Review Article Open Access
Nanotechnology (Nanohydroxyapatite Crystals): Recent Advancement in Treatment of Dentinal Hypersensitivity
Somya Khetawat and Surendra Lodha*
Department of Orthodontics and Dentofacial Orthopedics, Umrao Dental Care and Orthodontic Center, India
- Corresponding Author:
- Surendra Lodha
Department of Orthodontics andDentofacial Orthopedics
Private practice of orthodontics at 8A-26, Umrao Dental
Care and Orthodontic Center, R. C. Vyas Colony
Bhilwara, Rajasthan 311001 India
Tel: 01482 236 222;
E-mail: drsurendraalodha@gmail.com
Received date:May 21, 2014 ; Accepted date: June 12, 2015 ; Published date: June 18, 2015
Citation: Khetawat S, Lodha S (2015) Nanotechnology (Nanohydroxyapatite Crystals): Recent Advancement in Treatment of Dentinal Hypersensitivity. J Interdiscipl Med Dent Sci 3:181. doi: 10.4172/2376-032X.1000181
Copyright: © 2015 Khetawat S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Dentinal hypersensitivity is often a chief concern among patients. A wide range of commercially available products for the treatment of dentinal hypersensitivity contain potassium, strontium, oxalates, fluoride salts and recently calcium sodium phosphosilicate and proarginine. More recently, the toothpastes containing carbonated hydroxyapatite nanocrystals are being studied. These have high reactivity by which they bind to enamel and dentine apatite producing a biomimetic coating on enamel, contrasting plaque formation. They also prevent tooth from decay, rebuild and revitalize the teeth and seal dentinal tubules, annulling hypersensitivity. In near future new products of this kind will be a breakthrough in the treatment of dentinal hypersensitivity.
Keywords
Dentine hypersensitivity; Nanotechnology; Nanoparticles; Nanohydroxyapatite crystals
Introduction
Dentine hypersensitivity is characterized by short sharp pain arising from exposed dentine in response to stimuli typically thermal, evaporative, tactile, osmotic or chemical and which cannot be ascribed to any other form of dental defect or pathology [1]. Tooth hypersensitivity differs from dentinal and pulpal pain in that the patient’s ability to locate the source of pain is good. Dentinal hypersensitivity is a response from a non-noxious stimulus, is a chronic condition with acute episodes, whereas dentinal pain is a response from a noxious stimulus, and is usually an acute condition [2].
Varieties of treatment modalities are available for its treatment. Desensitizing agents tried clinically are summarized in Table 1 [3].
| Mode of Administration | |
|---|---|
| At home desensitizing agents | In office treatment |
| On the basis of mechanism of action | |
| Nerve desensitization | Potassium nitrate |
| Protein precipitation | Gluteraldehyde |
| Silver nitrate | |
| Zinc chloride | |
| Strontium chloride hexahydrate | |
| Plugging dentinal tubules | Sodium fluoride |
| Stannous fluoride | |
| Strontium chloride | |
| Potassium oxalate | |
| Calcium phosphate | |
| Calcium carbonate | |
| Bio active glasses (SiO2-P2O5-CaO-Na2O) | |
| Dentine Adhesive Sealers | Fluoride varnishes |
| Oxalic acid and resin | |
| Glass ionomer cements | |
| Composites | |
| Dentin bonding agents | |
| Lasers | Neodymium: yttrium aluminum garnet (Nd-YAG) laser |
| GaAlAs (galium-aluminium-arsenide laser | |
| Erbium-YAG laser | |
| Homeopathic medication | |
| Propolis |
Table 1: Classification of desensitizing agents [3].
Chemical agents incorporated into dentifrices accepted by the American Dental Association are strontium chloride and potassium nitrate. In office treatment options include noninvasive (oxalates, cavity varnishes, strontium chloride, composite resins, GLUMA, calcium hydroxide, lasers, iontophoresis) and invasive (pulpectomy, class V restorations, gingival graft surgery) methods. The availability of a wide variety of treatments certainly indicate that there is still no ideal desensitizing agent which can provide sustained or prolonged action for the treatment of dentine hypersensitivity. Most of the composite resins have limitation of handling and flow and microleakage due to shrinkage after polymerization. More recently, toothpastes containing carbonated hydroxyapatite nanocrystals are used to resolve this longstanding problem [4].
