Research Article Open Access
Neural and Vascular Invasions of Oral Squamous Cell Carcinomas
Cavalcante WS1,2*, Hsieh R1 , Lourenço SV1 , De Souza LNG2 , Almeida-Coburn KL2 and Barros LAP21University of São Paulo, Brazil
2Federal University of Espírito Santo, Brazil
- *Corresponding Author:
- Wanessa Siqueira Cavalcante
Rua José Alexandre Buaiz
160, Ed. London Office Tower, sala 101
Enseada do Suá, Brazil, Vitória, ES – CEP: 29050545
Tel: (55) 27 3315-5742; (55) 27 99892-1221
E-mail: wanessasiqueira@ gmail.com.
Received Date: August 13, 2015; Accepted Date: September 03, 2015; Published Date: September 07, 2015
Citation: Cavalcante WS, Hsieh R, Lourenço SV, De Souza LNG , Almeida-Coburn KL, et al.(2015) Neural and Vascular Invasions of Oral Squamous Cell Carcinomas. J Oral Hyg Health 3:187. doi: 10.4172/2332-0702.1000187
Copyright: © 2015 Cavalcante WS,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Objective: The aim of this retrospective study was to identify perineural and vascular invasions in Oral Squamous Cell Carcinoma lesions.
Methods: A series of 29 OSSC-diagnosed patients and their clinical and demographic data were collected. In order to identify invasive process, Hematoxiline and Eosin (HE) staining of all cases were analysed and CD31, CD34, S100 and PGP9.5 protein expression were performed by immunhistochemistry.
Results: All data were statistically assessed by Kappa test and McNemar’s. We identified vascular and perineural invasions in 17.2% and 31% of cases, respectively, by HE staining analysis. We found intense peritumoral microvascular density in 82.8% of cases, by CD31 immunostaining. The CD34 antibody was recorded only 6.9% of cases with vascular invasion. Perineural invasion was detected in 44.8% of S100 protein immunostained cases, and 58.6% of cases were immunostained by PGP9.5 protein.
Conclusion: According to our H/E and immunohistochemistry analysis, regarding the presence of perineural invasion, PGP9.5 protein was more effective than the others. It was concluded that the identification of vascular and perineural invasions is a useful tool for the prognosis of patients with oral squamous cell carcinoma, thus histopathological and immunohistochemistry methods should be combined for an effective analysis for this tumor.
Keywords
Oral squamous cell carcinoma; Neoplastic invasiveness; CD31 antigens; CD34 antigens; S100 proteins; PGP9.5 human protein; Human
Introduction
Oral cancer is a public health problem, representing the sixth most common malignant neoplasm and more than 90% of cases are Oral Squamous Cell Carcinoma (OSCC) diagnosis. Due to potential aggressiveness and propensity for early and extensive lymphatic metastasis, this cancer type has been widely investigated in recent decades [1-3].
The OSCC is defined as invasive epithelial neoplasia with varying degrees of squamous differentiation and propensity to early and extensive lymph node metastasis, it occurs mainly in adults ranging from 50 to 70 years and it could be associated to smoking and alcohol consumption [1-3]. This carcinoma can develop mainly in the oral and oropharynx mucosal membranes [1].s
Commonly OSCC diagnosis is done by histological and morphological analysis. Its histopathological features could define tumor grading, which is relevant for patient’s prognosis. However, not only tumor grade should be a concern during diagnosis, some process, such as connective tissue invasion, need to be a predictive factor for low survival of patients. Thus, OSCCs lymphovascular and perineural invasions have been associated with bad prognosis, as well as its aggressive behavior, increased of recurrence rates, low survival and metastasis [1,4,5]. Vascular invasion is defined by the presence of tumor cells nests in the vascular space, and perineural invasion is characterized by the presence of tumor cells nests in the perineural space [4-9].
Histopathological diagnosis of OSCC has been established by the HE staining analysis, and it has been considered as gold standard due to its low cost, easy to perform and useful in view of various components of tissues [10]. However, this technique offers little information about the chemical nature of the components of the tissue, thus this limitation has been overcome with the use of immunohistochemistry, which support the detection of proteins expression at different locations of a tissue. Basically, its mechanism consists in recognition of antigen by an antibody associated with tissue epitopes [11].
CD31, CD34, PGP9.5 and S100 biomarkers have been used in immunohistochemical studies to identify accurately vessels and nerves, supporting the presence of perineural and vascular invasion in different diseases, including the OSCC, moreover it results in deeper comprehension for morphological analysis related to its spread, and it contributes to better targeting for treatment and prognosis [9,12-15].
Therefore, studies seeking to deepen OSCC clinical and microscopic evaluation, supported by immunohistochemistry analysis, could contribute to this tumor pathogenesis understanding, because its evolution has a direct impact on patient’s quality of life, and also they can help specialized services in the prevention and diagnosis of oral cancer.
