Research Article Open Access
Natural Zeolite: A Coast-Effective Anatase Stabilizer in Glass-Ceramic Glaze
Ghafarinazari A1,2*, Amiri E1, Karbassi M3 and Soroor M11Research and Development Group, Loabiran Glaze Company, Shiraz, Iran
2Department of Biotechnology, University of Verona, Verona, Italy
3Department of Materials Engineering Science and Research Branch, IAU, Tehran, Iran
- *Corresponding Author:
- Ghafarinazari A
Department of Biotechnology
University of Verona
Verona, Italy
Tel: +39 3886598606
E-mail: ali.ghafarinazari@univr.it
Received Date: October 15, 2014; Accepted Date: November 27, 2014; Published Date: December 10, 2014
Citation: Ghafarinazari A, Amiri E, Karbassi M, Soroor M (2014) Natural Zeolite: A Coast-Effective Anatase Stabilizer in Glass-Ceramic Glaze. J Powder Metall Min 3:125. doi: 10.4172/2168-9806.1000125
Copyright: © 2014 Ghafarinazari A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
Zircon, as an opacifier material, is on the hazard of being replaced, which is mainly because of two reasons: anticipated shortage of high-quality grade zircon and high costs of the production of zircon as an opacifier material, resulting in upward pressure on zircon price. This study aimed to assess the influence of natural zeolite as an opacifier on both technological behaviors during processing and technical performances of ceramic glaze for tile manufacturers. Moreover, preliminary results show that potential of this category of tiles as a cost-effective antibacterial product as well.
Keywords
Glass-ceramic; Antibacterial tile; Titanium oxides; Zirconia; Zeolite; Frit; Natural minerals
Introduction
Ceramic tile industry has been progressively developing its worldwide production toward new materials with improved aesthetic and technical properties. Availability of huge amounts of these raw materials which is required by tile industry is a problem in many areas; however, in other contexts, its high price has been a disadvantage for tile manufacturing in competition with other producers of building materials. Thus, ceramic industry is continuously searching for cheap raw materials for replacing the traditional ones without altering the process and product characteristics [1].
In glaze production, among the commercial frits, zircon (ZrSiO4) is the most conventional opacifier and covering [2]. Obviously, these frits are expensive; therefore, recently, certain efforts have been made to reduce production expenditure by optimizing zircon concentration [3] or taking suitable glass–ceramic alternative systems [4]. Another common opacifier is TiO2; although refractive indices of titania (2.52 for anatase and 2.76 for rutile [5]) is higher than that of zirconium oxide (2.17 [1]), it has technical problems, the first of which is low chemical stability during melting. In addition, rutile, which is the stable structure of titania in standard conditions, is the main problem, because rutile phase in contrast to anatase is yellow and leads to increased surface roughness.
Zeolites are highly porous and crystalline alumino-silicates with a three-dimensional structure based upon the repeated units of silica and alumina tetrahedral [6]. Based on high-temperature phase transformations of natural zeolites [7], the possibility of using Iranian natural zeolite as the opacifier of tiles was investigated in this study. Zeolites belong to the tectosilicate mineral group and are built up by a framework of corner-sharing. The framework is arranged as such to form a mesoporous structure, with less than 2 nm diameters and large cages connected to channels. They possess special properties, such as ion exchange, molecular sieves, large surface area, and catalytic activity, which make them a preferable material for tremendous industrial applications in industries such as domestic and commercial water purification, softening, petrochemical industry, biogas industry, heating and refrigeration, detergents, medicine, and agriculture [8]. Nowadays, they are promising for the implementation at ceramic production, such as brick [9], ceramic pigments [10], porcelain and tiles bodies [11], and self-glazing ceramic tiles [12]. The main objective of this study was to use Iranian natural zeolite for developing a zeolite opacifier product for glazes in a cost effective way in comparison with zircon components.
