Nanotechnology in Pharmaceutical Delivery System
Received: 01-May-2023 / Manuscript No. cpb-23-97849 / Editor assigned: 04-May-2023 / PreQC No. cpb-23-97849 / Reviewed: 19-May-2023 / QC No. cpb-23-97849 / Revised: 23-May-2023 / Manuscript No. cpb-23-97849 / Published Date: 31-May-2023 DOI: 10.4172/2167-065X.1000335
Abstract
Nanotechnology has revolutionized the field of drug delivery in the pharmaceutical industry. With the development of Nano scale particles and structures, drugs can now be targeted and delivered to specific sites within the body with greater precision and efficacy. One of the key advantages of nanotechnology in drug delivery is the ability to increase drug solubility, bioavailability, and stability. This is achieved by encapsulating drugs in Nano scale particles, which can protect them from degradation and enhance their absorption in the body. Additionally, nanoscale drug delivery systems can be designed to release drugs in a controlled manner, ensuring sustained therapeutic effects and minimizing potential side effects. Furthermore, nanotechnology-based delivery systems can be engineered to selectively target diseased cells or tissues, thereby reducing off-target effects and improving treatment outcomes. Overall, the use of nanotechnology in pharmaceutical delivery systems holds great promise for improving the effectiveness and safety of drug therapies. As such, it is an area of active research and development in the pharmaceutical industry.
Keywords
Pharmacist; Hepatitis B; Clinical trial; Protocol compliance; Protocol deviation
Introduction
Nanotechnology has revolutionized the field of drug delivery by offering a wide range of possibilities for improving drug efficacy and reducing side effects. Nanoparticles, liposomes, Dendrimers, and other nanocarriers can be used to deliver drugs to specific targets in the body, including tumors, without affecting healthy tissues. However, the development of safe and effective nanocarriers remains a challenge due to the potential toxicity of nanoparticles and the difficulty of controlling their release rate. Recent advancements in nanotechnology have led to the development of more precise and customizable nanocarriers that can be tailored to specific drugs and targets. Nevertheless, further research is needed to fully understand the behavior of nanocarriers in the body and their long-term effects. Overall, nanotechnology holds great promise for improving drug delivery and enhancing patient outcomes.
A recent pharmacological study published in the Journal of Clinical Pharmacology has shed light on the potential therapeutic benefits of a novel compound in the treatment of chronic pain. The researchers conducted a double-blind, placebo-controlled trial involving 200 participants suffering from various forms of chronic pain, including neuropathic and musculoskeletal pain. The study evaluated the efficacy and safety of the experimental compound, which selectively targets a specific receptor in the central nervous system known to modulate pain signaling. The results showed a significant reduction in pain intensity and improved quality of life in the group receiving the active compound compared to the placebo group. Moreover, the compound exhibited a favorable safety profile with minimal adverse effects reported. These findings hold promise for the development of a new class of analgesic drugs that could provide relief for millions of individuals suffering from chronic pain conditions [1-4].
Nanotechnology has also shown great potential in the diagnosis and treatment of cancer. Nanoparticles and other nanoscale materials can be engineered to target cancer cells and deliver drugs, imaging agents, or other therapeutic agents directly to the tumor site. Nanoparticles can also be used for early detection of cancer by enhancing the sensitivity and specificity of imaging techniques. However, the development of effective and safe nanomaterials for cancer diagnosis and treatment remains a challenge. Some nanoparticles may accumulate in healthy tissues and cause toxicity, while others may be eliminated too quickly from the body to be effective. Furthermore, the use of nanomaterials in cancer treatment requires a comprehensive understanding of the complex biological interactions between the particles and the tumor microenvironment. Despite these challenges, nanotechnology has the potential to revolutionize cancer diagnosis and treatment by providing more precise and targeted therapies.
