, Rochester, New York, USA
7Department of Neurology Rochester, University of Rochester, New York, USA
- *Corresponding Author:
- Amy-Lee Bredlau
Medical University of South Carolina
135 Rutledge Avenue, MSC 558 Charleston
South Carolina, USA-29425
Tel: 843-876-2872
Fax: 843-792-8912
E-mail: bredlau@musc.edu
Received date: January 28, 2015; Accepted date: March 11, 2015; Published date: March 27, 2015
Citation: Bredlau AL, Harel BT, McDermott MP, Dworkin RH, Korones DN, et al. (2015) Neurocognitive Changes after Sustained Ketamine Administration in Children with Chronic Pain. J Palliat Care Med 5:215. doi: 10.4172/2165-7386.1000215
Copyright: © 2015, Bredlau AL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Introduction: Ketamine has received attention recently as an agent for chronic pain. There are concerns, however, regarding the neurocognitive changes patients might experience after ketamine exposure. Methods: This prospective, uncontrolled study describes the neurocognitive functioning of 11 children with chronic pain before and after 2 weeks of daily oral ketamine exposure. Neurocognitive assessment was performed at baseline, Week 2, and Week 14. We hypothesized that there would be declines in neurocognitive scores at either Week 2 or Week 14. Results: No decline in neurocognitive function was detected in the children investigated. Mean scores for tests measuring executive function and memory were improved at Weeks 2 and 14 compared to baseline. Discussion: This study did not detect any decline in neurocognitive scores in a small number of children exposed to 2 weeks of oral ketamine therapy. Randomized, controlled studies of the neurocognitive effects of ketamine in children are recommended to further investigate these preliminary findings.
Keywords
Pediatric; Neurologic; Memory; Cognitive function; Sedation
Introduction
Ketamine is an agent that has been used as a dissociative anesthetic during painful procedures and during induction of anesthesia in both adults and children for greater than 50 years [1]. However, there are concerns of the long-term neurocognitive effects of ketamine administration. Children who have had multiple surgeries requiring anesthesia with (and without) ketamine have been noted to have neurocognitive delays [2,3]. It has been impossible, to date, to determine if these neurocognitive delays are related to the ketamine, to the other anesthetic agents, or to a combination of both3.
Currently, ketamine is being investigated for control of chronic pain [4,5] and for treatment of severe depression [6]. These indications necessarily require prolonged ketamine exposure compared to that required for induction of anesthesia or for sedation through a procedure.
If ketamine is going to be further developed as a medication with prolonged exposures, it is important to have an understanding of the neurocognitive toxicities of ketamine. In light of the above-discussed risks for ketamine exposure in children, this study used dosages of ketamine below anesthetic levels. Here in we report data obtained on neurocognitive function in children aged 11-19 with chronic pain exposed to daily, oral ketamine for 14 days.
Methods
This study was approved by the Research Subjects Review Board of the University of Rochester and all participants completed an informed consent process and provided signed consent for their participation; parents provided written permission for the participation of minors (less than 18 years old). The study was registered on ClinicalTrials.gov before the first participant was enrolled (NCT01369680). The study conformed to the Food and Drug Administration guidelines for human subject’s protection (IND No. 110,951). Inclusion criteria were: Numerical Rating Scale (NRS) pain score ≥4, chronic pain for ≥3 months or persisting longer than expected for the underlying diagnosis, and age 8-22 years. The NRS pain had to be reported at least a 4 on the day of enrollment to meet eligibility criteria. This was reported by circling the number of “today’s pain” on a 10 cm NRS-11 pain scale [7]. The rationale for inclusion of children and adolescents aged 8-22 years old in this study was that they are still undergoing brain development. If this age group had notable lasting neurocognitive decline after daily exposure to low-dose ketamine, it would be reasonable to assume that younger children would be similarly harmed by daily exposure to low-dose ketamine. Chronic pain could be either idiopathic or related to a known diagnosis, including but not limited to cancer, rheumatologic disease, sickle cell anemia, cystic fibrosis, pancreatitis, and neuromuscular disease. Participants were ineligible for the study if there was a known or suspected history of: drug dependence or addiction, psychiatric disorder (depression, schizophrenia, bipolar disorder), or other medical problems thought to be unsafe during ketamine exposure. Participants were referred from pediatric services including neurology, orthopedic surgery, gastroenterology, rheumatology, and palliative care for participation in this clinical trial.