Experimental Procedure
In the first part of the experimental study, one single fast-firing opaque tile glaze was selected as the standard frit (Table 1). The basic standard frit composition was selected to consist of 6 wt.% suspending agent (kaolin), 0.2 wt.% deflocculated (sodium tri-poly-phosphate), and 0.1 wt.% ligand (carboxyl methyl cellulose, CMC) in an eccentric mill used at the selected suspension compositions. In order to investigate effect of others minerals (titania, zircon, zeolite and kaolin), they were added in the standard frit in compound state. In this research, all the materials were of commercial grades (less than 98 wt.% purity) from industrial clays.
| Component | SiO2 | Al2O3 | MgO | CaO | K2O | Na2O | ZnO | BaO | B2O3 |
|---|---|---|---|---|---|---|---|---|---|
| Content (mol.%) | 58.47 | 6.28 | 1.34 | 14.07 | 3.75 | 1.70 | 4.84 | 1.20 | 8.35 |
Table 1: Composition of the standard frit.
The frit suspensions applied on the surface of tile supports using a regulated glaze applicator for the deposition of raw layers with 0.4 mm thickness. The test specimens were then fired in a roller kiln with the temperature and rate that is industrially used to manufacture the product with the considered support and glaze. Heating and coolin rates were about 40ºC/min and soaking time of 1000ºC was 3 min.
Opacity of glaze was evaluated based on colorimetric analysis using a Minolta CM-2600d spectrophotometer. Results were expressed by the tri-chromatic coordinates: L* means degree of whiteness, a* indicates variation between green and red colors, and b* presents variation between blue and yellow colors; therefore, it is very important to investigate the amount of opacity of L*. In order to quantitatively evaluate shiny of the glazes, glossiness (β60) was determined by Zehntner ZGM1110 glossiness analyzer.
To complement the results, the glazes were micro-structurally characterized by X-ray diffraction (XRD) and field-emission scanning electron microscopy (FESEM). Crystalline phase identification was performed on the glazes prepared from the ground samples using a X-ray diffractometer (Philips PW 170) operating with Cu-Kα= 1.54056 Å, 35 kV, and 40 m A radiation in the range of 10 to 60°2θ using the following settings: 0.1 mm receiving slit and 0.4 s/0.04° 2θ counting time. FESEM images were taken by Hitachi S-4160.
As far as the method and results of experiments were concerned, particularly SEM, it was predicted that the new tile had the potential for antibacterial activity. The popular method for antibacterial activity is ½McFarland, which was described in detail for antibacterial tiles in [13]. But, briefly speaking, Escherichia coli and Streptococcus arureus were deposited on the tile slide (5 cm×5 cm) and each slide was placed in a sterile vial. Tryptone soy broth was then added to each vial. An aliquot (10 ml) of aureus or E. coli suspension was added to each vial containing the slides and the vials were incubated by agitation at 35°C and 220 rpm. Bacteriostatic activity was evaluated after 24 h and the percentage of bacterial reduction was calculated using the following equation [14]:
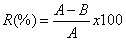 (1)
(1)
In which, R is reduction rate, A is the number of bacterial colonies from untreated tiles, and B is the number of bacterial colonies from the treated tiles.
Results and Discussion
In order to investigate the effect of zeolite on glaze in different chemical conditions, some experiments were carried out in the same firing profile. Output of glossiness and spectrophotometer in these experiments are shown in Table 2. Before going to the results, it is better to remind that, depending on the type of body, the acceptable amount of L* as opaque glaze is generally above 83; for instance a reference opaque frit has L* of 83.24 which contains 14 wt.% zircon. On the other hand, the minimum acceptable amount of glossiness for a shiny tile is 88.
| Formula | Glossiness | L* | a* | b* |
|---|---|---|---|---|
| Standard frit (S) | 102 | 69.4 | 6.79 | 13.59 |
| S + 10wt.% Zircon | 95 | 80.4 | 1.8 | 4.4 |
| S + 5wt.% TiO2 | 78 | 85.33 | 0.28 | 3.2 |
| S + 10wt.% TiO2 | 25 | 89.24 | -0.14 | 4.67 |
| S + 10wt.% Zeolite | 92 | 76.18 | 4.35 | 10 |
| S + 3 wt.% TiO2+ 7 wt.% Zeolite | 96 | 88.97 | 0.12 | 1.4 |
| S + 3 wt.% TiO2+ 7 wt.%Kaolin | 75 | 79.18 | 0.4 | 4 |
Table 2: Results of spectrophotometer and glossiness.
After checking standard frit, zircon and titania were added to frit as conventional opacifires. When 10 wt.% zircon was added to the frit as the state of compound, degree of whiteness and covering sharply increased without any significant effect on glossiness. Uniformity distribution of unfired zircon powders on the standard frit is exhibited in Figure 1.