The term “nano” is derived from the Greek word “dwarf ”. Nanotechnology is the science of manipulating matter measured in the billionths of meter or nanometer, roughly the size of 2 or 3 atoms [5]. Richard Feynman, a physicist, gave first idea of nanotechnology in 1959 in a lecture called “Plenty of Room at the Bottom” for which he won Noble Prize. Feynman's idea remained largely undiscussed until the mid-1980s, when the MIT educated engineer K Eric Drexler published “Engines of Creation”, a book to popularize the potential of molecular nanotechnology [6]. Prof. Kerie E. Drexler, a researcher and writer of nanotechnology [7], coined the term nanotechnology. First defined by Norio Taniguchi in 1974, “Nanotechnology mainly consists of the processing of separation, consolidation, and deformation of material by one atom or one molecule” [8].
Nanotechnology has achieved a tremendous progress in the past several decades. Nanomaterials are those materials with component less than 100 nm in at least one dimension, including clusters of atoms, grains less than 100 nm in size, fibers those are less than 100 nm diameter, films less than 100 nm in thickness, nanoholes, and composites. They have evoked a great amount of attention for improving disease prevention, diagnosis and treatment. From the definition provided by the National Nanotechnology Initiative, nanotechnology exploits specific phenomena and direct manipulation of materials on the nanoscale [9]. However, nanotechnology is much more than the study of small things; it is the research and development of materials, devices, and systems exhibiting physical, chemical, and biological properties that are different from those found on a larger scale [10].
In nanoland, tiny differences in size can add up to huge differences in function. Ted Sargent, author of the dance of Molecules, says matter is tunable at nanoscale. For example, quantum dot is a nanocrystal made of semiconductor materials that is small enough to exhibit quantum mechanical properties, changing the size of semiconductors, changes the spectrum of color light due to quantum confinement. Sergent made a three-nanometric dot that ‘glows’ blue, and four nanometer dot that glows red and a five nanometer dot that emits infrared rays or heat [11].
Nanotechnology will affect everything, as advocated by William Atkinson, author of Nanoscom. The unique quantum phenomena that happens at the nanoscale, draws researchers from many different disciplines including medicine, chemistry, physics, engineering, and dentistry [6].
In 2000, Freitas introduced the term nanodentistry and stated that “new treatment opportunities, permanent hypersensitivity cure, complete orthodontic realignments during a single office visit, covalently bonded diamondized enamel and continuous oral health maintenance through the use of mechanical dentifrobots [12].”
Properties of Nanoparticles [13]
1. Nanoparticles have significant surface effects, size effects, and quantum effects.
2. Nanoparticles have special properties, including chemical, optical, magnetic, and electro-optical properties, which differ from those of either individual molecules or bulk species.
3. Nanostructured materials are the development of selfassembly, an autonomous organization of components into patterns or structures without human intervention occurs.
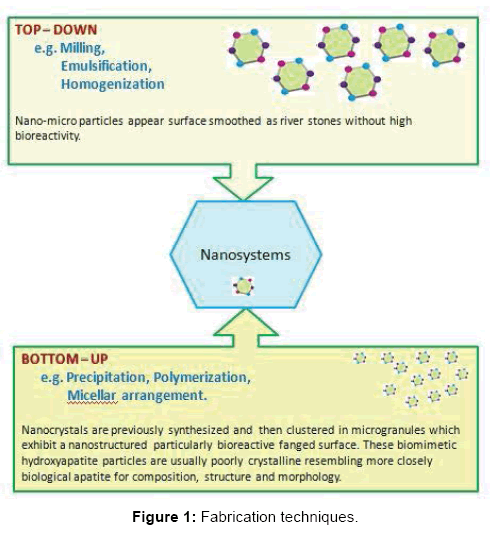
Fabrication Techniques
In ‘top - down’ techniques, the micro-nano dimensions of the hydroxyapatite are obtained by milling larger particles of commercial synthetic hydroxyapatite. Only recently, the development of nanotechnologies has opened new opportunities in obtaining cheap HA micro-nanoparticles using the ‘‘bottom up’’ methods, in order to improve the biological responses of natural HA.The bottom-up fabrication technique has been summerized in Figure 1.