When the primary tumor is associated with the invasions into nerves and vessels, the OSCC shows capacity to metastasize, increasing high recurrence rates and aggressive behavior. So perineural and vascular invasion are essential for early diagnosis and invasive conditions, because they will influence directly on the prognosis and survival of the patients [1,4,5]. The invasion of a primary malignant tumor and its growth in a distant site occurs from the basement membrane degradation, migration of tumor cells through connective tissue, spread through vascular path, colonization and growth of neoplasms in distant locations [16].
The CD31 protein, also known as PECAM-1, is a glycoprotein of molecular weight of 130 kDa, present in endothelial cells and platelets, regarded as a molecule of endothelial cell junction [17-20]. The CD31 molecule is found in continuous endothelium of all types of vessels, including arteries, arterioles, venules, and non-sine curves capillaries veins, making it a useful immunohistochemistry marker for blood vessels, it also can be expressed in leukocytes (monocytes, neutrophils and subgroups of T lymphocyte, especially TCD8+ cells), due to the aggregation between the circulating blood cells and the vascular endothelium [17-19,21]. Additionally, physiological protein roles were suggested in the inflammatory process and CD31 on angiogenesis [18,22]
Using polyclonal and monoclonal antibodies for identification of CD31, some authors [23] have demonstrated involvement in mediating tumor cell adhesion to vascular endothelium, one of the most important steps that lead to metastasis.
Another study [14] aimed to analyze the role of angiogenic and non- angiogenic mechanisms in OSCC evaluating microvessel density and intravascular tumor invasion using CD31 and CD34 immunohistochemical staining protein in 112 specimens. According to their results CD31 was detected in peritumoral microvascular density of all samples, ranging from 11 to 30 (average medium 20.4) microvessels per field in 400X magnification. Regarding the intravascular tumor invasion, CD31 showed less sensibility than CD34 .
The CD34 is a surface glycolphosphoprotein that is expressed in progenitor, endothelial and hematopoietic stem cells of blood capillaries [24-26]. Although several roles have been assigned to this family of proteins, their functions have not been determined yet. Among the functions described in the literature including the increase in proliferation and blockage of the stem cells differentiation, and promotion of lymphocyte adhesion to specialized vascular endothelium in lymphoid tissues [27]. Thus, the protein CD34 has been used in studies that evaluate the relationship between its immunolabeling and metastasis of OSCC to lymph nodes [16,28] and blood vessels [16].
A group of researchers [28] examined the relationship between the CD34 immunostaining patterns and metastasis of OSCC to lymph nodes in a series of 40 cases. They identified metastatic lymph nodes in 18 cases. They found many microvessels with strong remodeling activity as well as non-differentiated endothelial cells and immature endothelial cells in keratinocytes nest areas, and in the infiltrated marginal area of cancer, which seems to be related to metastasis. The lymph node metastasis was most common (63%) where the invasion pattern was the penetrating type, it also could be seen microvessels invading tumor stroma. It was concluded that this penetrating microvascular invasion pattern correlates with metastasis, suggesting that metastasis is closely associated with the distribution of blood vessels surrounding the nest of marginal regions of cancer, with the presence of immature endothelial cells.
Other authors [16] found that the immunohistochemical method with triple staining using the CD34, cytokeratin and podoplanin in a single histological section, which was more sensitive in detecting invasive events (lymphatic and blood vessel neoplastic invasions) than HE staining technique. This method allows us to study in more detail the vascular invasion process, thus providing better understand of the vessels phenotype that are being invaded by the tumor.
The S100 protein family is a group of low molecular weight acidic proteins enriched in neural tissue with two Ca2+ bound regions [29]. It is present mainly in muscle, glands and epithelial tissue [30,31]. Among its functions that have been proposed in the literature, including the translation of the Ca2+ signalizing producing a variety of intra and extracellular biological effects, which is related to the progression of the cell cycle, cell differentiation, interactions between cytoskeleton and membrane, muscle contraction and enzymatic activity. Several disorders, including neoplastic diseases, are related to increased levels of calcium ions [30-33]. The technology of genetic hybridization showed that four members of this family (S100A4, S100A6, S100A8 and S100A14) may be involved in the development of OSCC, with their down-regulated expressions [34]. Despite this information, further studies are needed to better understand the functional roles of the S100 protein in the progression of this type of cancer.
Researchers [9] conducted an important systematic review of 40 cases diagnosed with SCCs of the oral cavity in order to verify that the incidence of perineural invasion would increase by S100 immunohistochemistry detection. The average age of patients diagnosed was 58 years. Originally, the perineural invasion was found in 30% (12 cases) tumors, however, after reviewing the HE slides, the number of cases with perineural invasion increased to 62% (25 cases), later it has increased to 82% (33 cases) when S100 maker was used. Comparing the original slides, all 45 sections with S100 immunostained, no falsepositive cases were detected, but 21 false-negative cases. Thus, clearly the perineural invasion detection accuracy by HE method analysis was significantly lower than immunohistochemical method.