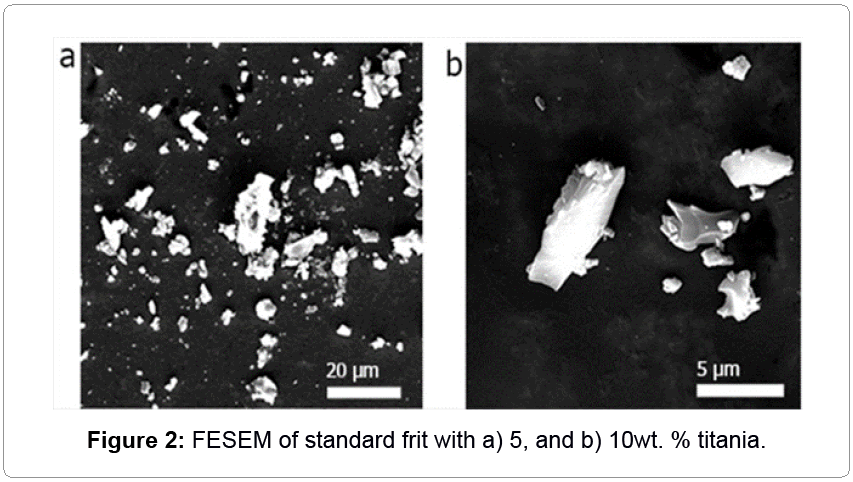
Another conventional opacifier, titania, was added with the amount of 5 and 10 wt.% to the standard frit. Results in Table 2 confirm that amounts of whiteness (L*) and covering (a*) were very higher than those of zircon; but, yellowish amount (b*) and decreased shininess demonstrated that titania was in a rutile crystallographic state. Morphology of titania on glaze directly depends on crystallographic state [15]. In fact, microstructural studies have confirmed that rutile crystals have always had an acicular morphology; however, anatase crystals are observed with cubic and rectangular morphologies [16]. Another stable phase of titanium in glaze is sphene (CaTiSiO5 [17]), which is completely sphere [4]. Visual effect of sphene and anatase is white; while anatase has benefits such as semiconductor and antibacterial effects. Bou et al. proposed that, in order to improve smoothness and glossiness, adding 1-3 wt.% P2O5 led to transferring rutile to sphene [4]. The problem of sphene is non-uniform distribution in glass matrix, which decreases chemical and mechanical resistance. Moreover, in terms of environmental safety, phosphorus pentoxide is extremely dangerous.
By making comparison Figure 2 with previous articles [4,14,15], existence of rutile was confirmed. This big crystal caused surface roughness and diminished glossiness to 25. One of the interesting results of FESEM of glaze containing titania was elemental distribution on glass-ceramic. In glassy matrix, atomic percentages of fluxes were 0.93, 4.87, 7.01, and 2.36% for Na, K, Ca, and Zn, respectively; but, these amounts were 3.46, 5.25, 12.35, and 3.11%; i.e. around crystalline part, flux materials, especially sodium and calcium, led to nucleation. Also, about 3 at .%, titanium was solved in glass, due to low chemical stability.
