N-butanol Preserved Maropitant Formulation Increases Local Tolerance in Dogs
Received: 01-Jan-2024 / Manuscript No. jvmh-24-125633 / Editor assigned: 04-Jan-2024 / PreQC No. jvmh-24-125633 (PQ) / Reviewed: 22-Jan-2024 / QC No. jvmh-24-125633 / Revised: 27-Jan-2024 / Manuscript No. jvmh-24-125633 (R) / Published Date: 30-Jan-2024
Abstract
Background: The antiemetic maropitant, with metacresol as preservative, is known to cause injection site pain in dogs and cats. Nowadays, generic formulations with different preservatives are authorized. The aim of this study was to compare local pain after subcutaneous injection of two maropitant formulations with different preservatives (metacresol and n-butanol), administered at refrigerated temperature and at room temperature to dogs.
Methods: A four-period, four-sequence, cross-over blinded study was conducted in 32 healthy beagle dogs, administered 1 mg/kg subcutaneously of two maropitant solutions for injection. Pain was evaluated and scored using visual analogue scale (VAS) immediately after dosing and simple descriptive scale (SDS) during two minutes after dosing. In addition to the local pain assessment, the dogs were observed for any other signs before and after the administration of the maropitant injection.
Results: Statistically significant lower VAS scores were observed after treatment with butanol-maropitant than after treatment with metacresol-maropitant. No differences between temperature, periods or sequences of administration were found with either of the formulations. The SDS scores showed significantly lower pain responses after injection of butanol-maropitant than after injection with metacresol-maropitant. No abnormal local reactions were observed.
Conclusion: It was demonstrated that n-butanol preserved maropitant was less painful than metacresol preserved maropitant after subcutaneous injection independent of temperature
Keywords
Maropitant; Injection site pain; Local tolerance; Dogs; Preservative; N-butanol; Metacresol; Subcutaneous injection; Antiemetic
Introduction
Maropitant is a potent, selective neurokinin-1 receptor antagonist that acts to prevent and treat vomiting in dogs and cats by blocking the binding of the key neurotransmitter substance P. Efficacy of maropitant in preventing vomiting caused by stimulation of either central or peripheral emetic pathways has been shown and underlines the broad-spectrum inhibition of emesis [1].
The first injectable formulation of maropitant (Cerenia®, Zoetis Belgium SA) contains maropitant (10 mg/ml), sulphobutylether- β-cyclodextrin (SBECD) and metacresol as preservative. With this formulation frequent occurrence of transient pain and vocalization during subcutaneous injection has been observed and reported [2,3]. A study suggested that free unbound maropitant, associated with higher temperature, is responsible for the local pain reactions. The formation of a maropitant-SBECD-complex increases with lower temperatures, which leads to reduction in pain associated with subcutaneous injection of maropitant with metacresol [4]. In addition, metacresol is considered to be more painful with respect to injection site pain than other preservatives [5,6]. It was reported that using an alternative preservative in a maropitant formulation may reduce the injection pain [7].
The objective of this study was to determine whether a maropitant formulation with the preservative n-butanol is less painful to injected dogs compared to metacresol-maropitant when administered at refrigerated temperature (approximately 4°C) and at room temperature (approximately 25°C).
Materials and Methods
Animals
The in-vivo study included 32 healthy beagle dogs (15 intact males, one male castrated and 16 intact females, between 1 and 6.75 years of age (mean 3.8 years), weighing between 10.35 and 18.40 kg). All dogs were group-housed during the study period under controlled conditions (temperature, humidity, light cycle) and underwent a veterinary clinical examination before being enrolled in the study. Dogs received their ration of diet once daily. Before administration they were fasted overnight and were fed four hours after injection. Water was provided ad libitum from a public water supply.
Study design
The study was a blinded, randomized, cross-over study according to a four-period, four-sequence design and was conducted in accordance with the principles of good laboratory practice (GLP). The personnel involved in the local pain assessment were blinded to the treatment group. The dogs were randomized by gender and body weight, then assigned to each of the four homogeneous study groups, according to a randomized block procedure using Microsoft Excel.