The PGP 9.5 is a protein first detected in human brain, with a molecular weight of 27 kDa. This cytoplasmic protein is specific to nerves and is widely distributed in the brains of vertebrates and diffuse neuroendocrine system cells in human [35-38]. Immunolabeling techniques using PGP 9.5 biomarker are able to demonstrate peripheral nerve fibers with impressive clarity and sensitivity [39], and it is possible to obtain information from nerve endings in human oral mucosa from different intraoral sites, such as vestibule, lip, gingiva, palate, tongue, except the sublingual region [40,41]. Thus, the PGP 9.5 was considered as the best biomarker for nerves and may demonstrate the nerves and their processes in all levels of the nervous system [39].
Some authors [42] have been using the PGP9.5 marker to evaluate the OSCC innervation pattern in 30 patients, and the aim is to find an explanation for the absence of pain symptoms in these cancers. Among their series only two (6.6%) nerve fibers were stained by PGP9.5, suggesting that no apparent pattern of innervation within the OSCC tumor tissue. However, PGP9.5 immunostained nerve fibers in adjacent tissues to carcinoma in 12 (40%) of 30 cases assessed. It was concluded that the nerve fibers are surrounding tumor areas.
Another group of researchers [14] compared the expression of the neuronal marker PGP9.5 to S100 protein expression in 16 cases of malignant nerve sheath tumors by immunohistochemistry, verified that the majority of cases (95%) showed positive expression of PGP9.5, although it is not a specific marker for these kinds of tumor, and the S100 protein has less sensitivity (38%). These results led the authors to conclude that the auxiliary PGP9.5 protein is a useful diagnostic tool to confirm the neural origin in sarcoma cell when no expression of the S100 protein.
Therefore, this present study evaluated perineural and vascular invasions rates in OSCCs by biomarkers staining, and also the correlation of their expression with clinical and histopathological features.
Materials and Methods
Tissue samples
This study was approved by the Research Ethics Committee at Federal University of Espirito Santo (UFES) under number 138/10. All patient’s sample were collected from Surgical Pathology Department, at Programm for Prevention and Early Diagnosis of Oral Cancer, Dental School, UFES, Brazil. Twenty nine formalin-fixed, paraffin-embedded OSCC tissues were selected according to the criteria as defined in the World Health Organization Histological Classification of Tumors [1]. The clinicopathological data such as age, sex, race, location of lesion, smoking, alcoholism, histopathological grading, peritumoral inflammatory infitration, intensity of the inflammatory infiltrate, neoplastic vascular invasion and perineural invasion were collected and are detailed in Table 1. Sections of 4μm thickness of the selected OSCCs were prepared for HE staining.
| (n) | (%) | |||
|---|---|---|---|---|
| CLINICOPATHOLOGICAL INFORMATION | Age (years) | 0-20 | 0 | 0 |
| 21-40 | 1 | 3.4 | ||
| 41-60 | 16 | 55.2 | ||
| 61-80 | 11 | 37.9 | ||
| >80 | 1 | 3.4 | ||
| Sex | Male | 24 | 82.8 | |
| Female | 5 | 17.2 | ||
| Race/color | White | 12 | 41.4 | |
| Black | 4 | 13.8 | ||
| Brown | 12 | 41.4 | ||
| Yellow | 0 | 0 | ||
| Indian | 0 | 0 | ||
| Not Informed | 1 | 3.4 | ||
| Location of the lesions | Mouth floor | 7 | 24.1 | |
| Tongue | 6 | 20.7 | ||
| Lip | 1 | 3.4 | ||
| Hard palate | 1 | 3.4 | ||
| Soft palate | 2 | 6.9 | ||
| Gingiva | 0 | 0 | ||
| Buccal mucosa | 1 | 3.4 | ||
| Alveolar ridge | 5 | 17.2 | ||
| Retromolartrigone | 0 | 0 | ||
| Association of two sites | 6 | 20.7 | ||
| Smoking | Yes | 24 | 82.8 | |
| No | 5 | 17.2 | ||
| Alcoholism | Yes | 14 | 48.3 | |
| No | 5 | 51.7 | ||
| Histopathological grading | Well differentiated | 20 | 69 | |
| Moderately differentiated | 9 | 31 | ||
| Little differentiated | 0 | 0 | ||
| Peritumoral inflammatory infiltrate | Difuse | 17 | 58.6 | |
| Juxtaposed | 12 | 41.4 | ||
| Intensity of the inflammatory infiltrate | Light | 3 | 10.3 | |
| Moderate | 17 | 58.6 | ||
| Intense | 9 | 31.1 | ||
| Neoplastic vascular invasion | Present | 5 | 17.2 | |
| Non-detected | 24 | 82.8 | ||
| Neoplastic perineural invasion | Present | 9 | 31 | |
| Non-detected | 20 | 69 | ||
| Total | 29 | 100 |
Table 1: Clinicopathological data of the OSCCs.