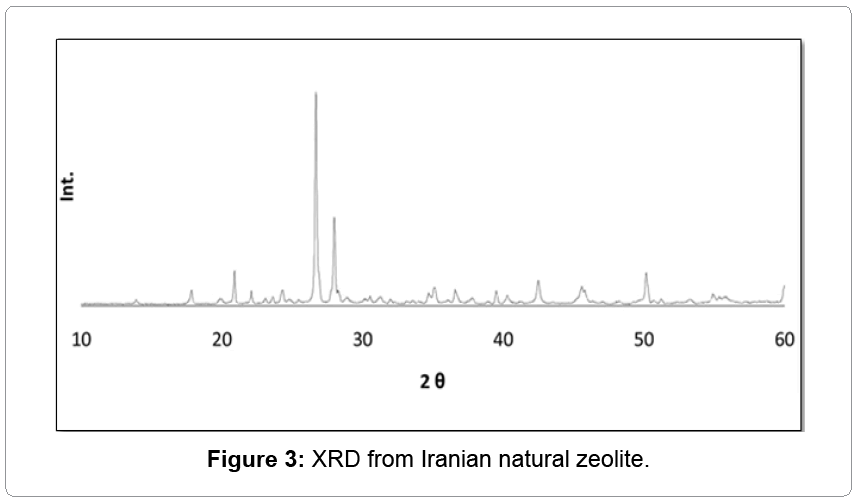
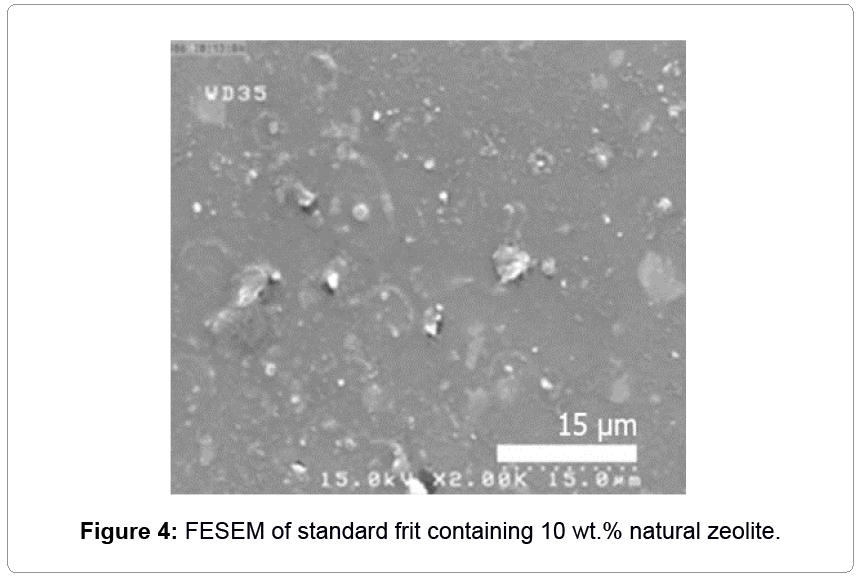
In order to investigate the effect of natural zeolite in glaze and compare it with other opacifiers, 10 wt.% natural zeolite was supplemented to the standard. Result of XRD showed that the zeolite had crystallinity based on having less amount of background with sharp peaks (Figure 3). Zeolite led to covering (absolute reduction in a* value in Table 2) and whitening (increase in L* amount) the surface without any significant effect on glossy. As can be seen in Figure 4, there were large amounts of unfired or crystalline materials in the glass matrix, which was due to the existence of zeolite. In contrast, for higher temperature (1100°C) and time duration (2 h), zeolite acted as a flux [1]; but, in this condition, it acted as a refractory. Although these results (Table 2) were comparable with those of zircon, no sufficient opaque glaze was obtained because of the refractive index of zeolite which was about 1.48-1.60 [18] and close to silicate-leadless glasses (1.5-1.7) [5].
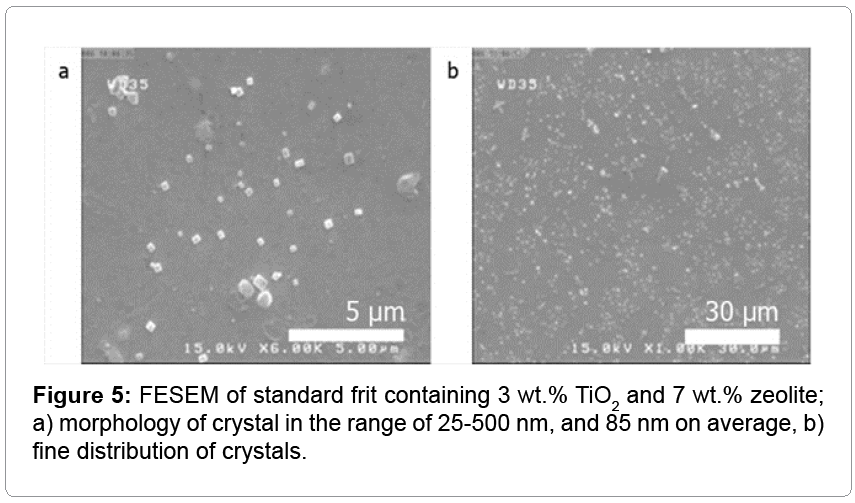
For sufficient covering with whitening in glaze by zeolite, the possibility of using zeolite as a nucleus for titanium oxides was inspected. For this purpose, 3 wt.% TiO2 and 7 wt.% zeolite were added to the standard frit. Table 2 exhibits that this glaze was acceptable as an opaque glaze. This glaze had a shiny surface, well-covered tile body, high whiteness, and especially low price due to cheap raw materials. To complement the results, FESEM was carried out on these tiles. Figure 5 shows rectangular nanoparticles (25-500 nm size distribution with average of 85 nm) with uniform distribution in glaze. Based on the previous studies, this structure is related to anatase [5]. In view of EDX analysis in position of Figure 2 (results does not show), elements distribution on matrix and ceramic are generally the same as those of the previous samples; but, percentage of Ti in matrix relented to 0.42 at.% (instead of 3 at.%). Indeed, natural zeolite is a cheap agent for the nucleation of nanocrystalline anatase without any side effects on other properties of the tiles.
