Near-infrared Irradiation and Graphene Oxide Film Fabricated on Dentin Surface Exhibit Photothermal and Antibacterial Effects
Received: 25-Nov-2017 / Accepted Date: 27-Dec-2017 / Published Date: 10-Jan-2018 DOI: 10.4172/2332-0702.1000231
Abstract
Background and objectives: Graphene oxide (GO) is a monolayer sheet of carbon with a thickness of 1 nm or less. Recent studies have revealed that GO exerts antibacterial properties, absorbs near-infrared (NIR) irradiation and generates heat. In this study, we fabricated a GO film on a human dentin block and investigated the photothermal and antibacterial effects of GO and NIR irradiation against Streptococcus mutans.
Methods: The dentin block was immersed in GO dispersion (concentration: 0, 1 and 10 μg/mL). GO-coated dentin blocks were observed using scanning electron microscopy (SEM) and characterized using the dentinal tubule sealing score. The temperature increase of the GO-coated dentin surface following NIR irradiation was examined by thermography. Furthermore, antibacterial effects of the combination of GO film and NIR irradiation against S. mutans were evaluated by SEM observation, turbidity measurement, colony formation assessment and live/dead staining.
Results: A thin GO film with a thickness of a few nanometers was successfully formed on the dentin surface. The dentinal tubule sealing score increased in a GO concentration-dependent manner. Even after ultrasonic cleaning, GO residue was frequently observed on the dentin surface. When the GO-coated dentin block was irradiated with NIR light, the temperature of the dentin block surface increased in a GO concentration- and time-dependent manner. In antibacterial assessments, turbidity and colony formation were suppressed by GO and NIR irradiation. In addition, dead bacteria were detected by live/dead staining.
Conclusion: A stable GO film was successfully formed on the dentin surface by immersion in GO dispersion. Photothermal and antibacterial effects were remarkably exhibited by GO and NIR irradiation.
Keywords: Antibacterial property; Dentin; Graphene oxide; Nanocarbon; Near-infrared light; Photothermal therapy; Streptococcus mutans.
Introduction
Graphene oxide (GO) exhibits a monolayer sheet of carbon with a thickness of 1 nm or less, which partly contains sp3 hybridized carbon atoms and oxygen functional groups, such as epoxy, carboxy and hydroxy groups. The presence of oxygen functional groups causes high hydrophilicity and dispersibility of GO. Recently, investigators have reported the antimicrobial properties of GO. Krishnamoorthy et al. showed that GO exerted antibacterial activity against Pseudomonas aeruginosa and Streptococcus iniae [1]. Gurunathan et al. demonstrated that GO and reduced GO dose-dependently inhibited the growth of P. aeruginosa and suggested that oxidative stress of reactive oxygen species (ROS) attacked bacterial cells [2]. Hu et al. reported that oxidized graphene sheets showed antibacterial activities, however, the cytotoxicity of GO sheets was slight [3]. Hence, GO may be relevant for the treatment of infectious diseases in various therapeutic fields.
Photothermal therapy (PTT) comprises light irradiation of a photosensitizer, which subsequently damages bacterial cells or cancer cells by heat generation via photoexcitation [4,5]. PTT has been focused on in the field of drug delivery to locally provide biological substances to targets [6,7]. Various photosensitizers, such as gold nanoparticles [8,9] and nanocarbons [10,11], have been widely investigated. In particular, GO may be a strong candidate for PTT. Robinson et al. reported that GO could generate fever after irradiation of near-infrared (NIR) light and successfully kill cancer cells [12]. In addition, chemo-photothermal therapy using GO loading of anticancer drugs was investigated [13]. Since long wavelengths showed low phototoxicity compared with short wavelengths such as UV light [14], we anticipate the application of NIR light is suitable for PTT using GO. We speculate that GO should adhere to the tooth surface and exhibit photothermal effects via NIR light irradiation. PTT using GO would likely exert strong bactericidal action against cariogenic bacterial cells. However, these hypotheses have not been investigated thus far. Accordingly, in this study, we evaluated whether GO films stably coated the surface of root dentin. In addition, we assessed the antibacterial effects of GO film irradiated by NIR light against an oral bacterium, Streptococcus mutans.