Immunohistochemistry
Four-micrometer sections from specimens were deparaffinized, rehydrated. For S100 and PGP9.5 proteins antigen retrieval, slides were heated in citrate buffer at 95oC for 45 min, followed by hydrogen peroxide incubation for 30 min to block endogenous peroxidase activity, before primary antibody incubation, on the other hand, for CD31 antigen retrieval, slides were heated in Tris-EDTA buffer at 95oC for 45 min. Finally, CD34 antigen retrieval was performed by trypsin enzyme incubation during 1 hour at 37oC. The sections were incubated with anti-CD31, anti-CD34, anti-S100 and anti-PGP9.5, information of these antibodies are described in Table 2.
| Primary serum | Clone | Manufacturer | Antigen retrieval | Titer |
|---|---|---|---|---|
| S100 | Z0311 | Dako (Glostrup, Denmark) | Citrate buffer pH 6.0 for 45 min at 95oC / Steamer | 1:10.000 |
| CD31 | M0823 | Dako (Glostrup, Denmark) | Tris-EDTA pH 9.0 for 45 min at 95oC/ Steamer | 1:2000 |
| CD34 | QNEnd/10 Leica | (Wetzlar, Hesse, Germany) | Enzyme trypsin pH 7.4 for 20 min at room temperature | 0.388889 |
| PGP9.5 | ab15503 | Abcam (Cambridge,England) | Citrate buffer pH 6.0 / Steamer | 1.777778 |
Table 2: Specificity of antibodies.
REVEAL SPRING system (Spring Bioscience, Pleasanton, CA) was used to detect the primary antibodies according to manufacture’s instructions. The DAB Substrate System solution - DAB Chromogen (Spring Bioscience, Pleasanton, CA) were used to show the staining by brownish color. The specimens were counterstained with Carazzi’s hematoxylin, dehydrated and mounted with Permount resin and glass cover slips.
All morphological, histopathological and immunohistochemical analysis were performed, simultaneously, by three blinded researchers in an optical microscope (Nikon Eclipse E200 Multiple Note - Lot: 851 979) and the results were registered by a digital camera (AxioCamMRc/ Zeiss – Lot: 2 04 07 0902).
Analysis
The microscopic criteria used for vascular invasion identification was defined by the presence tumor cells in the vascular space, lymph or blood vessel space, adhered to the vascular endothelium, maintaining or not architectural integrity of the invaded vessels [9].
Semi-quantitative analysis of peritumoral microvascular density was performed by CD31 immunostaining, it has been considered intense when found the presence of more than 10 microvessels per examined field in 400x magnification, and it was classified as moderate when up to 10 microvessels were observed. CD34 immunostaining analysis was evaluated by the presence of neoplastic invasion of blood vessels, the arrangement of the nest or neoplastic cells in isolated vascular structure, and compromising the integrity of the vascular morphology.