Administration and clinical assessment
Each dog received four subcutaneous administrations of 1 mg/kg of maropitant with a period of seven days between each injection. Two different formulations of maropitant 10 mg/ml solution for injection were used: metacresol preserved formulation (Cerenia®, Zoetis Belgium SA) and n-butanol preserved formulation (Vominil®, VetViva Richter GmbH, Wels, Austria). Both formulations were administered at refrigerated temperature (approximately 4°C) and at room temperature (approximately 25°C). Vials of each product were placed on an ice bath (approximately 4°C) and on a preheated water bath (approximately 25°C), respectively, approximately 1.5-2 h before administration.
Dose volume was determined based on the bodyweight measured during acclimatization and maintained throughout the four administrations. Two formulations (at refrigerated temperature of approximately 4°C and at room temperature at approximately 25°C) were administered subcutaneously between the scapulae (at approximately 2.5 cm from the spine of the scapula) using a 2/3 ml syringe and a 25 G needle. The following order of injection was used for administration: cranial right, caudal right, caudal left and cranial left. After insertion of the needle (for each administration with a new needle), the product was injected after approximately two seconds. Immediately after administration the dog stayed individually during local pain assessment (2 minutes after dosing).
The dogs were observed during the experimental period (predose and post-dose) including general observations (general appearance, physical condition, alertness, hydration state), digestive system (vomiting, diarrhoea), circulatory system (epistaxis) and local reactions (oedema, swelling or nodule) respectively pruritus (itching). In addition, during the study, the two observers had noted comments regarding clinical signs while evaluating and scoring.
Injection pain was assessed independently by two technicians (blinded to the treatment group) at the same time. Pain was evaluated and scored using VAS (visual analogue scale) immediately after dosing and SDS (simple descriptive scale) during the first two minutes after dosing. The VAS was performed by placing a horizontal line transecting a 10-cm long line scale, with 0 being no pain and 10 being the worst possible injection pain [8,9]. The more severe the response, the farther to the right the mark was made, with no pain or reaction marked at the far left on the scale and ‘worst possible injection site pain’ marked at the far right, without explicit definition of ‘worst possible injection site pain’. The SDS was performed by scoring between a range from 0 (no pain) to three (severe reaction) according to predefined clinical signs (Table 1).
| Score | Description |
|---|---|
| 0 | No pain |
| 1 | Mild reaction (Twitching of the skin, looking at injection site, one-time trying to lick or scratching of the injection site |
| 2 | Moderate reaction (examples included repeated licking or scratching, short-term vocalization, jumping or shuddering) |
| 3 | Severe reaction (examples included prolonged yelping, hiding or circling with tucked tail or aggression) |
Table 1: Simple descriptive scale scores.
Data Analysis
Arithmetic means, geometric means, standard deviations, medians, minima, maxima and coefficients of variation (CV%) were calculated for visual analogue scale scores (VAS) recorded immediately after dosing. Descriptive statistics were calculated by product (metacresol and n-butanol preserved maropitant) and temperature for each product (4° and 25°C) and by product (metacresol and n-butanol preserved maropitant), temperature (4° and 25°C) and period of administration (group).
Similarly, means, medians and range values of simple descriptive scale scores recorded during two minutes after administration, were calculated sorting by the same variables as VAS. SPSS ver25 and Phoenix-WinNonlin programs were used.
Comparison of log transformed values of VAS scores (geometric means of the two observer measurements) between products and temperatures were performed through a parametric statistical approach (analysis of variance) using the GLM procedure. SDS comparisons (median of two observer measurements) were performed through a logistic regression model. In both cases, product, temperature, period and sequence were used as fixed factors and the subject was considered as a random factor nested within the sequence. When statistically significant differences were found, pairwise comparisons using a twosided student-t-test were applied. Statistical significance was set at 0.05 in all the cases. SPSS ver25 program was used.