Materials And Methods
Preparation of dentin blocks
Dentin blocks were prepared from extracted vital third molars of patients (20–40 years of age) at Hokkaido University Hospital. The use of human teeth in this study was approved by the Institutional Review Board of Hokkaido University Hospital for Clinical Research (approval no. 12-46). The tooth root was cut with a sterilized diamond disc (87xFSI, Horico, Berlin, Germany) and sandpaper (#240 and #600) to prepare dentin blocks of 5 mm×5 mm size with 1-mm thickness. After ultrasonic cleaning twice with distilled water (DW) for 5 minutes, dentin blocks were sonicated with 3% ethylenediaminetetraacetic acid (EDTA) (Smearclean, Nippon Shika Yakuhin Co., Ltd., Shimonoseki, Japan) for 60 seconds. After ultrasonic cleaning with DW, dentin blocks were obtained for evaluation.
Fabrication and evaluation of GO film on dentin blocks
GO dispersion (nanoGRAX®, Mitsubishi Gas Chemical Co., Ltd., Tokyo, Japan) was produced by oxidation and chemical separation of graphite (Hummers-Offeman method [15]) (Figure 1A). Each dentin block was immersed in diluted GO dispersion (concentration: 0, 1 and 10 μg/mL) for 30 seconds and then immersed in DW for 5 minutes. To investigate GO film fabrication, some GO-coated dentin blocks were washed with ultrasonic waves (28 kHz, NS-100, Nippon Rikagaku Kikai Co., Ltd, Tokyo, Japan) for 20 seconds. Subsequently, dentin blocks were dehydrated in a graded ethanol series and then dried overnight. Dentin blocks were then coated with Pt-Pd and observed by scanning electron microscopy (SEM; S-4000, Hitachi Ltd., Tokyo, Japan) at 10 kV. Three area units (90 μm×120 μm) of SEM images were selected and then the ability of the GO film to cover dentinal tubules was scored from 1 to 4 as described previously with some modification [16-18] (Table 1).
| Score | Definition |
|---|---|
| 1 | Dentinal tubules were clearly opened. |
| 2 | Less than 50% of dentinal tubules were closed. |
| 3 | More than 50% of dentinal tubules were closed. |
| 4 | Dentinal tubules were completely closed. |
Table 1: Scoring system to evaluate the creation of GO film.
Assessment of surface temperature of dentin blocks after NIR irradiation
NIR irradiation of GO-coated dentin blocks was carried out using an NIR irradiation device (wavelength 800–1000 nm; LA-100 IR, Hayashi Watch-Works Co., Ltd., Tokyo, Japan). The irradiation distance was set to 1, 2 and 3 cm, and the irradiation time was set to 0, 10, 20 and 30 seconds. After NIR irradiation and a 5-second pause, the surface temperature was measured with thermography (FLIRix, FLIR Systems, Inc., Wilsonville, OR, USA). Furthermore, the temperature of the GO-coated dentin surface was measured after repeated NIR irradiation. The irradiation distance and time were set to 1 cm and 10 seconds, respectively. After NIR irradiation and a 5-second pause, the surface temperature was determined with thermography. After 30 seconds for cooling of the dentin block, NIR irradiation and subsequent temperature measurement were performed in the same manner. The same procedure was repeated again. The surface morphologies of dentin blocks receiving single and 3-times irradiation were then observed by SEM.
Antibacterial effects of GO film and NIR irradiation
A suspension of facultative anaerobic bacteria, S. mutans ATCC 35668, was cultured and kept frozen until analysis. Bacterial stocks were incubated in brain heart infusion broth (BHI; Pearlcore®, Eiken Chemical, Co., Ltd., Tokyo, Japan) supplemented with 0.1% antibiotic (gramicidin D and bacitracin, Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 1% sucrose (Wako Pure Chemical Industries, Ltd.). GO-coated dentin blocks were placed into micro plates and then the suspension of S. mutans (final concentration: 1.5×106 colony-forming units (CFU)/mL) was dispensed. After anaerobic incubation at 37°C for 5 hours, dentin blocks were irradiated by NIR light for 30 seconds. Control specimens received no irradiation. Dentin blocks were then cultured again for 5 hours and the surface of the dentin blocks was observed by SEM. Some specimens were cultured for 24 hours after NIR irradiation to measure turbidity using a turbidimeter (CO7500 Colourwave, Funakoshi Co., Ltd, Tokyo, Japan) at 590 nm. In addition, S. mutans suspensions cultured for 12 hours after NIR irradiation were diluted 10-fold in fresh BHI broth, spread onto BHI agar plates (Eiken Chemical Co., Ltd.) and incubated at 37 °C for 48 hours to determine S. mutans colony counts.