Perineural analysis was considered when presence and/or involvement of isogenous keratinocytes groups, i.e. nests of tumor cells, in the perineural space [5]. The perineural space consists of connective tissue surrounding the nerve [43], with full or partial inclusion of the identified nerve [9].
For S100 and PGP9.5 protein expression analysis, the presence of neoplastic perineural invasion, the arrangement of the neoplastic cells in nest or isolated compromising the nervous structure and the integrity of nerve morphology were evaluated.
All results from the HE staining and immunohistochemical analysis were tested statistically. The statistical package IBM SPSS Statistics version 20.0 (IBM) was used.
Subsequently, an analysis of agreement between HE histological technique and immunohistochemistry methods (S100, PGP9.5) were performed using the Kappa coefficient, regarding sensitivity and specificity parameters. The sensitivity is reflected in the likelihood of a method to correctly identify the presence of neoplastic perineural invasion, while the specificity means the likelihood of a method to be negative to the neoplastic perineural invasion actually not detected.
The comparison of presence of neoplastic perineural invasion percentage between HE, S100 and PGP 9.5 staining was performed by nonparametric McNemar’s.
Results
Histopathological findings
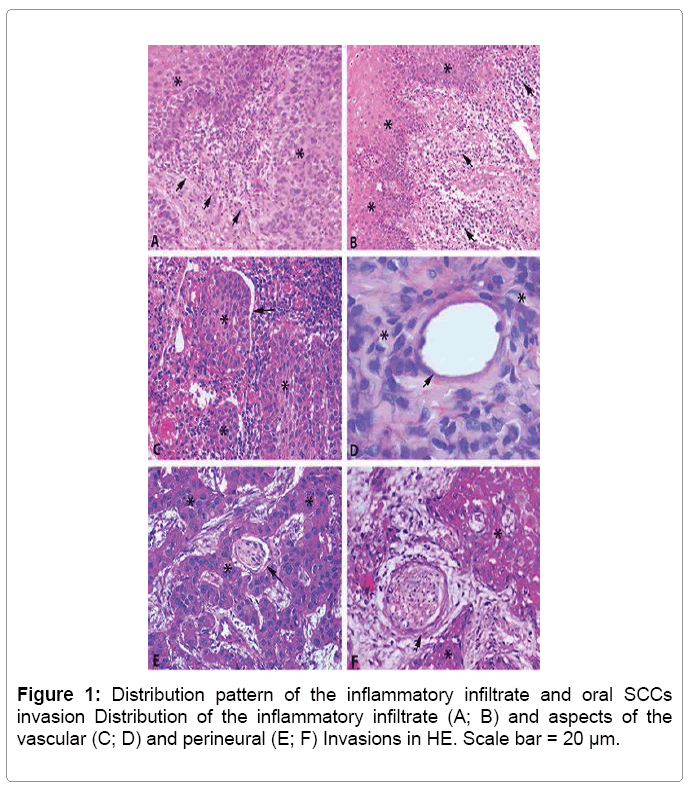
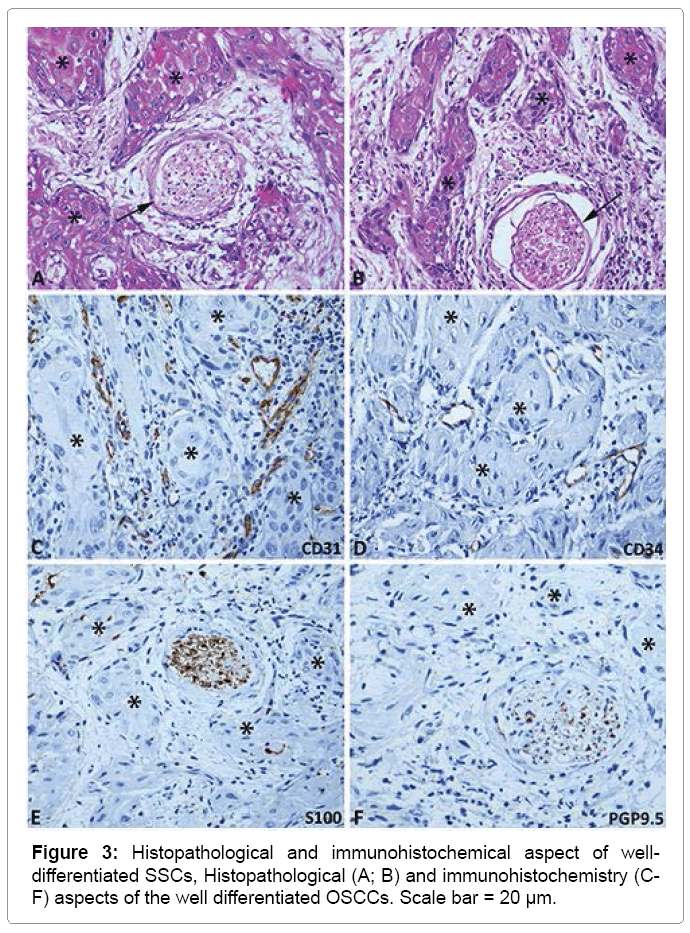
The histopathological analysis for OSCCs classification showed, in most cases, well differentiated grading (69%). The peritumoral inflammatory infiltrate distribution pattern was most prevalent diffuse (58.6%) and moderate intensity (58.6%) (Table 1 and Figure 1).
According to HE stained sections analysis, it was possible to observe the presence of neoplastic invasion of vessels by keratinocytes, arranged in nest form, with no commitment of vascular morphology in 17.2% of cases. The neoplastic perineural invasion was found in 31% of cases, and the morphology of the evaluated nerves was fully preserved (Table 1 and Figure 1).
Immunohistochemical findings
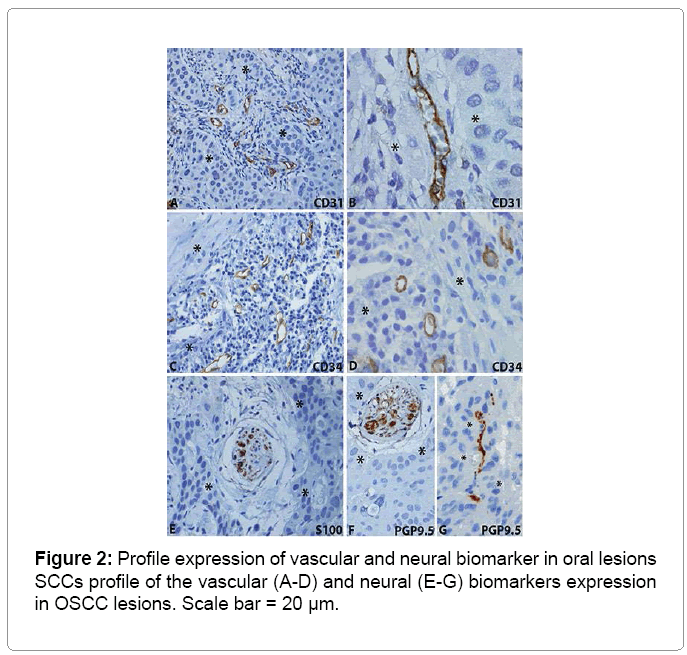
The immunohistochemical results are summarized in Table 3 and illustrated in Figures 2 and 3. The analysis of the CD31 protein expression showed intense peritumoral microvascular density in 82.8% of the cases. Neoplastic invasion from blood vessels was found in 6.9% of cases immunostained by CD34. The neoplastic perineural invasion by nests of keratinocytes was present in 44.8% of cases immunostained by the S100 protein, moreover in 58.6% of cases immunostained by PGP9.5 protein (Table 3, Figures 2 and Figure 3).
