Case Report Open Access
Neoadjuvant Chemotherapy in the Management of Extraovarian Yolk Sac Tumor: A Case Report and A Review of the Literature
Susana Mustafa, Amnon Amit, Ari Reiss and Zeev Weiner*Department of Obstetrics and Gynecology, Rambam Health Care Campus, Ruth and Bruce Rappaport, Faculty of Medicine Technion-Israel Institute of Technology,Haifa, Israel
- *Corresponding Author:
- Zeev Weiner
MD, Department of Obstetrics and Gynecology
Rambam Health Care Campus, Ruth and Bruce Rappaport
Faculty of Medicine Technion-Israel Institute of Technology, Haifa, Israel
Tel: 9728542536
Fax: 9728542453
E-mail: z_weiner@rambam.health.gov.il
Received date: September 17, 2014; Accepted date: October 16, 2014; Published date: October 18, 2014
Citation: Mustafa S, Amit A, Reiss A, Weiner Z (2014) Neoadjuvant Chemotherapy in the Management of Extraovarian Yolk Sac Tumor: A Case Report and A Review of the Literature. J Clin Exp Pathol 4:198. doi:10.4172/2161-0681.1000198
Copyright: ©Mustafa S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Extraovarian yolk sac tumor (YST) is an extremely rare malignancy occurring mainly in girls and young women of childbearing ages and commonly treated with fertility-sparing surgery and postoperative adjuvant chemotherapy. We report a case treated with neoadjuvant chemotherapy (NACT) followed by cytoredutive surgery and adjuvant chemotherapy.
A 32-year-old single woman came for a second opinion following explorative laparotomy secondary to pelvic mass and histologic results of an undetermined malignancy. Our evaluation revealed a yolk sac tumor involving the cecum with a cutaneous fistula and pelvic infection. The patient was treated with three courses of Bleomycin, Etoposide and Cisplatin (BEP) followed by an optimal cytoreductive surgery and another two courses of Etoposide and Platinum-AQ (EP). There is no evidence of disease during a two years follow-up.
Conclusion: NACT followed by fertility sparing surgery should be considered as an alternative to primary debulking surgery in advanced YSTs.
Keywords
Neoadjuvant chemotherapy; Germ cell tumor; Extraovarian yolk sac tumor
Introduction
Yolk Sac Tumor (YST), formerly known as endodermal sinus tumor, is a rare malignant germ cell neoplasm, primarily occurring in young women and adolescents. Although YST is the third most common malignant germ cell tumor of the ovary it consists of only 1% of all ovarian malignancies [1]. Extragonadal YST is an extremely rare malignancy, representing 2-5% of all adult germ-cell malignancies [2].
During embryogenesis, at the fourth through the sixth weeks of development, germ cells migrate along the urogenital ridge. Misplacement and a remnant tissue anywhere along the migration course may play a role in the pathogenesis of extra gonadal YSTs. Although the exact mechanism of this misplacement is poorly understood, malignant transformation of these cells leads to primary germ cell tumors at these sites [3,4].
Since it occurs mainly in girls and young women of childbearing ages, the standard of care consists of fertility-sparing surgery and postoperative adjuvant chemotherapy with Bleomycin, Etoposide, and Cisplatin (BEP). BEP has become the most effective regimen and therefore the first line of chemotherapy since 1990s [5].
In patients with an advanced stage of disease, a cytoreductive surgery with fertility sparing is often impossible. In these cases, neoadjuvant chemotherapy (NACT) may be appropriate although this approach has not been widely investigated.
We report a case of extra gonadal YST treaded with NACT, followed by cytoreductive surgery and adjuvant chemotherapy. We discuss the role of NACT treatment in germ cell tumors reviewing the literature.
Case Report
A 32-year-old nulligravida underwent explorative laparotomy secondary to abdominal pain and pelvic mass. Explorative laparotomy revealed a pelvic mass involving the intestine with difficulties to isolate the uterus and right ovary secondary to extensive adhesions. The biopsies indicated malignancy from an unknown origin.
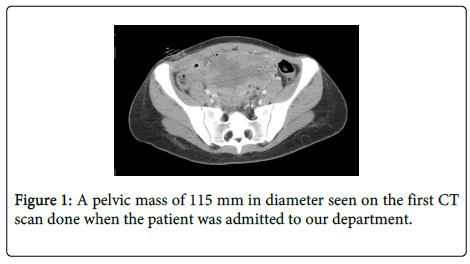
The patient presented to our department for a “second opinion”. On admission, she was febrile, discharging pus from an infected surgical wound. A review of the histologic specimen suggested Hepatic Yolk Sac Tumor. The CT scan demonstrated a solid mass (Figure 1) and fluid collection in the pelvis with a fistula extending from the fluid collection to the abdominal wall.