Dentin blocks not incubated or incubated for 5 hours after NIR irradiation (10 or 30 seconds) were stained by the Live/Dead® BacLight™ Bacterial Viability Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Dentin blocks were observed using fluorescence microscopy (Biorevo BZ-9000, Keyence Co., Osaka, Japan). In the incubated group, dead bacteria (red) and live bacteria (green) were counted in three area units (40 μm × 50 μm per unit) and the percentage of dead bacteria was calculated.
Statistical analysis
Statistical analysis was performed by Scheffe’s test. P-values
Results
Evaluation of GO film fabrication on dentin blocks
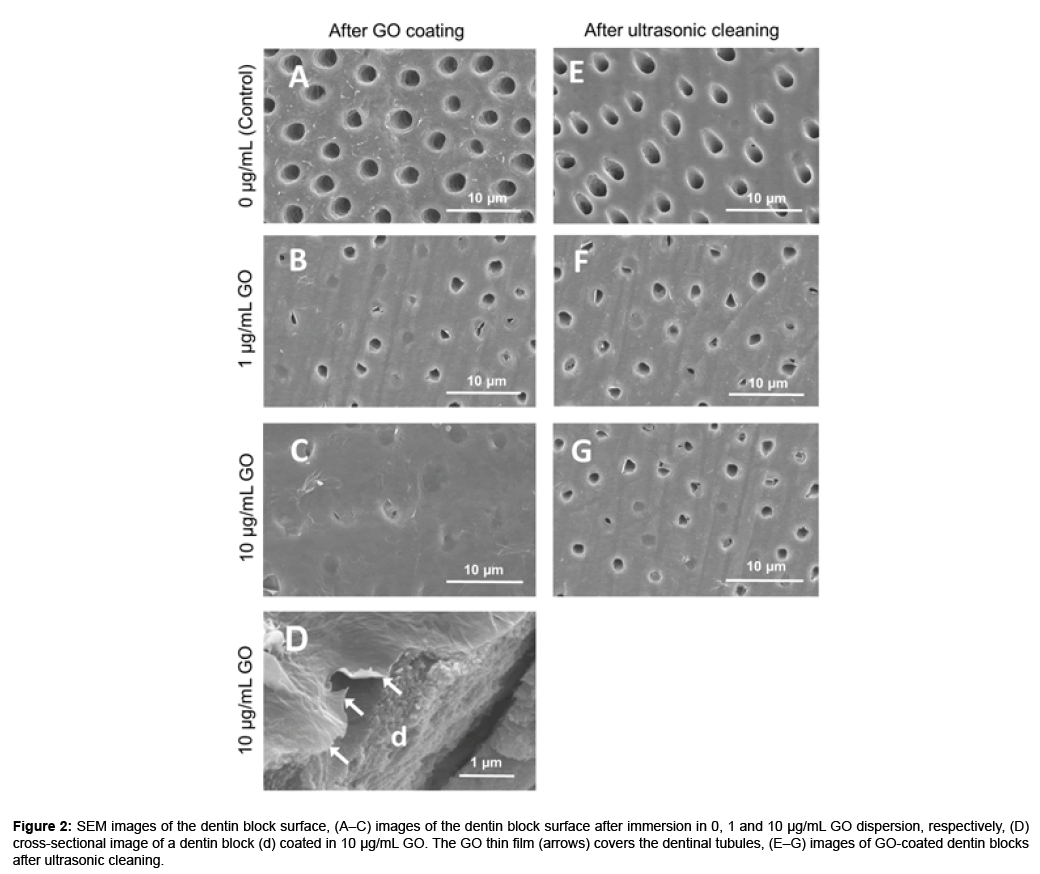
The color of the dentin blocks did not change after GO application (Figures 1B and 1C). In SEM observation, dentin block surfaces showed open dentinal tubules after EDTA treatment (Figure 2A). After immersion in GO solution, a thin wrinkled film of nanoscale thickness was observed on the dentin surface that sealed dentinal tubules (Figures 2B–2D). The dentinal tubule sealing score [mean ± standard deviation (SD)] was 1.02 ± 0.03, 3.02 ± 0.03 and 3.78 ± 0.18 after GO coating using 0 (control), 1 and 10 μg/mL GO solution, respectively (Table 2). GO coating increased the sealing score and the sealing ability of 10 μg/ mL GO was significantly greater than that of 1 μg/mL GO (p<0.05). After ultrasonic cleaning, a part of the GO film disappeared and open dentinal tubules were frequently observed (Figures 2F and 2G). However, residual GO film was observed on the intertubular dentin. After ultrasonic washing, the dentinal tubule sealing score (mean ± SD) was 1.76 ± 0.17 and 2.10 ± 0.36 for 1 and 10 μg/mL GO, respectively (Table 2). High concentrations of GO possessed greater coatability compared with low concentrations of GO (Figures 1A-2D).