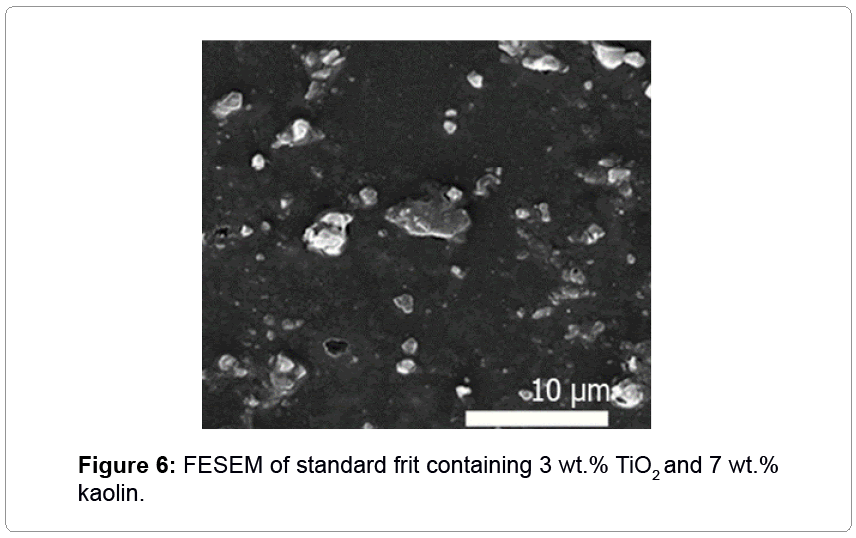
A proposed mechanism for this phenomenon from thermodynamic point of view is that zeolite leads to the stability of anatase. Also, in the past, the proposed alumina and silica were the nucleation for anatase [9]. Sincezeolite is alumino-silica, this reasoning was checked by kaolin, as mineral clay mainly containing alumina and silicates, but with different morphologies. Results of this experiment are illustrated in Table 2 and Figure 6. Kaolin had no effect on the phase and morphology of rutile and led to increasing roughness and firing temperature of glaze. In conclusion, the ability of natural zeolite in making glassceramic by anatase nanoparticles is unique owing to its high porosity and crystalline (Figure 3) structure.
In the case of 3 wt.% TiO2 and 7 wt.% Zeolite, owing to the fact that there are anatase nanoparticles in the glaze, it has a promising antibacterial activity [19]. This phenomenon was investigated by ½McFarland method, as mentioned in experimental procedures.
In this case, it has been demonstrated that obtaining stable nanostructural anatase is not enough for antibacterial activity [20]. On the other hand, anatase must be excited by ultraviolet illumination. However, this tile has just about 35% bacteriostatic, at ½ McFarland conditions, which is not significant in comparison by naked eyes and it should be measured by a microscope and specified experiment. This result is much lower than that of other components, which was more than 95% [13].
It would be obvious that titania at glaze was initially melted and then solidification was carried out in the form of anatase. The increased zeolite of anti-bacteria agent was performed by achieving smaller anatase particles and better distribution, which can be realized by the chemistry of glass network. However, due to the fact that the recommendation of this research was just opacity, this result was also very interesting and beyond the present specified goal.