Full details of the dose-escalation protocol and rationale, eligibility criteria, and preparation and delivery of oral ketamine have been previously published [4]; the current report extends this work with special focus on the intermediate-term neurocognitive safety data. The study included 4 cohorts of 3 participants each, who were given dosages of oral ketamine of 0.25, 0.5, 1, or 1.5 mg/kg/dose three times a day. Participants in each cohort completed neurocognitive testing before, immediately after and 3 months after exposure to oral ketamine. Oral ketamine was administered three times per day for 14 consecutive days.
Neurocognitive measures
The study was principally concerned with investigating safety and tolerability of ketamine, including potential cognitive effects of the drug. Therefore, participants completed a neurocognitive test battery that included assessments of attention and processing speed, memory, and executive function. The tests were selected because of prior literature [8-12] that suggested that these cognitive domains might be affected by exposure to ketamine.
Neurocognitive assessment was completed three times: at Baseline (prior to, but on the same day as, the first dose of ketamine), at Week 2 (between 24-48 hours after the final dose of ketamine), and at Week 14 (3 months after the final dose of ketamine). None of the participants took ketamine within 24 hours of the neurocognitive testing.
Most tests were drawn from CogState [13], a computer-administered, repeatable battery of neuropsychological tests. These tests were selected because they are brief, resistant to practice effects, and have well-established reliability and validity for the repeated assessment of cognitive function in children [14-16]. The entire battery took approximately 30 minutes for most participants and was administered in a quiet, private room by the study team. The CogState tasks are computer-administered; each task is preceded by scripted, written instructions that are also presented on the computer screen. Study team personnel read these standardized Cog State instructions to each participant for each testing session, unless the participant elected to read the directions on their own. Parents, family members, and friends were asked to step out of the private room for the duration of testing. CogState tasks and variables were chosen to evaluate selected cognitive domains previously suggested to be affected by ketamine exposure and are fully described in Appendix 1.
In addition to CogState, participants completed verbal fluency tasks to assess fluency for phonemic (first letter) and semantic categories. These tasks required participants to generate words as quickly as possible within 60-second trials. The phonemic fluency task utilized three trials, each requiring participants to generate words beginning with a specific letter of the alphabet (Controlled Oral Word Association; F-A-S). The dependent variable was the total number of correct words generated across all three trials. The semantic (Category) fluency task required participants to generate words within two specific categories (animals; food/drinks). The dependent variable for Category fluency was the total number of correct words generated over these two trials.
In addition to the formal neurocognitive assessment, cognitive symptoms were also included among the possible adverse events (AEs) and dose limiting toxicities (DLTs) that could be experienced. AEs, DLTs, and compliance with study medication were monitored at each visit and also weekly by phone. Cognitive DLTs for which participants were specifically monitored included hallucinations, delirium, confusion, mania, anxiety, amnesia, insomnia, agitation, dizziness, and depressed level of consciousness.
Statistical analysis
Boxplots were obtained and descriptive statistics generated to evaluate all neurocognitive outcome variables for normality and outliers. Formal analyses were performed using three composite outcomes based on the following neurocognitive domains: Processing Speed (Detection response speed, Identification response speed, Chase moves per section); Memory (ISL Task correct responses; ISL Recall correct responses); and Executive Function (One Back response speed, Set Shifting accuracy, Groton Maze Learning Test total errors, Controlled Oral Word Association Test words generated, Category Fluency Test words generated). To create the composite variables, each individual variable within a cognitive domain at each study visit was transformed to a z-score using the mean and standard deviation from the baseline study visit to allow comparison of scores with different normal distributions. The assumption of normality was made for these analyses. These z-scores were then averaged across tests, resulting in a single composite score for Processing Speed, Memory, and Executive Function for each participant at each study visit. The null hypothesis tested was that there would be no decline in neurocognitive composite scores on average at Week 2 or Week 14. Formal analyses used a linear mixed model with time (visit week) as the independent variable (categorical) and neurocognitive domain score as the dependent variable. This model uses a direct-likelihood approach to accommodate missing data that is valid under the “missing at random” assumption [17]. The comparisons of primary interest were those representing differences between Baseline and Week 2 and between Baseline and Week 14. For each composite score, the magnitudes of the mean differences between Baseline and Week 2 and between Baseline and Week 14 were expressed using Cohen’s d [18]; this measure of effect size is defined as the mean difference divided by the residual standard deviation from the linear mixed model. All statistical analyses were performed with SPSS (version 20). No power analysis was performed for this study, as it was a pilot study.