| Biomarkers | Evaluated Criteria | Variables | Values | |
|---|---|---|---|---|
| (n) | (%) | |||
| CD31 | Peritumoral microvascular density | Moderate | 5 | 17.20% |
| Intense | 24 | 82.80% | ||
| CD34 | Neoplastic blood vessels invasion | Present | 2 | 6.90% |
| Non-detected | 27 | 93.10% | ||
| Morphology of the vessel | Integrate | 1 | 3.40% | |
| Partially destroyed | 1 | 3.40% | ||
| Not evaluated | 27 | 93.10% | ||
| S100 | Neoplastic perineural invasion | Present | 13 | 44.80% |
| Non-detected | 16 | 55.20% | ||
| Arrangement of the neoplastic perineural cell | Isolate | 0 | 0.00% | |
| Nest | 13 | 44.80% | ||
| Not evaluated | 16 | 55.20% | ||
| Morphology of the nerve | Íntegrate | 13 | 44.80% | |
| Partially destroyed | 0 | 0.00% | ||
| Not evaluated | 16 | 55.20% | ||
| PGP9.5 | Neoplastic perineural invasion | Present | 17 | 58.60% |
| Non-detected | 12 | 41.40% | ||
| Arrangement of the neoplastic perineural cell | Isolate | 0 | 0.00% | |
| Nest | 17 | 58.60% | ||
| Not evaluated | 12 | 41.40% | ||
| Morphology of the nerve | Íntegrate | 17 | 58.60% | |
| Partially destroyed | 0 | 0.00% | ||
| Not evaluated | 12 | 41.40% | ||
| Total | 29 | 100.00% | ||
Table 3: Assessment of vascular and perineural invasion of the osccs subjected to immunohistochemistry technique for CD31, CD34, S100 and PGP9.5.
The agreement of the S100 protein expression with the HE method was strong (kappa = 0.713), however PGP9.5 protein expression with the HE method was moderate (kappa = 0.482). It means that the S100 protein obtained more compatible responses with HE technique, while PGP9.5 resulted in less compatible responses with the mentioned technique (Table 4).
| Neoplastic Perineural Invasion | Neoplastic Perineural Invasion (HE) | ||||||
|---|---|---|---|---|---|---|---|
| Present | Non-Identified | Total | Sensitivity | Especificity | Kappa | ||
| S100 | Present | 9 | 4 | 13 | 100.00% | 80.00% | 0.713* |
| Non-Identified | 0 | 16 | 16 | ||||
| PGP9.5 | Present | 9 | 8 | 17 | 100.00% | 60.00% | 0.482* |
| Non-Identified | 0 | 12 | 12 | ||||
| Total | 9 | 20 | 29 | ||||
* Statistically significant
Table 4: Analysis of agreement between histopathological and immunohistochemistry methods.
Finally, the McNemar test was carried out between the three methods used in the research in order to compare the percentages of presence of neoplastic perineural invasion in the studied cases, showing that the PGP9.5 expression was identified in more cases (58.6%) than the HE method (31%) and S100 (44.8%) immunostaining. Although the difference between PGP9.5 and HE methods was statistically significant, the percentage of cases evaluated by the HE technique did not differ significantly from those by S100 immunostaining (Table 5).
| Categories | HE | S100 | PGP9.5 |
|---|---|---|---|
| Present | 9(31,0%)A | 13(44,8%)A,B (58,6%)B |
17 |
| Non-Identified | 20(69,0%) | 16(55,2%) (41,4%) |
12 |
| Total | 29(100,0%) | 29(100,0%) | 29 (100,0%) |
* Percentages followed by different letters differ significantly by McNemar test, at a significance level of 5%
Table 5: Comparison of the rate of invasion among three methods.
Discussion
One of the main aspects for the prognosis of patients with OSCC is the identification of neoplastic vascular and perineural invasions, since they are associated with bad prognosis, as well as the increased rates of recurrence, aggressive behavior, low survival and metastases [1,4,5]. In this study, the vascular and perineural invasions rates resulting from HE and immunohistochemical techniques analysis showed that IHC was more efficient in detection of perineural invasion, what is the relevant factor to the prognosis of the patients with OSCCs.
HE staining analysis identified 17.2% of neoplastic vascular invasion by nests of keratinocytes, either in space or lymphatic vessel, sometimes resulting thrombus within these vessels. This result is close to that of another group [44] who found 21.6% of vascular invasion using the same technique. However, other authors [9] presented higher values to those already mentioned, having been initially identified 30% of vascular invasion in cases already diagnosed with OSCCs, which number increased to 35% after the revision of the HE stained slides. With the same technique of histological identification, the presence of perineural invasion by nests of keratinocytes was found in 31% of cases, which are in agreement with a study that found 31.1% [44], but other than that it was reported in other works, 52% [45,46] and 62% [9].
From the analysis of the expression of CD31 protein, there was intense density (> 10 vessels per field examined in 400x magnification) microvascular peritumoral. It has been reported that the CD31 protein has a role in the inflammatory process and angiogenesis [18,19], which is responsible for the nutrition of tumor cells, thereby contributing to tumor progression, at the same time the tumor growth stimulates the formation of new microvessels [43]. The analysis of CD34 immunostaining showed low spread of OSCCs by blood vessels (6.9%). We suggest a more detailed investigation of these invasions with D240 and cytokeratin antibodies.