In addition, an involvement of the cecum was also suspected. Serum alpha-fetoprotein (AFP) was elevated (105.5 ng/ml, normal values <10 ng/ml) with normal values of cancer antigen 125 (CA-125). There was a leukocytosis with a left shift and the culture of the purulent discharge revealed mixed organisms. Antibiotics were administered according to sensitivity.
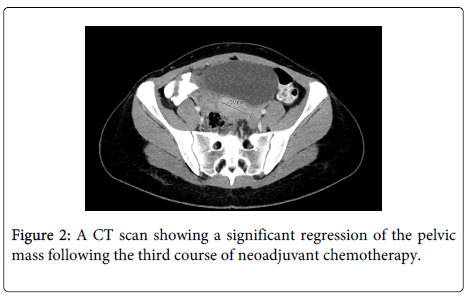
Due to the difficulties to obtain significant debulking and the occurrence of infection involving the abdominal scar, we decided to administer neoadjuvant chemotherapy (NACT) using BEP along with an aggressive antibiotic treatment. The patient received three courses of BEP with total doses of Bleomycin 270 IU, Etoposide 1700 mg, and Cisplatin 432 mg. Following the third course of chemotherapy AFP decreased to normal values (5 ng/ml). The repeated CT scan showed significant regression of the pelvic mass with a spontaneous resolution of the fistula (Figure 2).
Following the response to chemotherapy, we decided to perform surgery in order to achieve a complete debulking. On the the explorative laparotomy the following findings were noted: the omentum was attached to the surgical scar, the tumor was located in the mesocolon and involved the terminal ileum, the cecum and the sigmoid colon. The conglomerate was attached to the uterus and the right adnexa. However, separation of the tumor mass from the attached organs was feasible with no evidence of macroscopic tumor in the uterus and ovaries. Biopsies from both ovaries did not reveal any evidence of malignancy.
Right hemicolectomy, resection of a small bowel loop, and partial sigmoidectomy were performed with iliocolic and sigmoido-rectal anastomosis. Omentectomy was also performed while the uterus and ovaries were preserved.
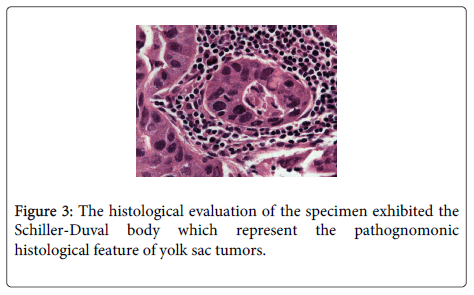
The histologic results revealed a Yolk sac tumor originated from the mesocolon with severe cellular atypia. The tumor was detected on the surface of the colon and one out of 51 lymph nodes revealed necrotic metastasis. The histological evaluation of the specimen presented the Schiller-Duval body (Figure 3).
Following surgery, two additional cycles of chemotherapy consisting of Etoposide 170 mg and Platinum-AQ 34 mg (EP) were administrated. Two years following diagnosis the patient is healthy with normal CT scan and normal AFP values.
Discussion
YSTs usually occur in the gonads and extragonadal YSTs, although rare, have been reported in several cases [6-10]. The most common sites for extragonadal YSTs are the sacrococcygeal region, the mediastinum [6], the retro peritoneum, and the pineal gland [2]. Another common location is the female reproductive tract such as the vagina, the vulva, the cervix, and the uterine corpus [11,12]. The mesentery as a primary site of an extragonadal YST, has been reported in one case describing two YSTs presented in the mesentery of the jejunum and the transverse colon in two male patients (2 and 17 years old). Both patients received chemotherapy postoperatively [13].
Prior to the introduction of chemotherapy, YSTs had an unfavorable prognosis with 80-90% mortality rate within two years of diagnosis [14,15]. The introduction of platinum based chemotherapy changed significantly the overall survival rate for all stages in the range of 85% to 94% [16,17]. Patients who do not respond to chemotherapy die within three years of diagnosis [18]. Serum AFP is a reliable marker for monitoring the response to chemotherapy and to detect tumor recurrence.
Due to their low incidence, no prospective randomized studies have been published about treatment and the management of these tumors is based on the results of retrospective studies and on the management of ovarian epithelial cancer (EOC) [19]. Similar to EOC, cytoreductive surgery strongly affects the prognosis of YSTs [18]. As in other germ cell tumors, the standard of care consists of surgery followed by postoperative chemotherapy. Since most of YSTs originate from the ovaries and many of these patient are of childbearing ages it is acceptable to preserve fertility by sparing the uterus and at least one of the ovaries. In case of extra gonadal YSTs there is no indication to remove the uterus or ovaries if biopsies exclude malignancy as in our case.