Results
Participant details
Participants were aged 11-19 years (mean=16 years) and reported abdominal pain (n=3), joint or musculoskeletal pain (n=7), headaches (n=3), or allodynia (n=1). Participants did occasionally endorse pain in multiple sites and some had idiopathic musculoskeletal pain.
Sample size and follow-up
In the first two cohorts (0.25 mg/kg and 0.50 mg/kg), 100% of participants (3 per group) were present for all three neurocognitive assessments. However, there was one non-completion for the Groton Maze Chase Test (GMCT) and the International Shopping List Test (ISRL) at Baseline do to computer malfunction. In the third dosage cohort (1.0 mg/kg), one participant did not complete the Week 14 evaluation due to transportation difficulties. In the fourth cohort (1.5 mg/kg), two of three participants were not evaluated at Week 14. Unwillingness to return for evaluations was thought to be related to these participants’ experience of adverse events (see below). In addition, the reliability of data obtained from one participant was deemed questionable due to motor and speech disabilities. Consequently, all data for this participant were removed from all analyses. Thus, the final sample size for neurocognitive assessment was N=11 participants (Table 1), though all 12 enrolled and treated participants are included in discussion of adverse events and pain scores.
| Age (years) | |
| Range | 11-19 |
| Median | 16 |
| Sex | |
| Female | 8 |
| Male | 3 |
| Race | |
| African-American | 1 |
| Caucasian | 9 |
| More than One Race | 1 (White and African-American) |
| Ethnicity | |
| Hispanic | 1 |
| Not Hispanic | 10 |
Table 1: Baseline demographic characteristics of all participants contributing to neurocognitive data. (Modified with permission).
Neurocognitive assessment
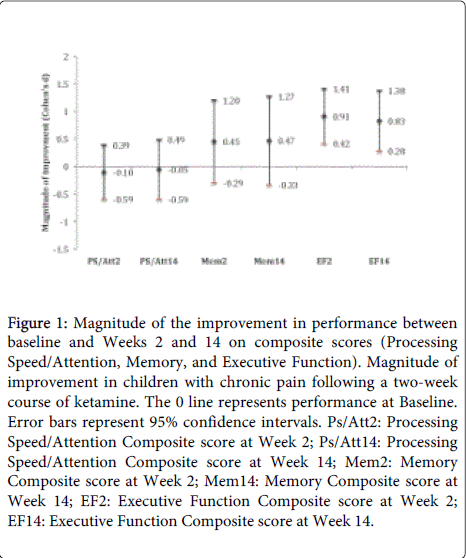
Summary longitudinal data for all neurocognitive outcomes are provided in Table 2. Linear mixed model analyses indicated that there were significant main effects of time on the Executive Function Composite (F (2,18)=8.66, p=0.002) but no significant main effects of time on the Processing Speed Composite (F(2,18)=0.10, p=0.91) or the Memory Composite (F(2,18)=1.06, p=0.37). The results of the planned comparisons (Week 2 vs. Baseline and Week 14 vs. Baseline), which are summarized in Figure 1, indicate that mean composite scores at Week 2 and Week 14 were not statistically different from those at Baseline for either the Processing Speed domain (Week 2: p=0.67, d (Cohen’s d)=-0.10; Week 14: p=0.84, d= -0.05) or the Memory domain (Week 2: p=0.22, d=0.45; Week 14: p=0.23, d=0.47). Significant mean improvements in the Executive Function Composite score were observed at Week 2 (p=0.001, d=0.91) and Week 14 (p=0.005, d=0.83). Improvements in Executive Function Composite scores at both Week 2 and Week 14 were noted.