Figure 2: SEM images of the dentin block surface, (A–C) images of the dentin block surface after immersion in 0, 1 and 10 μg/mL GO dispersion, respectively, (D) cross-sectional image of a dentin block (d) coated in 10 μg/mL GO. The GO thin film (arrows) covers the dentinal tubules, (E–G) images of GO-coated dentin blocks after ultrasonic cleaning.
| Parameters | 0 μg/mL GO | 1 μg/mL GO | 1 μg/mL GO | 10 μg/mL GO | 10 μg/mL GO | |
|---|---|---|---|---|---|---|
| (control) | (after UC) | (after UC) | ||||
| Score (mean ± SD) | 1.02 ± 0.03 | 3.02 ± 0.03 | 1.76 ± 0.17 | 3.78 ± 0.18 | 2.10 ± 0.36 | |
| Mean difference | 0.03 | 0.04 | 0.21 | 0.23 | 0.45 | |
| P-value | vs. 0 μg/mL GO (control) | NA | p<0.001* | p=0.002* | p<0.001* | p<0.001* |
| vs. 1 μg/mL GO | p<0.001* | NA | p<0.001* | p=0.002* | p<0.001* | |
| vs. 1 μg/mL GO (after UC) | p=0.002* | p<0.001* | NA | p<0.001* | p=0.230 | |
| vs. 10 μg/mL GO | p<0.001* | p=0.002* | p<0.001* | NA | p<0.001* | |
| vs. 10 μg/mL GO (after UC) | p<0.001* | p<0.001* | p=0.230 | p<0.001* | NA | |
Note: GO- Graphene Oxide; UC- ultrasonic cleaning; SD- standard deviation; NA- not applicable; *p<0.05
Table 2: Mean dentinal tubule sealing scores (n=4).
Assessment of the temperature of the dentin surface after NIR irradiation
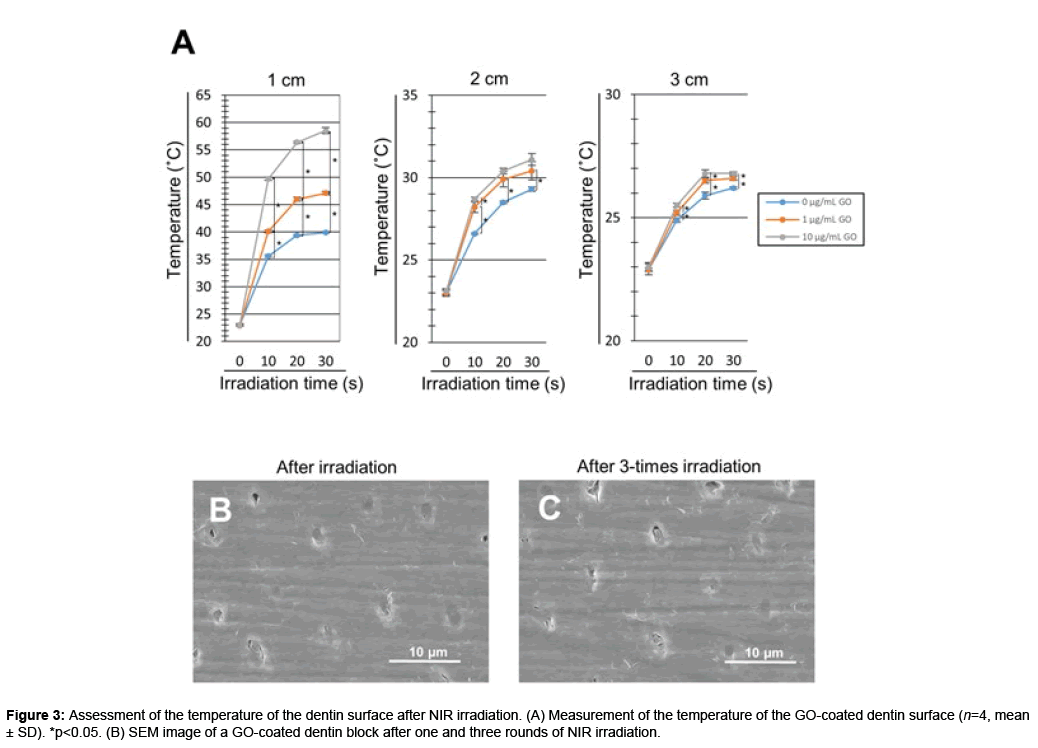
The temperature of the GO-coated dentin surface increased after NIR irradiation. The dentin surface temperature increased in a GO concentration- and time-dependent manner. In addition, NIR irradiation decreased the surface temperature in a distance-dependent manner. NIR irradiation at a distance of 1 cm significantly increased the surface temperature of GO-coated dentin blocks