Various Nanostructures [10]
1. Nanopores
2. Nanotubes
3. Quantum dots
4. Nanoshells
Dendrimers
A nanopores is a small hole with an internal diameter of the order of 1 nanometer. A nanotube is a nanometer scale tube like structure. Quantum dots also known as nanocrystals, are nanosized semiconductors, depending upon their size, they can emit light of all colours of the rainbow. Nanoshells also referred to as core-shells, and are spherical cores of a particular compound surrounded by a shell or outer coating, which is few nanometers thick. Dendrimers are highly branched structures gaining wide use in nanomedicine because of the multiple molecular "hooks" on their surfaces that can be used to attach cell-identification tags, fluorescent dyes, enzymes and other molecules. The first dendritic molecules were produced around 1980, but interest, knowledge and advancement in nanotechnology has blossomed more recently with the discovery of their biotechnological uses.
Nanoparticles
Composite resins are also used for curing dentinal hypersenitivity by sealing dentinal tubules. Nanocomposites have shown better results in comparision to the hybrid or last generation of the resins. Nonagglomerated discrete nanoparticles are homogenously manufactured in resin or coating to produce nanocomposite. The nanofiller used included an aluminosilicate powder having a mean particle size of about 80nm and 1: 4 M ratio of alumina to silica. The deliberate placement, manipulation and higher level of control on the sub 100nm scale particles in the resin matrix that display the polish of a microfill but the strength and wear resistance of hybrid composite [14].
Gary and Leinfelde (2009) have summerized the types of nanoparticles in composite resins as below [15]
Type I: Subtype A: Nanomeric particles dispersed as a single unit within the resin matrix.
Type I: Subtype B: Consists of agglomerated cluster of nanoparticles.
Type II: Consists of cage like structure that is composed of eight silicon atoms and twelve oxygen atoms.
Characteristics of nanofillers in dental composites make the nanocomposites superior to the conventional composites and blend with natural tooth structure much better.
- Superior hardness
- Superior flexural stength
- Superior modulus of elasticity
- Superior translucency, esthetic appeal, excellent color density, high polish and polish retention
- About 50% reduction in filling shrinkage
- Excellent handling properties
Nano-resins are “utility in fielder” with ability to flow over the walls of the cavity preparation, wetting the surface of dentine to ensure better adaptation. These materials can be used as sealants and as cement for thin staked porcelain veneers. Septodont’s N’Durance- Dimer flow, is a new flowable composite, which features unique chemistry and nanotechnology formulation. Nanocomposite have minimum shrinkage, has ease of handling, and is highly esthetic material. Nanotechnology in dentistry is giving significant occupany in different forms as stated in Table 2.
| Bottom up approach | Top down approach |
|---|---|
| Local anesthesia | Nano composites |
| Hypersensitivity cure Nanorobotic dentifrice Dental durability & cosmetics Orthodontic treatment Photosensitizers & carriers Diagnosis & treatment of oral cancer |
Nano solutions Impression materials Nanoencapsulation Nanoneedles Bone replacement materials |
Table 2: Application of nanotechnology in dentistry.
Teeth Remineralization by Nanotechnology
Hydroxyapatite Ca10(PO4)6(OH)6 building block of enamel, are the main constituent of dental tissues representing in enamel and dentin 95% - 97% wt and 75 % respectivily and responsible for mechanical behaviour of dental tissues. Hydroxyapatite (HA) is the most stable form of calcium phosphate. Enamel prismatic HA crystals consist of a weaving of prisms ranging from 3 to 5 μm in diameter. A single prism reveals a highly organized array of fastened needle like HA crystallites (approximately 30 nm thick, 60 nm wide, and several millimetres long). Unlike bone, in enamel and dentine when HA is dissolved or abraded, it cannot spontaneously remineralize because enamel is deprived of regenerative cells and contrarily dentine apposition occurs only towards the pulp tissues [16].