Dilution experiments
For the local tolerance study both maropitant formulations were used undiluted. Additionally, dilution experiments for n-butanol preserved maropitant were undertaken. The n-butanol preserved maropitant was diluted 1: 5 in various commercially available infusions for injection, sodium chloride 0.9% (Fresenius Kabi), Ringers’ solution (B. Braun), Ringers’ lactate solution (B. Braun), glucose 5% (B. Braun) and electrolyte solution (B. Braun). Samples were stored in polyethylene or glass, respectively. Subsequently, the general characteristics of the dilutions (appearance, clarity and visible particles) were evaluated, the content of maropitant and related impurities were determined by High Pressure Liquid Chromatography.
Results
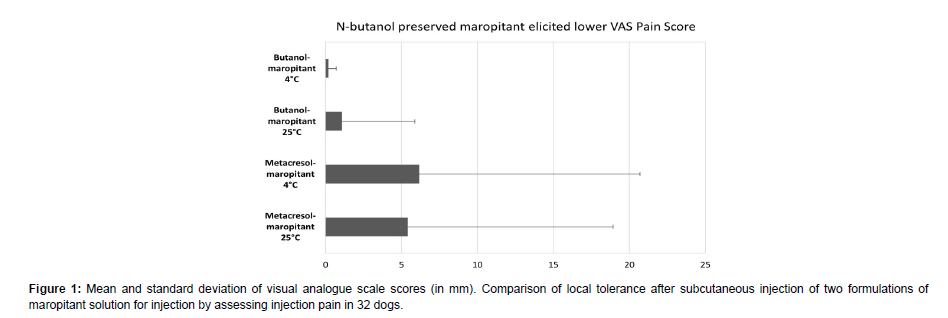
Thirty-two dogs were enrolled and completed the study. The mean VAS scores per group are depicted in Figure 1. The highest score of arithmetic means from the two observers for metacresol-maropitant were 51.0 (at 4°C) and 60.5 (at 25°C), for butanol-maropitant the highest score at 4°C was 2.0. At 25°C there was one outlier for butanolmaropitant (26.5), next to two low scores (6.5, 2.0) and all the remaining dogs were scored at 0. Overall, one observer had a tendency to score higher than the other.
The injection of butanol-maropitant was associated with seven pain scores >0 compared to metacresol-maropitant for which 17 pain scores >0 (generally significantly higher) were recorded.
The statistical analysis showed lower VAS scores for butanolmaropitant compared to metacresol-maropitant in terms of arithmetic means, standard deviations, median, minima, maxima values and coefficients of variation of VAS scores, when sorted by formulation, temperature within each formulation.
Statistically significant differences were found between formulations when the log transformed values of geometric means from the two observers for VAS scores were compared (p=0.003) with statistically significant lower VAS scores after treatment with butanol-maropitant (4°C and 25°C) than after treatment with metacresol-maropitant (4°C and 25°C). No statistically significant differences were found between administration days and injection sites, or between temperatures, periods or sequences of administration.
Mean and median (range) of the SDS scores are summarized in Table 2. Statistically significant differences were found between formulations when medians from the two observers for SDS scores were compared (p=0.004). Statistically lower SDS scores were observed after treatment with butanol-maropitant (4°C and 25°C) than after treatment with metacresol-maropitant (4°C and 25°C). Only 8 scores (median) associated with pain were assessed for butanol-maropitant (only one score of 2, two scores of 1.5, one score of 1 and four scores of 0.5), however 18 scores for metacresol-maropitant (with significantly higher scores: six times 2, also six times 1.5, five times 1 and once 0.5). No differences between temperatures, periods or sequences of administration were found.
| Mean | Median (range) | |
|---|---|---|
| Butanol-maropitant 4°C | 0.13 | 0 (0-2) |
| Butanol-maropitant 25°C | 0.13 | 0 (0-1.5) |
| Metacresol-maropitant 4°C | 0.39 | 0 (0-2) |
| Metacresol-maropitant 25°C | 0.44 | 0 (0-2) |
Table 2: Simple descriptive scale scores (mean, median and range) for 32 dogs receiving subcutaneous injections of two formulations of maropitant solution for injection at 4°C and 25°C.