compared with untreated dentin blocks (p<0.05). In contrast, irradiation at distances of 2 and 3 cm tended to decrease the degree of temperature rise. For NIR irradiation of 30 seconds at a distance of 1 cm, the surface temperature was 39.9, 47.1 and 58.5°C on 0 (control), 1 and 10 μg/mL GO-coated dentin blocks, respectively (Figure 3A).
We next confirmed the stability of the GO film after repeated NIR irradiation of GO-coated dentin blocks (n=4). The dentin surface temperature (mean ± SD) was 49.7 ± 0.50, 50.3 ± 0.49 and 50.9 ± 0.70°C after single, 2-times and 3-times irradiation, respectively. No significant difference was found between the three groups. SEM images showed GO films exhibited no morphological change. Dentinal tubules were completely sealed by GO film even after repetitive NIR irradiation (Figures 3A-3C).
Antibacterial evaluation of GO film and NIR irradiation
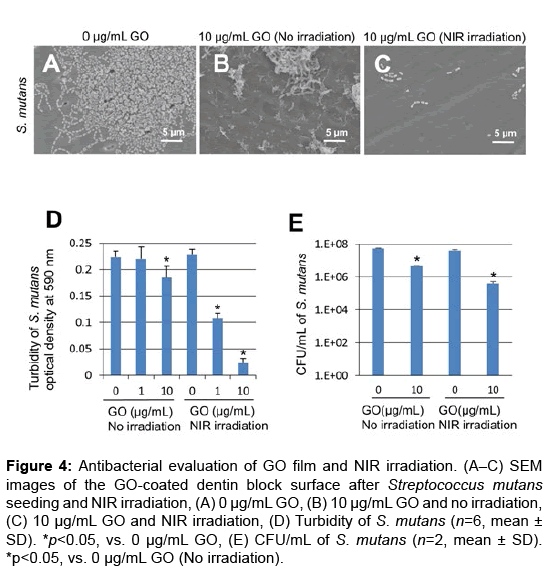
In SEM observation, bacterial aggregation on dentin block surfaces was remarkable in control specimens receiving no GO or NIR irradiation (Figure 4A). In contrast, slight S. mutans growth appeared on GO-coated dentin blocks (Figures 4B and 4C). Furthermore, GO and NIR light application strongly suppressed bacterial growth and colonization (Figure 4C).
Figure 4: Antibacterial evaluation of GO film and NIR irradiation. (A–C) SEM images of the GO-coated dentin block surface after Streptococcus mutans seeding and NIR irradiation, (A) 0 μg/mL GO, (B) 10 μg/mL GO and no irradiation, (C) 10 μg/mL GO and NIR irradiation, (D) Turbidity of S. mutans (n=6, mean ± SD). *p<0.05, vs. 0 μg/mL GO, (E) CFU/mL of S. mutans (n=2, mean ± SD). *p<0.05, vs. 0 μg/mL GO (No irradiation).