Biogenic Hydroxyapatite V/S Biomimetic Synthetic Hydroxyapatite (Nanohydroxyapatite)
Biogenic carbonate hydroxyapatite (CHA) nanocrystals, constituents mineral phase of calcified tissues such as bone, dentin and enamel. They contain 4-8 wt % of carbonate anions, approximately 25 nm wide, 2-5 nm thick and 60 nm in length. They exhibit a nonstoichiometric composition and have low degree of crystallinity.
Synthetic biomimetic CHA nanocrystals are very similar to biogenic CHA nanocrystals. Biomimetic CHA nanocrystals have been synthesized, containing 4 ± 1 wt% of carbonate ions, about 20 and 100 nm in size with an acicular and plate morphology respectively. They are nearly stoichiometric in bulk Ca/P molar ratio of about 1.6-1.7 [17]. Synthetic bioresorbable biomimetic hydroxyapatite nano and micro crystals exhibit excellent properties like bone filler biomaterial, such as biocompatibility, bioactivity, osteoconductivity, direct bonding to bone, etc., exciting new applications of HA in the fields of bone tissue engineering and orthopaedic therapies.
Recently, synthetic CHA biomimetic nanocrystals have been shown to produce, In Vitro, re-mineralization of the altered enamel surfaces and closing of dentinal tubules, thus showing a potential use in desensitizing dentifrices. Hefferren et al. have suggested that, increased re-mineralization occurs more with apatite particles sizes <4 μm [18]. The potential desensitizing effect of biomimetic CHA nanocrystals, is due to the progressive closure of the tubular openings of the dentine with plugs within a few minutes until the regeneration of a mineralized layer has occurred within a few hours.
Natural hypersensitive teeth have eight times higher surface density of dentinal tubules and diameter with twice as large than nonsensitive teeth [19]. Reconstructive dental nanorobots, using native biological materials, could selectively and precisely occlude specific tubules within minutes, offering patients a quick and permanent cure [20]. Field Emission Scanning Electron Microscope (FE-SEM) observation of the nano-HAP-treated dentine surface showed that nano-HAP uniformly occluded the dentinal tubules with a dentinal plug and a protective layer on the surface of the dentine was also formed [21].
Nanotechnology Offers a Unique Approach to Overcome the Shortcomings of Many Conventional Materials
- Fluoride remineralisation, is based mainly on surface enamel apatite modification, whereas biomimetic hydroxyapatite forms a new coating.
- Potassium reduces nerve excitability whereas biomimetic hydroxyapatite acts as alternate substance, not only alleviate pain but also cures sensitivity.
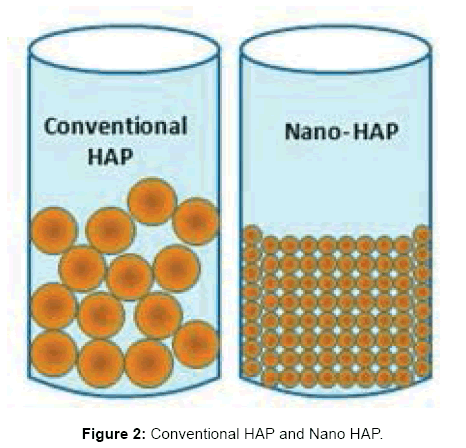
- Conventional hydroxyapatite has poor affinity and is randomly oriented resulting in easy loss of crystals, whereas biomemmitic hydroxyapatite has high affinity and self-assembly property (Figure 2).