Figure 1: Magnitude of the improvement in performance between baseline and Weeks 2 and 14 on composite scores (Processing Speed/Attention, Memory, and Executive Function). Magnitude of improvement in children with chronic pain following a two-week course of ketamine. The 0 line represents performance at Baseline. Error bars represent 95% confidence intervals. Ps/Att2: Processing Speed/Attention Composite score at Week 2; Ps/Att14: Processing Speed/Attention Composite score at Week 14; Mem2: Memory Composite score at Week 2; Mem14: Memory Composite score at Week 14; EF2: Executive Function Composite score at Week 2; EF14: Executive Function Composite score at Week 14.
| Task | Measure | Cognitive Domain | Baseline (mean, SD) | Week 2 (mean, SD) | Week 14 (mean, SD) |
|---|---|---|---|---|---|
| n=11 | n=11 | n=9 | |||
| DET | Speed, log10ms | Processing Speed-Attention | 2.46 (.06) | 2.48 (.06) | 2.42 (.17) |
| IDN | Speed, log10ms | Processing Speed-Attention | 2.68 (.06) | 2.70 (.08) | 2.74 (.12) |
| GMCT | Moves per second | Processing Speed-Attention | 1.38 (.40)* | 1.52 (.31) | 1.53 (.28) |
| ISLT | Total words recalled | Memory | 25.90 (2.77) | 28.55 (2.77) | 27.89 (4.14) |
| ISRL | Total words recalled | Memory | 9.80 (1.23)* | 9.73 (1.62) | 10.22 (1.56) |
| ONB | Speed, log10ms | Executive Function | 2.93 (.06) | 2.85 (.06) | 2.87 (.08) |
| SETS | Accuracy, arcsine proportion correct | Executive Function | 1.12 (.11) | 1.18 (.08) | 1.15 (.11) |
| GMLT | Total errors | Executive Function | 49.73 (12.87) | 41.55 (7.53) | 39.00 (12.76) |
| COWA | # words generated | Executive Function | 30.55 (8.50) | 34.64 (9.12) | 40.14 (13.42) |
| Category Fluency | # words generated | Executive Function | 37.27 (9.93) | 39.73 (8.97) | 39.57 (13.84) |
Table 2: Summary of tasks, associated outcome measures and cognitive domains, and scores at baseline, 2 weeks and 14 weeks; DET: Detection Task; IDN: Identification Task; GMCT: Groton Maze Chase Test; ISLT: International Shopping List Test, Immediate Recall; ISRL: International Shopping List Test, Delayed Recall; ONB: One Back task; SETS: Set Shifting task; GMLT: Groton Maze Learning Test; COWA: Controlled Oral Word Association test. Note: *There was one non-completion for the GMCT and the ISRL at Baseline.
Neurocognitive Adverse Events and Dose Limiting Toxicities
Eleven of 12 participants in the study experienced adverse events while on study drug. Three participants discontinued participation prior to Week 14, all of whom discontinued study drug, due to an adverse event (new pain, decreased level of consciousness, and anorexia) prior to Week 2.
Two of these three adverse events were considered to be dose-limiting toxicities, both of which resolved within 2 days of discontinuing ketamine. Grade 1 adverse events involving neurologic changes included dizziness (N=4 participants) and confusion (N=4 participants). One participant experienced memory impairment (Grade 1 AE), and one experienced the dose limiting toxicity (Grade 2 AE) of depressed level of consciousness (complete sedation), which lasted for less than one hour and completely resolved following cessation of ketamine. Other non-cognitive DLTs are described in Bredlau et al. [4].
Pain scores
Of the 12 participants treated in this study, 2 had complete resolution of pain by Week 2. Three other participants had decreases of 2 or more points on the NRS pain scale at Week 2. One participant had an increase in pain, and the other six participants had no change in pain scores.