Assessment of S. mutans turbidity and colony count was carried out to determine the antibacterial capability of GO film and NIR irradiation. Under no irradiation, the optical density (mean ± SD) was 0.22 ± 0.01, 0.22 ± 0.01 and 0.19 ± 0.01 in 0, 1 and 10 μg/mL GO-coated dentin, respectively. The optical density of 10 μg/mL GO-coated dentin samples was significantly decreased compared with untreated dentin samples (p<0.05) (Figure 4D). When NIR irradiation was applied, the optical density (mean ± SD) was 0.23 ± 0.01, 0.11 ± 0.02 and 0.02 ± 0.02 in 0, 1 and 10 μg/mL GO-coated dentin, respectively. The optical density of GO-coated samples was significantly lower than that of untreated samples (p<0.05). The number of S. mutans colonies of 10 μg/mL GO-coated dentin samples was reduced compared with that of untreated dentin samples. In addition, the combination of GO and NIR irradiation strongly inhibited the formation of bacterial colonies (Figures 4A-4E).
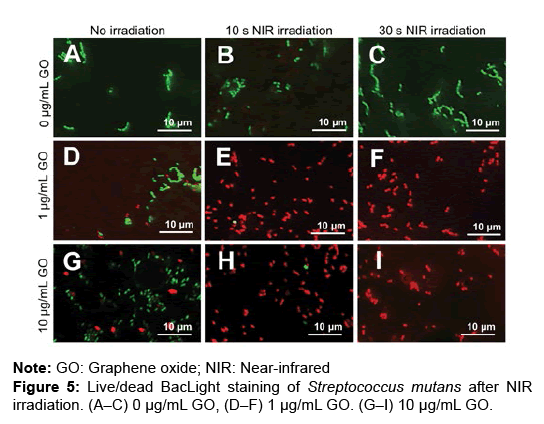
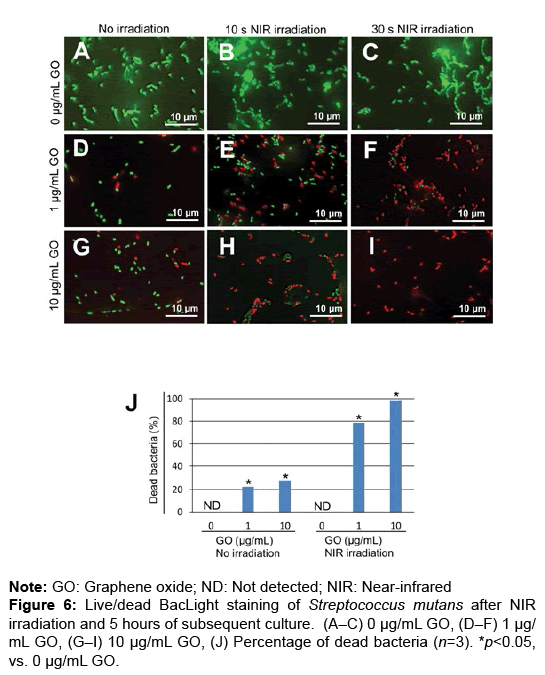
To assess the bactericidal effects of GO film and NIR irradiation, live/dead staining of S. mutans was carried out. GO coating and NIR irradiation increased the number of dead bacteria after 0 and 5 hours of incubation (Figures 5A–I and 6A–I). In the assessment of the percentage of dead bacteria after incubation for 5 hours, no dead bacteria were found in untreated dentin, regardless of application of NIR irradiation. When NIR irradiation was not applied, the mean percentage of dead bacteria was 22.6 and 28.4% in 1 and 10 μg/mL GO-coated dentin, respectively. In contrast, with NIR irradiation for 30 seconds, the mean percentage of dead bacteria was 78.9% and 98.3% in 1 μg/mL and 10 μg/ mL GO-coated dentin, respectively (Figures 5A–6J).
Discussion
SEM observation of GO-coated dentin revealed that the wrinkled film structure sealed dentinal tubules on dentin block surfaces. The cross-sectional SEM image of GO-coated dentin revealed a very thin film on the dentin. In contrast, control specimens showed that dentinal tubules were fully opened. Thus, dispersed GO easily aggregated to form thin GO films on the dentin, consequently sealing dentinal tubules. Regarding the concentration of the GO dispersion, a high concentration of GO (10 μg/mL) remarkably covered the dentin surface compared with a low concentration of GO (1 μg/mL), suggesting that GO coating covered dentinal tubules in a concentration-dependent manner. Ultrasonic cleaning of GO-coated dentin resulted in partial removal of the GO film to open dentinal tubules. However, as compared with the control, GO-coated dentin showed a high sealing ratio of dentinal tubules and GO film strongly remained on the intertubular dentin after ultrasonic cleaning. Therefore, GO film can stably cover the surface of root dentin. Some investigators showed that GO possesses protein absorption capability [19,20]. GO has a large amount of hydrophilic oxygen functional groups on its surface because of oxidation processes [21]. GO-dentin binding force may be enhanced by covalent bonding between epoxy groups of GO and dentin protein exposed by EDTA treatment [22]. Due to these attractive forces, GO film would readily form on the dentin surface by only the immersion process. The formation of a stable GO film on the root surface would be beneficial for dental GO therapy against root caries and periodontal diseases.