In histopathological diagnosis, attention should be given to peritumoral fibrosis, which refers to the presence of concentric rings of fibrous tissue, when associated to nests of tumor cells, may mimic perineural invasion, making it difficult to distinguish from nerve tissue without the aid immunomarkers such as S100 and PGP9.5. A cancer that gains the ability to invade the perineurium finds a path of low resistance of the protected host defenses, which facilitates the spread of the cancer along the nerve, reaching various parts of the body [5]. A more sensitive biomarker to identify the nerves is the PGP 9.5 than the S100, which demonstrates the nerve structures at all levels of the nervous system [39]. In addition, isolated nerve fibers, nerve bundles, around blood vessels, and they innervate muscles and glands [40]. PGP 9.5 identifies nerve endings organized in various regions of the mouth [41].
In addition to the above analysis, HE staining still has been considered as gold standard than immunohistochemical technique. However, it was noted that the data obtained from S100 immunostaining was compatible with those obtained from the technique of HE. On the other hand, the same was not observed with the expression of PGP 9.5, resulting in less compatible responses between the both techniques.
However, when the presence of neoplastic perineural invasion was compared among the three methods, the PGP 9.5 identified more cases than the method HE and the S100, detecting approximately twice as many neoplastic perineural invasion that the technique used routinely in pathological services. PGP 9.5 was able to mark nerve fibers of the oral cavity of all sizes with stunning clarity, demonstrating that this antibody is the best marker to nerves when compared to S100, corroborating with other results found in the literature [14,39,41].
In summary, the record of the presence of perineural and vascular invasion during microscopic analysis of OSCCs can be accomplished through conventional HE staining, but vascular and neural structures immunostaining extends this analysis to prognosis, and they should also be performed for a more effective regular pathological diagnosis
References
- Barnes L, Eveson JW, Reichart P, Sidransky D (2005) Pathology and genetics of head and neck tumors. WHO classification of tumors, Lyon, IARC.
- Zigon G, Berrino F, Gatta G, Sánchez MJ, van Dijk B, et al. (2011) Prognoses for head and neck cancers in Europe diagnosed in 1995-1999: a population-based study.Ann Oncol 22: 165-174.
- Gaitán-Cepeda LA, Peniche-Becerra AG, Quezada-Rivera D (2011) Trends in frequency and prevalence of oral cancer and oral squamous cell carcinoma in Mexicans. A 20 years retrospective study.Med Oral Patol Oral Cir Bucal 16: e1-5.
- Niimi K, Yoshizawa M, Nakajima T, Saku T (2001) Vascular invasion in squamous cell carcinomas of human oral mucosa. Oral Oncol 37: 357-364.
- Dunn M, Morgan MB, Beer TW (2009) Perineural invasion: identification, significance, and a standardized definition. DermatolSurg 35: 214-221.
- Bryne M, Koppang HS, Lilleng R, Kjaerheim A (1992) Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol 166: 375-381.
- Pindborg JJ, Reichart PA, Smith CJ, Van der Waal I (1997) Histological typing of cancer and precancer of the oral mucosa. WHO, Berlin, Springer.
- Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, et al. (2005) Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J SurgPathol 29: 167-178.
- Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RS (2005) Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med 129:354-359.
- Junqueira LC, Carneiro J (2008) Histologia e seusme´todos de estudo. In: ______. Histologiaba´sica. (11thedn) Rio de Janeiro: Guanabara Koogan.
- Stricker TP, Kumar V (2010) Neoplasia. In: Mitchell RN, Kumar V, Abbas AK, Fausto N, Aster JC. Robbins &Cotranpatologia: bases patológicas das doenças. (8thedn). Elsevier, Rio de Janeiro, page: 259-330.
- Ramos-Vara JA (2005) Technical aspects of immunohistochemistry. Vet Pathol 42: 405-426.
- Ralhan R, Swain RK, Agarwal S, Kaur J, Nath N, et al. (1999) P-glycoprotein is positively correlated with p53 in human oral pre-malignant and malignant lesions and is associated with poor prognosis. Int J Cancer 84: 80-85.
- Hoang MP, Sinkre P, Albores-Saavedra J (2001) Expression of protein gene product 9.5 in epithelioid and conventional malignant peripheral nerve sheath tumors. Arch Pathol Lab Med 125: 1321-1325.
- Shieh YS, Lee HS, Shiah SG, Chu YW, Wu CW, et al. (2004) Role of angiogenic and non-angiogenic mechanisms in oral squamous cell carcinoma: correlation with histologic differentiation and tumor progression. J Oral Pathol Med 33: 601-606.
- O’Donnell RK, Feldman M, Mick R, Muschel RJ (2008) Immunohistochemical method identifies lymphovascular invasion in a majority of oral squamous cell carcinomas and discriminates between blood and lymphatic vessel invasion. J HistochemCytochem 56:803-810.
- Albelda SM, Muller WA, Buck CA, Newman PJ (1991) Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol 114: 1059-1068.