Challenges Faced by Nanodentistry [21]
• Precise positioning and assembly of molecular scale part
• Economical nanorobot mass production technique
• Biocompatibility
• Simultaneous coordination of activities of large numbers of independent micron-scale robots
• Social issues of public acceptance, ethics, regulation and human safety
Problems for Research in Nanotechnology in India [21]
• Painfully slow strategic decisions
• Sub-optimal funding
• Lack of engagement of private enterprises
• Problem of retention of trained manpower
Conclusion
Carbonated hydroxyapatite nanocrystals synthesized with tailored biomimetic characteristics for composition, structure, size and morphology can chemically bind themselves on the surfaces of teeth tissues, filling the scratches, producing a bound biomimetic apatitic coating and protecting the enamel surface structure. Only recently, the development of nanotechnologies has opened new opportunities in obtaining cheap hydroxyapatite micro-nano particles by the “bottom up” methods. These hydroxyapatites are surface nanostructured and have higher surface area and consequently higher reactivity, allowing them to bind to enamel and dentine apatite producing a biomimetic coating on enamel, contrasting plaque formation and sealing dentine tubules and annulling hypersensitivity.
References
- Dowell P, Addy M (1983) Dentine hypersensitivity--a review. Aetiology, symptoms and theories of pain production. J ClinPeriodontol 10: 341-350.
- Frederick A (1990) Tooth Hypersensitivity. The Dental Clinics of North America 34.
- Miglani S, Aggarwal V, Ahuja B (2010) Dentin hypersensitivity: Recent trends in management. J Conserv Dent 13: 218-224.
- Ciancio SG (1995) Chemical agents: plaque control, calculus reduction and treatment of dentinal hypersensitivity. Periodontol 2000 8: 75-86.
- Kaehler T (1994) Nanotechnology: basic concepts and definitions. ClinChem 40: 1797-1799.
- Patil M, Mehta DS, Guvva S (2008) Future impact of nanotechnology on medicine and dentistry. J Indian SocPeriodontol 12: 34-40.
- Verma SK, Prabhat KC, Goyal L, Rani M, Jain A (2010) A critical review of the implication of nanotechnology in modern dental practice. Natl J MaxillofacSurg 1: 41-44.
- Taniguchi N (1974) On the Basic Concept of 'NanoTechnology'. Proc Intl Conf Prod Eng Tokyo, Part II, Japan Society of Precision Engineering.
- Huang Z, Chen H, Chen Z, Roco MC (2004) International nanotechnology development in 2003: Country, institution, and technology field analysis based on USPTO patent database. J Nanoparticle Res 6: 325-354.
- FreitasJr RA (1999) Nanomedicine, Volume I: Basic Capabilities. Nanomedicicne 1.
- Sargent T (2006) The Dance of Molecules: how nanotechnology is changing our lives. Thunder's Mouth Press 304.
- Freitas RA Jr (2000) Nanodentistry. J Am Dent Assoc 131: 1559-1565.
- Kong LX, Peng Z, Li SD, Bartold PM (2006) Nanotechnology and its role in the management of periodontal diseases. Periodontol 2000 40: 184-196.
- Swift EJ (2006) Nanocomposites. J Esth Rest Dent 17: 3-4.
- Radz GM, Leinfelde KF (2008) The current state of composite resins. Inside Dentistry 4.
- Roveri N, Foresti E, Lelli M, Lesci IG (2009) Recent advancements in preventing teeth health hazard: the daily use of hydroxyapatite instead of fluoride. Recent patents on biomedical engineering 2: 197-215.
- Orsini G, Procaccini M, Manzoli L, Giuliodori F, Lorenzini A, et al. (2010) A doubleblind randomized-controlled trial comparing the desensitizing efficacy of a new dentifrice containing carbonate/hydroxyapatite nanocrystals and a sodium fluoride/potassium nitrate dentifrice. J ClinPeriodontol 37: 510-517.
- Hefferren JJ (1976) A laboratory method for assessment of dentrifriceabrasivity. J Dent Res 55: 563-573.
- Addy M, Mostafa P, Newcombe RG (1990) Effect of plaque of five toothpastes used in the treatment of dentin hypersensitivity. ClinPrev Dent 12: 28-33.
- Ohta K, Kawamata H, Ishizaki T, Hayman R (2007) Occlusion of Dentinal Tubules by Nano-Hydroxyapatite. J Dent Res 86: 21-24.
- Saravana KR, Vijayalakshmi R (2006) Nanotechnology in dentistry. Indian J Dent Res 17: 62-65.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 19508
- [From(publication date):
June-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 14436
- PDF downloads : 5072