The temperature of GO-coated dentin surfaces time-dependently increased via NIR light irradiation, however, dentin without GO coating exhibited no temperature rise after NIR irradiation. It was suggested that both application of GO film and NIR irradiation increased the surface temperature of dentin. Robinson et al. detected the absorption spectrum of GO and revealed the NIR absorption ability of GO [12]. Li et al. showed that the temperatures of a solution containing GO was increased by NIR irradiation [23]. Taken together, GO film formed on dentin possesses NIR absorption ability and releases thermal energy to generate heat after NIR irradiation. In the present study, the morphology of GO film remained unchanged after repetitive (3 rounds) NIR irradiation (Figures 3B and 3C). It is considered that the film stably covered the dentin even with heat generation.
Live/dead staining showed that dead bacteria were significantly observed on GO-coated dentin that received subsequent NIR irradiation. In addition, S. mutans turbidity and colony formation were significantly reduced by GO and NIR irradiation. It was suggested that the GO-NIR system provided thermal damage to S. mutans and consequently facilitated bacterial sterilization. Ma et al. demonstrated that the growth of mesophilic bacteria, such as S. mutans, occurs at 30– 47°C [24]. In the present study, the surface temperature of GO-coated dentin via NIR irradiation was higher than 50°C. Therefore, S. mutans would likely receive profound damage, inhibiting bacterial proliferation. The GO-NIR system conclusively exhibited great antibacterial effects against S. mutans.
Although NIR irradiation was not performed, S. mutans was sparsely observed on dentin blocks immersed in 10 μg/mL GO dispersion compared with biofilms grown on control specimens in SEM images. In addition, GO application with no irradiation reduced S. mutans turbidity and colony formation compared with the control. These findings suggested that GO-coated dentin independently exhibited antibacterial effects. The antimicrobial properties of GO against various bacteria, such as P. aeruginosa, Escherichia coli and S. iniae, have been investigated [1,2]. Some investigators concluded that the antimicrobial properties of GO were exerted by ROS production, which caused bacterial cell damage [2]. It was considered that GO should gradually and continuously produce ROS because of GO reduction and release the oxygen functional groups by temporal degradation. Accordingly, GO films on the tooth surface would exert a long-term antibacterial effect.
Conclusion
We investigated the possibility of GO film coverage on the surface of root dentin and the effectiveness of photothermal effects by NIR irradiation on oral bacterium S. mutans. The stable, thin GO film formed on the dentin surface to cover dentinal tubules in a concentrationdependent manner. Furthermore, the combination of GO film and NIR irradiation remarkably exhibited photothermal and bactericidal effects. The GO-NIR system is anticipated to be an effective photothermal antibacterial therapy against oral infections.
Acknowledgements
The authors would like to thank Mitsubishi Gas Chemical Co., Ltd. for providing nanoGRAX® and Nippon Shika Yakuhin Co., Ltd. for providing Smearclean. This work was supported by JSPS KAKENHI (grant nos. 25463210, 16H06604 and 16K11822). The authors report that they have no conflicts of interest related to this study.
References
- Krishnamoorthy K, Umasuthan N, Mohan R, Lee J, Kim SJ (2012) Antibacterial activity of graphene oxide nanosheets. Sci Adv Mat 4: 1111-1117.
- Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim JH (2012) Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomedicine 7: 5901-5914.
- Hu W, Peng C, Luo W, Lv M, Li X, et al (2010). Graphene-based antibacterial paper. ACS Nano 4: 4317-4323.
- Shahnawaz KM, Abdelhamid HN, Wu HF (2015) Near infrared (NIR) laser mediated surface activation of graphene oxide nanoflakes for efficient antibacterial, antifungal and wound healing treatment. Colloids Surf B Biointerfaces 127: 281-291.
- Li JL, Hou XL, Bao HC, Sun L, Tang B, et al. (2014) Graphene oxide nanoparticles for enhanced photothermal cancer cell therapy under the irradiation of a femtosecond laser beam. JBiomed Mater Res A 102: 2181-2188.