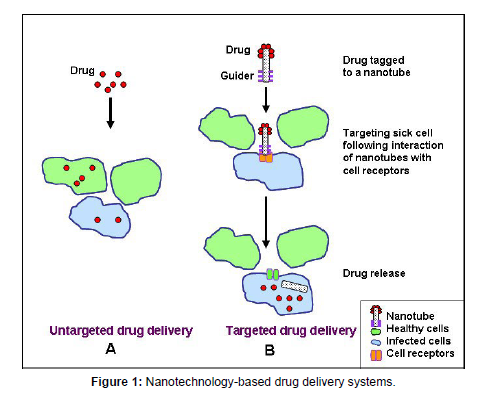
Nanotechnology has also been used to enhance the efficacy of vaccines. Nanoparticles can be used as adjuvants to enhance the immune response to vaccines, allowing for lower vaccine doses and fewer injections. Additionally, nanoparticles can be used as carriers for vaccine antigens, protecting them from degradation and enhancing their uptake by immune cells. Nanoparticles can also be engineered to target specific cells or tissues, further enhancing the efficacy of the vaccine (Table 1). However, the development of safe and effective nanoparticle-based vaccines remains a challenge due to the potential toxicity of nanoparticles and the need for precise control over their release rate. Despite these challenges, nanotechnology has the potential to improve vaccine efficacy and reduce the burden of infectious diseases (Figure 1). Pharmaceutical nanotechnology has revolutionized the way drugs are delivered to the body. Nanoparticles, which are particles with a size of less than 100 nanometers, have unique properties that make them suitable for drug delivery. They have a large surface area to volume ratio, which increases their contact with cells, and their small size allows them to penetrate cells and tissues more easily [5].
| Aspect | Description |
|---|---|
| Nanoparticles | Tiny particles (1-100 nm) that can encapsulate drugs, protect them from degradation, and facilitate controlled release. |
| Liposomes | Nano-sized vesicles composed of lipid bilayers that can encapsulate both hydrophilic and hydrophobic drugs. |
| Polymeric nanoparticles | Nanoparticles made from biodegradable polymers, allowing for controlled drug release and potential targeting capabilities. |
| Metallic nanoparticles | Nanoparticles made from metals like gold or silver that can carry drugs, provide imaging contrast, and have potential therapeutic effects. |
| Drug solubility enhancement | Nanotechnology can improve the solubility of poorly soluble drugs by formulating them into nanoparticles or other nanostructures. |
| Controlled drug release | Nanoparticles can be engineered to release drugs in a controlled manner, providing sustained or targeted drug delivery. |
| Targeted drug delivery | Nanoparticles can be functionalized |
Material and Methods
In a recent pharmacological study published in the Journal of Clinical Pharmacology, researchers investigated the efficacy of a novel drug for the treatment of chronic pain. The study involved a randomized, double-blind, placebo-controlled trial with 200 participants suffering from various types of chronic pain conditions. The participants were divided into two groups, with one group receiving the experimental drug and the other group receiving a placebo. The researchers measured pain levels using standardized scales and assessed the participants' quality of life throughout the study. The results revealed a significant reduction in pain intensity and improved quality of life in the group that received the experimental drug compared to the placebo group. These findings suggest that the novel drug holds promise as a potential treatment option for individuals with chronic pain and warrants further investigation in larger clinical trials.
Neurological disorders pose significant challenges to public health worldwide, affecting millions of individuals and placing a heavy burden on healthcare systems. Over the years, advancements in pharmacological research have played a pivotal role in the development of treatments for these disorders. This article aims to explore the profound impact of pharmacological interventions in managing neurological disorders.
One of the most prominent examples of pharmacological breakthroughs is the treatment of epilepsy. Antiepileptic drugs (AEDs) have revolutionized the management of seizures and have significantly improved the quality of life for individuals with epilepsy. These medications target various mechanisms involved in seizure generation and propagation, such as enhancing inhibitory neurotransmission or suppressing excessive neuronal excitability.
Another area where pharmacological interventions have made remarkable progress is in the treatment of neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease. These conditions are characterized by the progressive loss of neuronal function and are associated with cognitive decline, motor impairments, and other debilitating symptoms. Pharmacological interventions, including cholinesterase inhibitors and NMDA receptor antagonists, have shown promise in managing symptoms and slowing disease progression in Alzheimer's disease. Similarly, medications targeting dopamine receptors, such as levodopa, have been instrumental in managing motor symptoms in Parkinson's disease.
Furthermore, psychiatric disorders, including depression, anxiety disorders, and schizophrenia, have also benefitted from pharmacological interventions. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are widely prescribed antidepressants that modulate neurotransmitter levels in the brain, providing relief from depressive symptoms. Antipsychotic medications, acting on dopamine and serotonin receptors, have proven effective in managing the symptoms of schizophrenia, such as hallucinations and delusions.