Due to the fact that YSTs are highly aggressive with 30% to 40% of them being in stage III or IV at the time of diagnosis [20], optimal debulking is not always feasible or may be associated with a significant increase in morbidity
Several studies demonstrated that using NACT in EOC is associated with less morbidity and an increase probability to achieve optimal cytoreduction, especially in patients with an advanced stage and low performance status [21-26].
Unlike EOC, the data is limited regarding the role of NACT in advanced malignant germ cell tumors of the ovary (MOGCT). In a recent study addressing the role of NACT in the management of advanced ovarian YST reported by Yan Lu et al. [27] 21 patients with stage III or IV diseases received NACT compared with 32 patients who underwent primary debulking surgery followed by adjuvant chemotherapy. The results showed that after NACT the overall status of the patients was improved and patients treated with NACT had a higher rate of optimal cytoreduction with less peri-operative morbidities. As previously mentioned, only few studies examined the role of NACT in the management of advanced MOGCT [27,28].Talukdar et al.[28] reported the results of 23 patients diagnosed with MOGCT with advanced bulky disease and poor performance status who received NACT, compared with 43 patients with advanced disease who underwent upfront surgery followed by adjuvant chemotherapy. Following NACT, 21 patients responded, achieving a complete response (CR) in 16 with pathological CR in 13 of them. Other small series reported good outcome using preoperative chemotherapy [29-31]. The excellent results of chemotherapy in these advance cases should encourage us to use more liberally the approach of NACT in patients with germ cell tumors. Furthermore, even if the disease confines to one ovary, NACT may decrease significantly the tumor size allowing us to improve fertility sparing outcome by conversion of the traditional explorative laparotomy to laparoscopic surgery. In addition to its traditional advantages (painless, cosmetic result, fewer complications, faster return to normal activities, and shorter hospital stay), laparoscopy may create fewer post-operative adhesions, which are essential for this population [32,33].
Recently Gremeau et al. [34] reported the results of a retrospective study comparing open versus laparoscopic surgery in the management of non-epithelial ovarian cancer. The results showed that laparoscopy may be a good alternative to laparotomy for the initial management of non-epithelial ovarian malignancies with no differences in the rate of complications, the number of lymph nodes removed, and the need for adjuvant treatment.
Our and other success in taking care of complicated case, should lead us to use more widely NACT in the management of patients with germ cell tumor. We conclude that NACT followed by fertility sparing surgery should be considered as an alternative to primary debulking surgery in advanced YSTs.
References
- Smith HO, Berwick M, Verschraegen CF, Wiggins C, Lansing L, et al. (2006) Incidence and survival rates for female malignant germ cell tumors. ObstetGynecol 107: 1075-1085.
- Wada S, Yoshimura R, Nishisaka N, Kishimoto T, Ikehara T, et al. (2001) Primary retroperitoneal pure yolk-sac tumor in an adult male. Scand J UrolNephrol 35: 515-517.
- Flanagan CW, Parker JR, Mannel RS, Min KW, Kida M (1997) Primary endodermal sinus tumor of the vulva: a case report and review of the literature. GynecolOncol 66: 515-518.
- Park NH, Ryu SY, Park IA, Kang SB, Lee HP (1999) Primary endodermal sinus tumor of the omentum. GynecolOncol 72: 427-430.
- Bokemeyer C, Nichols CR, Droz JP, Schmoll HJ, Horwich A, et al. (2002) Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J ClinOncol 20: 1864-1873.
- Clement PB, Young RH, Scully RE (1988) Extraovarian pelvic yolk sac tumors. Cancer 62: 620-626.
- Pasternack T, Shaco-Levy R, Wiznitzer A, Piura B (2008) Extraovarian pelvic yolk sac tumor: case report and review of published work. J ObstetGynaecol Res 34: 739-744.
- Dede M, Pabuccu R, Yagci G, Yenen MC, Goktolga U, et al. (2004) Extragonadal yolk sac tumor in pelvic localization. A case report and literature review. GynecolOncol 92: 989-991.
- Tseng MJ, Jung SM (2007) Primary extra-gonadal yolk-sac tumour of pelvis with unusual presentation. Lancet Oncol 8: 179-180.
- Huntington RW Jr, Bullock WK (1970) Yolk sac tumors of extragonadal origin. Cancer 25: 1368-1376.
- Joseph MG, Fellows FG, Hearn SA (1990) Primary endodermal sinus tumor of the endometrium. A clinicopathologic, immunocytochemical, and ultrastructural study. Cancer 65: 297-302.
- Copeland LJ, Sneige N, Ordonez NG, Hancock KC, Gershenson DM, et al. (1985) Endodermal sinus tumor of the vagina and cervix. Cancer 55: 2558-2565.