- Wu MC, Deokar AR, Liao JH, Shih PY, Ling YC (2013) Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS Nano 7: 1281-1290.
- Qin XC, Guo ZY, Liu ZM, Zhang W, Wan MM, et al. (2013) Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J Photochem Photobiol B: Biol 120: 156-162.
- Huang XH, Jain PK, El-Sayed IH, El-Sayed MA (2007) Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostic and therapy. Nanomedicine 2: 681-693.
- Miyata S, Miyaji H, Kawasaki H Yamamoto M, Nishida E, et al. (2017) Antimicrobial photodynamic activity and cytocompatibility of Au25(Capt)18 clusters photo excited by blue LED light irradiation. Int J Nanomedicine 12: 2703-2716.
- Kam NWS, O’Connell M, Wisdom JA, Dai HJ (2005) Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 102: 11600-11605.
- Kim JW, Shashkov EV, Galanzha EI, Kotagiri N, Zharov VP (2007) Photothermal antimicrobial nanotherapy and nanodiagnostics with self-assembling carbon nanotube clusters. Lasers Surg Med 39: 622-634.
- Robinson JT, Tabakman SM, Liang Y, Wang H, Sanchez Casalongue H, et al. (2011) Ultra small reduced graphene oxide with high near-infrared absorbance for photo-thermal therapy. J Am Chem Soc 133: 6825-6831.
- Cheon YA, Bae JH, Chung BG (2016) Reduced graphene oxide nanosheet for chemo-photothermal therapy. Langmuir 32: 2731-2736.
- Chui C, Hiratsuka K, Aoki A, Takeuchi Y, Abiko Y, et al. (2012) Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers Surg Med 44: 856-864.
- Hirata M, Gotou T, Horiuchi S, Fujiwara M, Ohba M (2004) Thin-film particles of graphite oxide 1: High-yield synthesis and flexibility of the particles. Carbon 42: 2929-2937.
- Zand V, Bidar M, Ghaziani P, Rahimi S, Shahi S (2007) A comparative SEM investigation of the smear layer following preparation of root canals using nickel titanium rotary and hand instruments. J Oral Sci 49: 47-52.
- Shahi S, Yavari HR, Rahimi S, Reyhani MF, Kamarroosta Z, et al. (2009) A comparative scanning electron microscopic study of the effect of three different rotary instruments on smear layer formation. J Oral Sci 51: 55-60.
- Mahajan V, Kamra A, Dahiwale S (2010) The effect of 17% EDTA and MTAD on smear layer removal and on erosion of root canal dentin when used as final rinse: an in vitro SEM study. J Int Clin Dent Res Org 2: 113-118.
- Li H, Fierens K, Zhang Z, Vanparijs N, Schuijs MJ, et al. (2016) Spontaneous protein adsorption on graphene oxide nanosheets allowing efficient intracellular vaccine protein delivery. ACS Appl Mater Interfaces 8: 1147-1155.
- Nishida E, Miyaji H, Kato A, Takita H, Iwanaga T, et al. (2016) Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int J Nanomedicine 11: 2265-2277.
- Pei S, Cheng HM (2012) The reduction of graphene oxide. Carbon 50: 3210-3228.
- Kim DS, Park SH, Choi GW, Choi KK, Kim SY (2011) Effect of EDTA treatment on the hybrid layer durability in total-etch dentin adhesives. Dent Mater J 30: 717-722.
- Li M, Yang X, Ren J, Qu K, Qu X (2012) Using graphene oxide high near-infrared absorbance for photothermal treatment of alzheimer’s disease. Adv Mat 24: 1722-1728.
- Ma Y, Marquis RE (1997) Thermo-physiology of Streptococcus mutans and related lactic-acid bacteria. Antonie Van Leeuwenhoek 72: 91-100.
Citation: Nagao K, Miyaji H, Nishida E, Akasaka T, Miyata S, et al. (2018) Nearinfrared Irradiation and Graphene Oxide Film Fabricated on Dentin Surface Exhibit Photothermal and Antibacterial Effects. J Oral Hyg Health 6: 231. DOI: 10.4172/2332-0702.1000231
Copyright: © 2018 Nagao K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6395
- [From(publication date): 0-2018 - Apr 24, 2025]
- Breakdown by view type
- HTML page views: 5396
- PDF downloads: 999