- DeLisser HM, Newman PJ, Albelda SM (1994) Molecular and functional aspects of PECAM-1/CD31. Immunol Today 15: 490-495.
- Jackson DE (2003) The unfolding tale of PECAM-1. FEBS Lett 540: 7-14.
- Privratsky JR, Paddock CM, Florey O, Newman DK, Muller WA, et al. (2011) Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. J Cell Sci 124: 1477-1485.
- Newman PJ (1997) The biology of PECAM-1. J Clin Invest 100: S25-29.
- DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, et al. (1997) Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 151: 671-677.
- Tang DG, Chen YQ, Newman PJ, Shi L, Gao X, et al. (1993) Identification of PECAM-1 in solid tumor cells and its potential involvement in tumor cell adhesion to endothelium. J BiolChem 268: 22883-22894.
- Civin CL, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, et al. (1984) Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol 133:157-165.
- Tindle RW, Nichols RA, Chan L, Campana D, Catovsky D, et al. (1985) A novel monoclonal antibody BI-3C5 recognisesmyeloblasts and non-B non-T lymphoblasts in acute leukaemias and CGL blast crises, and reacts with immature cells in normal bone marrow. Leuk Res 9: 1-9.
- Furness SG, McNagny K (2006) Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res 34: 13-32.
- Nielsen JS, McNagny KM (2008) Novel functions of the CD34 family. J Cell Sci 121: 3683-3692.
- Nagatsuka H, Hibi K, Gunduz M, Tsujigiwa H, Tamamura R, et al. (2005) Various immunostaining patterns of CD31, CD34 and endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med 34: 70-76.
- Kligman D, Hilt DC (1988) The S100 protein family. Trends BiochemSci 13: 437-443.
- Zimmer DB, Cornwall EH, Landar A, Song W (1995) The S100 protein family: history, function, and expression. Brain Res Bull 37: 417-429.
- Schäfer BW, Heizmann CW (1996) The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends BiochemSci 21: 134-140.
- Donato R (2001) S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 33: 637-668.
- Salama I, Malone PS, Mihaimeed F, Jones JL (2008) A review of the S100 proteins in cancer. Eur J SurgOncol 34: 357-364.
- Sapkota D, Bruland O, Bøe OE, Bakeer H, Elgindi OA, et al. (2008) Expression profile of the S100 gene family members in oral squamous cell carcinomas. J Oral Pathol Med 37: 607-615.
- Jackson P, Thompson RJ (1981) The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J NeurolSci 49: 429-438.
- Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J (1983) PGP 9.5--a new marker for vertebrate neurons and neuroendocrine cells.Brain Res 278: 224-228.
- Day IN, Thompson RJ (1987) Molecular cloning of cDNA coding for human PGP 9.5 protein. A novel cytoplasmic marker for neurones and neuroendocrine cells. FEBS Lett 210: 157-160.
- Day IN, Hinks LJ, Thompson RJ (1990) The structure of the human gene encoding protein gene product 9.5 (PGP9.5), a neuron-specific ubiquitin C-terminal hydrolase. Biochem J 268: 521-524.
- Wilson PO, Barber PC, Hamid QA, Power BF, Dhillon AP, et al. (1988) The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br J ExpPathol 69: 91-104.
- Hilliges M, Hellman M, Ahlström U, Johansson O (1994) Immunohistochemical studies of neurochemical markers in normal human buccal mucosa. Histochemistry 101: 235-244.
- Hilliges M, Astbäck J, Wang L, Arvidson K, Johansson O (1996) Protein gene product 9.5-immunoreactive nerves and cells in human oral mucosa. Anat Rec 245: 621-632.
- Habash FS, Hantash RO, Yunis MA (2012) Assessment of the innervation pattern of oral squamous cell carcinoma using neural protein gene product (9.5)-An immunocytochemical study. J Oral MaxillofacPathol 16: 16-21.
- Mastoraki A, Ioannidis E, Apostolaki A, Patsouris E, Aroni K (2009) PGP 9.5 and cyclin D1 coexpression in cutaneous squamous cell carcinomas. Int J SurgPathol 17: 413-420.
- Tai SK, Li WY, Chu PY, Chang SY, Tsai TL, et al. (2012) Risks and clinical implications of perineural invasion in T1-2 oral tongue squamous cell carcinoma. Head Neck 34: 994-1001.
- Fagan JJ, Collins B, Barnes L, D'Amico F, Myers EN, et al. (1998) Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 124: 637-640.
- Ahmad A , Hart IR (1997) Mechanisms of metastasis. Crit Rev OncolHematol 26: 163-173.
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Children’s Oral Health
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Fluoride Treatments
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral and Maxillofacial Pathology
- Oral Hygiene
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Microbiome
- Oral Rehydration
- Oral Surgery Special Issue
- Orthodontistry
- Periodontal Disease Management
- Periodontistry
- Root Canal Treatment
- Tele-Dentistry
Recommended Journals
Article Tools
Article Usage
- Total views: 17782
- [From(publication date):
November-2015 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 12844
- PDF downloads : 4938