The clinical signs observed during and within two minutes after injection are summarized in Table 3. More clinical signs were observed after injection of metacresol-maropitant than butanol-maropitant (n=61 versus n=20) (not statistically analyzed). Scratching at the injection site, vocalization and looking at the injection site were mainly observed. Scratching was observed in 21 dogs after injection with metacresol-maropitant (n=9 for 4°C and n=12 for 25°C) and in five dogs after injection with butanol-maropitant (n=1 for 4°C and 4 for 25°C). Vocalization was observed in 19 dogs after injection with metacresolmaropitant (n=12 for 4°C and n=7 for 25°C) and in only five dogs after injection with butanol-maropitant at 4°C). Looking at the injection site was observed in 15 dogs after injection with metacresol-maropitant (n=8 for 4°C and n=7 for 25°C) and in six dogs after injection with butanol-maropitant (n=5 for 4°C and n=1 for 25°C).
| Clinical signs | Butanol-maropitant 4°C 25°C | Metacresol-maropitant 4°C 25°C | ||
|---|---|---|---|---|
| Looking at injection site | ||||
| Once | 5 | 1 | 8 | 5 |
| Repeated (twice) | 0 | 0 | 0 | 2 |
| Discomfort | 3 | 0 | 0 | 4 |
| Licking of the injection site | 0 | 1 | 0 | 0 |
| Vocalization | ||||
| Short-term/slight | 5 | 0 | 6 | 4 |
| Continued (slight/moderate/crying/yelping) | 0 | 0 | 6 | 3 |
| Scratching of the injection site | ||||
| Once | 0 | 3 | 4 | 4 |
| Repeated (twice, three times) | 1 | 1 | 5 | 8 |
| Trying to bite during administration | 0 | 0 | 0 | 1 |
| During administration the dog moves and the administration must be performed in three times | 0 | 0 | 0 | 1 |
| Total | 14 | 6 | 29 | 32 |
Table 3: Frequency of clinical signs observed during and within two minutes after subcutaneous injection of two formulations of maropitant solution to 32 dogs at 4°C and 25°C.
Within twenty-four hours after injection no abnormal observation were made (no local reactions like oedema, swelling or nodule; no pruritus). No concomitant treatments have been used during the study. No illness or disorders in the animals have been observed and no treatment has been administered. In conclusion, the statistical results of the in vivo study found that butanol-maropitant is significantly less painful than metacresol-maropitant independent of temperature. The additional experiment, where butanol-maropitant was diluted in various infusions for injections, confirmed stability of the dilutions for up to 24 h. The diluted solutions remained clear, colourless and no visible particles were observed. The maropitant content was not decreased during storage and no formation of maropitant-related impurities was detected (Table 4).
| N-butanol preserved maropitant diluted in | Primary packaging material | Sampling point hours | Content% |
|---|---|---|---|
| maropitant | |||
| Sodium chloride 0.9% | glass | 0 | 99.5% |
| polyethylene | 0 | 99.2% | |
| glass | 24 | 99.2% | |
| polyethylene | 24 | 99.5% | |
| Ringers’ solution | glass | 0 | 99.1% |
| polyethylene | 0 | 99.4% | |
| glass | 24 | 100.0% | |
| polyethylene | 24 | 99.7% | |
| Ringers’ lactate solution | glass | 0 | 100.5% |
| polyethylene | 0 | 99.9% | |
| glass | 24 | 99.8% | |
| polyethylene | 24 | 99.8% | |
| Glucose 5% | glass | 0 | 99.6% |
| polyethylene | 0 | 99.8% | |
| glass | 24 | 99.7% | |
| polyethylene | 24 | 98.9% | |
| Electrolyte solution | glass | 0 | 99.4% |
| polyethylene | 0 | 99.7% | |
| glass | 24 | 99.8% | |
| polyethylene | 24 | 98.4% |
The total impurities for all dilutions were < LOQ (Limit of quantification); no visible particles were seen in any dilution; the clarity was consistently clear.
Table 4: Results of stability evaluation of n-butanol preserved maropitant in various commercially available solutions for infusion.
Discussion
Our study showed no obvious differences in pain response to the injection of metacresol-maropitant using refrigerated material, this is in keeping with a previous study [7].