Three of the six participants who had not had any change in pain scores were the three who did not complete the two-weeks of oral ketamine due to dose-limiting toxicities4. Table 3 lists pain scores for individual participants before and after ketamine exposure, along with executive function composite scores at the same time points.
| Participants | Baseline EC (z-score) | Baseline NRS pain score | Week 2 EC (z-score) | Week 2 NRS pain score |
|---|---|---|---|---|
| 1001 | -.238 | 5 | .236 | 0 |
| 1002 | .030 | 9 | .934 | 7 |
| 1003 | .364 | 10 | .916 | 8 |
| 1004 | .630 | 5 | 1.474 | 5 |
| 1005 | -.960 | 4 | -.202 | 9 |
| 1006 | .392 | 5 | 1.316 | 5 |
| 1007 | .438 | 4 | 1.008 | 0 |
| 1008 | -.102 | 5 | -.042 | 1 |
| 1009 | -.018 | 6 | 1.044 | 6 |
| 1010 | -1.092 | 0 | -.268 | 0 |
| 1011 | .338 | 6 | .664 | 6 |
Table 3: Summary of tasks, associated outcome measures and cognitive domains, and scores at baseline, 2 weeks and 14 weeks; EC: Executive Composite score; NRS: Numerical rating scale (0=no pain, 10-worst imaginable pain).
Discussion
This article describes the neurocognitive changes in children with chronic pain who received 14 days of oral ketamine. No evidence of decline in neurocognitive abilities was observed in this small cohort of children. However, given that this was a preliminary uncontrolled study with a small sample size, it is possible that subtle neurocognitive deficits were missed. Interestingly, and unexpectedly, we observed a statistically significant improvement in executive functioning. However, when directly queried, neither any child nor their parents reported a noticeable change in the child’s executive functioning.
Intuitively, it seems possible that children with chronic pain could experience decrements in neurocognitive test performance that is improved with appropriate pain control, as manifested by the increased (improved) executive function test scores in the current sample. Although it is known that children with chronic pain experience academic disruption (e.g., absenteeism) [19,20], that in turn may impact learning and cognitive performance, there are no studies that have definitively established a direct causal association between chronic pain and cognitive test performance in this diverse pediatric population. A recent study reported that lower intellectual ability in childhood (age 11 years) independently predicted higher chronic widespread pain over 30 years later, but noted that this relationship was mediated by both physiological (body mass index) and sociological (socioeconomic status) factors. In addition, the authors noted that “the poorer cognitive performance often reported in people with chronic pain might… long predate the development of that pain” (p. 2342) [21]. Similarly, in studies of adults with chronic pain, the association between chronic pain and cognitive performance is not yet clear, and is potentially confounded by motor slowing, mood dysfunction, fatigue, and motivational issues [22,23].
While it is recognized that individuals with chronic pain may have difficulty, relative to those without chronic pain, on highly demanding attention or executive function tasks, performance on less cognitively demanding tasks is not impaired. Moreover, psychosocial factors, including psychological distress and educational level, may significantly mediate this relationship, as may a participant’s interest in secondary gain. In another investigation of the relationship between pain and cognition in adults, there was no association between pain ratings and performance on tests of working memory (an executive function skill) among individuals with chronic pain, nor among healthy volunteers exposed to a new pain event (cold presser task). In addition, in both groups, neuropsychological test performance was normal. By contrast, among healthy volunteers who were instructed to malinger, or among chronic pain patients with a known history of malingering, cognitive test performance was impaired. While the authors noted that chronic pain might disrupt attentional control, they also reported that fewer than 5% of the non-malingering, chronic pain participants obtained cognitive test scores in the impaired range [24].
Given the previous literature on ketamine abuse, it is surprising that these children had an improvement in executive functioning after ketamine use. In fact, ketamine abuse (in adults with chronic, high-dose ketamine exposure) has been shown to be deleterious for executive functioning [9]. It is important to note, however, that these studies involved transiently induced pain in adults and were not “real-world” studies of children with chronic pain. In addition, these studies often examined the cognitive performance of participants during the active high-dose drug state. We are reassured that ketamine exposure at the dosages and delivery method used in the current study did not appear to have a sustained negative effect on cognition. Furthermore, consistent with prior studies, pain relief may improve cognitive control and/or reduce psychosocial distress, which is also implicated in the relationship between cognition and pain, as noted above.