- Jones MA, Clement PB, Young RH (1994) Primary yolk sac tumors of the mesentery. A report of two cases. Am J ClinPathol 101: 42-47.
- Kurman RJ, Norris HJ (1977) Malignant germ cell tumors of the ovary. Hum Pathol 8: 551-564.
- Mitchell PL, Al-Nasiri N, A'Hern R, Fisher C, Horwich A, et al. (1999) Treatment of nondysgerminomatous ovarian germ cell tumors: an analysis of 69 cases. Cancer 85: 2232-2244.
- Chan JK, Tewari KS, Waller S, Cheung MK, Shin JY, et al. (2008) The influence of conservative surgical practices for malignant ovarian germ cell tumors. J SurgOncol 98: 111-116.
- de La Motte Rouge T, Pautier P, Duvillard P, Rey A, Morice P, et al. (2008) Survival and reproductive function of 52 women treated with surgery and bleomycin, etoposide, cisplatin (BEP) chemotherapy for ovarian yolk sac tumor. Ann Oncol 19: 1435-1441.
- Nawa A, Obata N, Kikkawa F, Kawai M, Nagasaka T, et al. (2001) Prognostic factors of patients with yolk sac tumors of the ovary. Am J ObstetGynecol 184: 1182-1188.
- Pectasides D, Pectasides E, Kassanos D (2008) Germ cell tumors of the ovary. Cancer Treat Rev 34: 427-441.
- Dällenbach P, Bonnefoi H, Pelte MF, Vlastos G (2006) Yolk sac tumours of the ovary: an update. Eur J SurgOncol 32: 1063-1075.
- Hou JY, Kelly MG, Yu H, McAlpine JN, Azodi M, et al. (2007) Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. GynecolOncol 105: 211-217.
- Kuhn W, Rutke S, Spathe K, Schmalfeldt B, Florack G, et al. (2001) Neoadjuvant chemotherapy followed by tumordebulking prolongs survival for patients with poor prognosis in International Federation of Gynecology and Obstetrics Stage IIIC ovarian carcinoma. Cancer 92: 2585-2591.
- Lee SJ, Kim BG, Lee JW, Park CS, Lee JH, et al. (2006) Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epithelial ovarian cancer who are inadequate for optimum primary surgery. J ObstetGynaecol Res 32: 99-106.
- Loizzi V, Cormio G, Resta L, Rossi CA, Di Gilio AR, et al. (2005) Neoadjuvant chemotherapy in advanced ovarian cancer: a case-control study. Int J Gynecol Cancer 15: 217-223.
- Vergote I, Tropé CG, Amant F, Ehlen T, Reed NS, et al. (2011) Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J ClinOncol 29: 4076-4078.
- Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, et al. (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363: 943-953.
- Lu Y, Yang J, Cao D, Huang H, Wu M, et al. (2014) Role of neoadjuvant chemotherapy in the management of advanced ovarian yolk sac tumor. GynecolOncol 134: 78-83.
- Talukdar S, Kumar S, Bhatla N, Mathur S, Thulkar S, et al. (2014) Neo-adjuvant chemotherapy in the treatment of advanced malignant germ cell tumors of ovary. GynecolOncol 132: 28-32.
- Bafna UD, Umadevi K, Kumaran C, Nagarathna DS, Shashikala P, et al. (2001) Germ cell tumors of the ovary: is there a role for aggressive cytoreductive surgery for nondysgerminomatoustumors? Int J Gynecol Cancer 11: 300-304.
- Raveendran A, Gupta S, Bagga R, Saha SC, Gainder S, et al. (2010) Advanced germ cell malignancies of the ovary: should neo-adjuvant chemotherapy be the first line of treatment? J ObstetGynaecol 30: 53-55.
- Gueye A, Narducci F, Baranzelli MC, Collinet P, Farine O, et al. (2007) [Malignant ovarian germ cell tumours: a trial of 36 cases]. GynecolObstetFertil 35: 406-419.
- Gutt CN, Oniu T, Schemmer P, Mehrabi A, Büchler MW (2004) Fewer adhesions induced by laparoscopic surgery? SurgEndosc 18: 898-906.
- Peccatori F, Bonazzi C, Chiari S, Landoni F, Colombo N, et al. (1995) Surgical management of malignant ovarian germ-cell tumors: 10 years' experience of 129 patients. ObstetGynecol 86: 367-372.
- Gremeau AS, Bourdel N, Jardon K, Rabischong B, Mage , et al. (2014) Surgical management of non-epithelial ovarian malignancies: advantages and limitations of laparoscopy. Eur J ObstetGynecolReprodBiol 172: 106-110.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17543
- [From(publication date):
December-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 12947
- PDF downloads : 4596