The preservative n-butanol was used because it does not interact with maropitant or with the solubilizer and still ensures adequate preservation. Our study showed significantly lower pain scores (SDS & VAS) after subcutaneous administration of n-butanol preserved maropitant at both temperatures (4°C and 25°C) than the metacresol preserved maropitant. Further, it may not be necessary to inject butanol-maropitant at refrigerated temperature because of the absence of statistical significance between pain results at refrigerated temperature (4°C) and room temperature (25°C).
The assumption that unbound maropitant which increases with temperature is responsible for injection pain, could not be supported in the current study. A previous study [4], however, used a parallel design while our study was conducted according to a cross-over design. Furthermore, pretreatment VAS (injection with saline at 25°C on day- 1) was used as a covariate for the analysis of VAS for pain, making comparison between the two studies difficult.
The site of injection was not randomized, however no period effects could be determined. Therefore, the site of injection had no influence and consequently constituted no limitation for the study.
Furthermore, the tendency of one observer to score higher than the other, due to presumed subjective perception, had no impact on the outcome of the study because of consistent effects in all groups.
The beagle breed is considered to be a representative breed for the general dog population to allow conclusions about results in veterinary practices. The individuality of dogs in a certain setting may play an important role and should therefore be kept in mind.
Conclusions
In healthy beagle dogs, the maropitant solution for injection with n-butanol as preservative was less painful than with metacresol as the preservative after subcutaneous injection independent of temperature (4°C/25°C) based on statistically significant differences in VAS and SDS between the two formulations. The temperature was not associated with the pain, rather the preservative was presumed to be a key factor.
Author Contributions
Conceptualization, S.S. and B.F.; methodology, S.S., J.U. and S.E.; writing—original draft preparation, K.H.; writing—review and editing, S.E., S.S., K.H.; All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors would like to thank ISOQUIMEN (Barcelona, Spain) for the performance of this study and Michaela Ranner for her technical assistance.
Conflicts of Interest Statement
K.H., J.U., S.E., B.F. and S.S are employees of VetViva Richter GmbH. VetViva Richter GmbH developed the generic maropitant formulation.
Funding Information
The study was financially supported by Richter Pharma AG.
Ethics Statement
The animal study protocol was approved by the Ethics and Animal Experimentation Committee at ISOQUIMEN, as well as the Department of Territory and Sustainability, Directorate General of Environmental Policy of the Generalitat de Catalunya (Autonomous Govern of Catalonia). This procedure was registered with the DAAM order number 11786.
References
- Comparative efficacy of maropitant and selected drugs in preventing emesis induced by centrally or peripherally acting emetogens in dogs. Vet Pharm & Therapeutics 31: 533–537.
- European Medicines Agency (EMA) (2006) Cerenia: EPAR - Scientific Discussion
- European Medicines Agency (EMA) (2009) Cerenia: EPAR - Product Information
- Effect of refrigeration of the antiemetic Cerenia (maropitant) on pain on injection. Veterinary therapeutics: research in applied veterinary medicine 10: 93–102.
- Usach I, Martinez R, Festini T, Peris J-E (2019) Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Advances in therapy 36(11): 2986–2996.
- Liquid growth hormone: preservatives and buffers. Hormone research 62 Suppl 3: 98–103.
- Comparison of pain response after subcutaneous injection of two maropitant formulations to beagle dogs. Veterinary record open 5: 1–6.
- Guidelines for recognition, assessment and treatment of pain: WSAVA Global Pain Council members and co-authors of this document. The Journal of small animal practice 55(6): E10-68.
- Review of different methods used for clinical recognition and assessment of pain in dogs and cats. International Journal of Veterinary Science and Medicine 7: 43–54.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Citation: Hofstetter K, Urich J, Eichhorn S, Follrich B, Schwab S (2024) N-butanol Preserved Maropitant Formulation Increases Local Tolerance in Dogs. J Vet Med Health 8: 214.
Copyright: 2024 Hofstetter K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 876
- [From(publication date): 0-2024 - Mar 12, 2025]
- Breakdown by view type
- HTML page views: 783
- PDF downloads: 93