Most of the participants in this study were not taking other medications that could affect their cognition, including executive function. Given that most of these children had chronic pain that was not responsive to medications; most of them were not taking opioid pain medications. Other medications to which the participants were exposed are not associated with changes in executive function4 and were stable in dosage during the 2 weeks of oral ketamine exposure and cognitive function testing. The one exception was Participant 1001, who was on daily extended-release morphine, which provided minimal pain control prior to study participation. The participant’s pain was completely resolved on ketamine, and morphine was stopped on day 3 to avoid sedation from morphine while exposed to oral ketamine. A methadone taper was begun, due to the participant’s long-term exposure to morphine.
The small sample in this investigation is a limitation that might have diminished the ability to detect subtle neurocognitive change in participants. The sample was further reduced due to dropout of one subject who experienced ketamine toxicity. This was a transient, fully-reversible, dose-related effect (sedation). In retrospect it would have been interesting to pursue further cognitive assessment in this participant to ascertain whether or not neurocognitive changes occurred following cessation of ketamine. Another important limitation is the use of a non-randomized, uncontrolled design. The improvement in executive functioning scores could be spurious due to practice effects (i.e., improvement due to repeat exposure(s) to a task over a short period of time). Although the CogState tasks selected for this study were designed to minimize practice effects [16,25] it is possible that the Executive Function Composite score improved as a result of such effects, particularly in light of the inclusion of paper and pencil tests (COWAT and CFT) in the composite score [26,27]. It is difficult to evaluate this possibility, however, in the absence of a control group. In addition, the small sample size per dose group and lack of randomization precluded our ability to evaluate potential dose-response relationships.
The cognitive test data in this study were obtained principally as a component of secondary safety monitoring for the preliminary dosage-ranging trial of ketamine. The results will need to be verified with a larger sample size in a randomized, controlled trial before conclusions regarding the effects of exposure to 14 days of oral ketamine in children with chronic pain can be reached. In addition, those children experiencing dose-limiting toxicities were not available for Week 14 testing, yet they could have been the participants most likely to experience lasting neurocognitive decline on the study. Future studies will need to pay close attention to retention of participants, especially those experiencing dose-limiting toxicities.
The fact that participants did not complete cognitive testing during active ketamine exposure could also be considered a limitation. This study instead evaluated whether there were carryover neurocognitive changes following relatively short- and long-term discontinuation of ketamine. However, this approach can be viewed as providing more clinically relevant information, as the model for our ketamine therapy trial was a short-term (2 week) exposure for the treatment of chronic pain, as this is thought to be a sufficient duration for ketamine therapy in pediatric chronic pain [28,29]. It is encouraging that there were no detectable long-term effects of ketamine (3 months after its discontinuation) on cognition.
It is also possible that long-term neurocognitive decline may not be detectable in this study sample after only 3 months off therapy. However, given retention problems as outlined above, we thought it most reasonable to include assessments after a relatively short duration.
On the basis of prior preclinical and preliminary clinical findings, the primary concern for administering low dosage ketamine to children has been the possibility of a resulting decrement in neurocognitive function. The data presented here do not support the hypothesis that oral ketamine administered three times daily at low dosages for 14 days results in a sustained decrement in neurocognitive function, though participants did experience transient neurocognitive or neurologic adverse events while actively exposed to ketamine4. It is intriguing that executive function scores improved after administration of oral ketamine; however, this is in contradiction to previous data in ketamine drug abusers [8,10] and is not consistent with the preclinical data [30,31]. Data from randomized, controlled clinical trials are necessary to further characterize the impact (if any) of oral ketamine in children with chronic pain.
Funding
This work was supported by the University of Rochester CTSA award number UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health; and the K24 RR024198-02, Multi-criteria clinical decision support: A comparative evaluation, from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. There are no potential conflicts of interest.
Disclosures
Dr. Harel is a full time employee of CogState, a cognitive test company that provided the cognitive tests used in this study.
Acknowledgments
The authors would like to thank Karl Kieburtz, MD and Susan Fisher, PhD for their guidance in the design and analysis of the trial.
References
- Morgan CJ, Curran HV; Independent Scientific Committee on Drugs (2012) Ketamine use: a review. Addiction 107: 27-38.