Conclusion
Natural zeolite is a new mineral source for construction and ceramic glass materials. The present research showed that zeolite was economically expedient and natural zeolite can be used for fabricating opaque glaze. Indeed, natural zeolite led to the stabilized nanocrystalline of anatase. This glaze had the potential for antibacterial tiles. Initial investigation showed its potential of antibacterial activity in a cost-effective way. At the moment we just mention to showing Zeolite has a good alternative for zircon and has potential also for obtaining more properties like antibacterial activities.
References
- DemirkiranAS, Artir R, Avci E (2008) Effect of natural zeolite addition on sintering kinetics of porcelain bodies. JMater Proc Tech 203: 465-470.
- Beals M, Blair LR, Foraker RW, Lasko LR (1951) Study of Particle Size of the Opacifying Phase in Titania Enamels: I, Change of Particle Size with Change of Concentration of Dissolved TiO2. J Am Ceram Soc 45:403.
- Bish DL, Carey JW (2001) Thermal behavior of natural zeolites. RevMineral Geochem 45: 403.
- BouE, Moreno A, Escardino A, Gozalbo A (2007) Microstructural study of opaque glazes obtained from frits of the system: SiO2-Al2O3-B2O3-(P2O5)-CaO-K2O-TiO2. J Eur CeramSoc 27: 1791-1796.
- Casasola R, Rincón JM, Romero M (2012) Glass–ceramic glazes for ceramic tiles: a review. J Mater Sci 47: 553-582.
- EpplerR (1969) Crystallization and Phase Transformation in TiO2Opacified Porcelain Enamels: 11, Comparison of Theory with Experiment. J Am Ceram Soc 52: 94-99.
- Diop MB,Grutzeck MW (2008) Sodium silicate activated clay brick. Bull Eng Geol Environ 67: 499-505.
- Osman Gencel, MucahitSutcu, ErtugrulErdogmus, VahdettinKoc, VedatVeli Cay, et al. (2013) Properties of bricks with waste ferrochromium slag and zeolite. J Cleaner Prod 59: 111-119.
- Hanaor DAH, Sorrell CC (2011) Review of the anatase to rutile phase transformation. J Mater Sci 46: 855-874.
- Pekkan K, KarasuB (2009) Production of opaque frits with low ZrO2 and ZnO contents and their industrial uses for fast single-fired wall tile glazes. J Mater Sci 44: 2533-2540.
- Pogrebenkov VM, Sedel'nikova MB,Vereshchagin VI (1998) Zeolites: Raw material for ceramic pigments. Glass Ceram 55: 55-56.
- Pogrebenkov VM, Mel'nik ED, Vereshchagin VI (1997) Use of Siberian mineral raw materials in the manufacture of self-glazing ceramic tile. Glass Ceram 54: 373-375.
- Ghafarinazari A, Moztarzadeh F, Rabiee SM, Rahabloo T, Mozafari M, et al. (2012) Antibacterial activity of silver photodepositednepheline thin film coatings. CeramInt 38:5445-5451.
- Nazari AG, Tahari A, Moztarzadeh F, Mozafari M (2011) Ion exchange behaviour of silver-doped apatite micro- and nanoparticles as antibacterial biomaterial. Micro Nano Lett 6: 713-717.
- Gong X, Kang SB, Li S, Cho JH (2009) Enhanced plasticity of twin-roll cast ZK60 magnesium alloy through differential speed rolling. Mater Design 30: 3345-3350.
- Teixeira S, BernardinAM (2009) Development of TiO2 white glazes for ceramic tiles. Dyes Pigments 80: 292-296.
- Frost BR, Chamberlain KR, Schumacher JC (2001)Sphene (titanite): phase relations and role as a geochronometer. ChemGeol 172: 131-148.
- Larlus O,Mintova S, Valtchev V, Jean B, Metzger TH, et al. (2004) Silicalite-1/polymer films with low-k dielectric constants. Appl Surf Sci226: 155-160.
- Saeki Y (2011) Application of Antibacterial and Self-Cleaning Effects to NoncementitiousConstruction Materials, in Applications of Titanium Dioxide Photocatalysis to Construction Materials. Springer 17-22.
- Niederhãusern S, Bondi M, Bondioli F (2013) Self-Cleaning and Antibacteric Ceramic Tile Surface. Int J Appl Ceram Tech 10: 949-956.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 15269
- [From(publication date):
December-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10671
- PDF downloads : 4598