- Mellon RD1, Simone AF, Rappaport BA (2007) Use of anesthetic agents in neonates and young children. AnesthAnalg 104: 509-520.
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, et al. (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110: 796-804.
- Bredlau AL, McDermott MP, Adams HR, Dworkin RH, Venuto C, et al. (2013) Oral ketamine for children with chronic pain: a pilot phase 1 study. J Pediatr 163: 194-200.
- Kajiume T, Sera Y, Nakanuno R, Ogura T, Karakawa S, et al. (2012) Continuous intravenous infusion of ketamine and lidocaine as adjuvant analgesics in a 5-year-old patient with neuropathic cancer pain. J Palliat Med 15: 719-722.
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, et al. (2014) Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry 75: e417-423.
- von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, et al. (2009) Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain 143: 223-227.
- Curran HV, Monaghan L (2001) In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction 96: 749-760.
- Morgan CJ, Muetzelfeldt L, Curran HV (2009) Ketamine use, cognition and psychological wellbeing: a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction 104: 77-87.
- Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A (1998) Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry 43: 811-816.
- Krystal JH, Karper LP, Seibyl JP et al. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of general psychiatry 51:199-214.
- Malhotra AK, Pinals DA, Weingartner H et al. (1996) NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 14:301-307.
- Maruff P, Thomas E, Cysique L et al. (2009) Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 24:165-178.
- Bangirana P, Giordani B, John CC, Page C, Opoka RO, et al. (2009) Immediate neuropsychological and behavioral benefits of computerized cognitive rehabilitation in Ugandan pediatric cerebral malaria survivors. J DevBehavPediatr 30: 310-318.
- Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, et al. (2010) A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology 24: 667-673.
- Mollica CM, Maruff P, Collie A, Vance A (2005) Repeated assessment of cognition in children and the measurement of performance change. Child Neuropsychol 11: 303-310.
- Molenberghs G, Kenward MG (2007) Missing Data in Clinical Studies. Chinchester, UK: John Wiley and Sons.
- Cohen J (1992) A power primer. Psychol Bull 112: 155-159.
- Gorodzinsky AY, Hainsworth KR, Weisman SJ (2011) School functioning and chronic pain: a review of methods and measures. J PediatrPsychol 36: 991-1002.
- Dick BD, Pillai Riddell R (2010) Cognitive and school functioning in children and adolescents with chronic pain: a critical review. Pain Res Manag 15: 238-244.
- Gale CR, Deary IJ, Cooper C, Batty GD (2012) Intelligence in childhood and chronic widespread pain in middle age: the National Child Development Survey. Pain 153: 2339-2344.
- Oosterman J, Derksen LC, van Wijck AJ, Kessels RP, Veldhuijzen DS (2012) Executive and attentional functions in chronic pain: does performance decrease with increasing task load? Pain Res Manag 17: 159-165.
- Hart RP, Martelli MF, Zasler ND (2000) Chronic pain and neuropsychological functioning. Neuropsychol Rev 10: 131-149.
- Etherton JL, Bianchini KJ, Ciota MA, Heinly MT, Greve KW (2006) Pain, malingering and the WAIS-III Working Memory Index. Spine J 6: 61-71.
- Falleti MG, Maruff P, Collie A, Darby DG (2006) Practice effects associated with the repeated assessment of cognitive function using the Cog State battery at 10-minute, one week and one month test-retest intervals. Journal of clinical and experimental neuropsychology 28:1095-1112.
- Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H (2010) Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci 11: 118.
- Beglinger LJ, Gaydos B, Tangphao-Daniels O, et al. (2005) Practice effects and the use of alternate forms in serial neuropsychological testing. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists 20:517-529.
- Chazan S, Ekstein MP, Marouani N, Weinbroum AA (2008) Ketamine for acute and subacute pain in opioid-tolerant patients. J Opioid Manag 4: 173-180.
- Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, et al. (2009) Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain 147: 107-115.
- Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, et al. (2009) Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J DevNeurosci 27: 727-731.
- Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, et al. (2009) Potential neurotoxicity of ketamine in the developing rat brain. ToxicolSci 108: 149-158.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 15217
- [From(publication date):
March-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10614
- PDF downloads : 4603