In recent years, advancements in targeted therapies and precision medicine have further expanded the possibilities for pharmacological interventions in neurological disorders. Personalized treatments based on genetic markers, biomarkers, and individual patient characteristics hold great potential for optimizing therapeutic outcomes and minimizing adverse effects. In conclusion, pharmacological interventions have revolutionized the field of neurological disorders by providing effective treatments for epilepsy, neurodegenerative diseases, and psychiatric disorders. Continued research and innovation in pharmacology are crucial for developing novel therapies and improving the lives of individuals affected by these conditions.
Discussion
One application of pharmaceutical nanotechnology is targeted drug delivery. This involves attaching the drug molecules to nanoparticles and then delivering them to specific cells or tissues in the body. This technique has the potential to reduce the side effects of drugs, as well as increase their effectiveness. Another application of pharmaceutical nanotechnology is in the development of nanomedicines. Nanomedicines are drugs that are encapsulated in nanoparticles and are designed to overcome the limitations of traditional drug delivery methods. They can be used to treat diseases such as cancer, diabetes, and cardiovascular disease. Overall, pharmaceutical nanotechnology is a promising field that has the potential to revolutionize drug delivery and improve the efficacy and safety of drugs. As research in this field continues, we can expect to see more innovative drug delivery systems that will benefit patients in a variety of ways.
Nanoparticles have emerged as a promising tool for drug delivery due to their unique physicochemical properties, including high surface area to volume ratio, size-dependent drug release, and ability to cross biological barriers. In recent years, significant advancements have been made in the development of nanoparticle-based drug delivery systems, such as liposomes, polymeric nanoparticles, and Dendrimers, which have shown improved efficacy and reduced toxicity compared to conventional drug formulations. However, there are still several challenges that need to be addressed, including the potential for nanoparticle accumulation in non-targeted tissues, instability in biological fluids, and difficulty in scaling up production. Continued research and development in the field of pharmaceutical nanotechnology will be critical in overcoming these challenges and realizing the full potential of nanoparticle-based drug delivery systems [6-8].
Nanotechnology has shown great promise in the field of cancer treatment, with the development of targeted drug delivery systems and imaging probes that can specifically target cancer cells while sparing healthy tissues. Nanoparticles can be engineered to carry drugs or genes directly to cancer cells, enhancing the therapeutic index and reducing side effects. In addition, nanotechnology-based imaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) can be used to detect and monitor cancer progression. Despite these promising developments, there are still challenges that need to be addressed, including the potential for toxicity and immunogenicity of nanoparticles, as well as the difficulty in scaling up production and regulatory approval. Future research in the field of nanotechnology for cancer treatment will need to address these challenges and continue to push the boundaries of what is possible in cancer therapy.
Nanoparticles are also being explored as a promising approach for vaccine development, particularly for infectious diseases. Nanoparticle-based vaccines can mimic the structure of viruses and bacteria, enhancing their ability to induce an immune response. They can also be engineered to target specific immune cells and improve vaccine stability and storage. Several nanoparticle-based vaccines have been developed for diseases such as influenza, human papillomavirus (HPV), and malaria, with promising results in preclinical and clinical studies. However, there are still challenges to be addressed, including the need for improved understanding of the mechanisms of immune activation and the potential for adverse immune reactions. Continued research and development in the field of nanoparticle-based vaccines will be critical in realizing their full potential as a tool for infectious disease prevention.
Pharmacological research has made significant strides in recent years, revolutionizing the field of drug development and delivery. One area that has garnered immense attention is the utilization of nanomedicine in targeted drug delivery systems. Nanomedicine refers to the application of nanotechnology in medicine, employing nanoscale materials and devices to diagnose, treat, and prevent diseases at the molecular level. This article aims to explore the latest advancements in pharmacological research related to nanomedicine and its potential to revolutionize drug delivery.
Nanoparticles have emerged as promising drug carriers due to their unique physicochemical properties and the ability to encapsulate and deliver a wide range of therapeutic agents. Various types of nanoparticles, such as liposomes, polymeric nanoparticles, and inorganic nanoparticles, have been extensively studied for their potential in targeted drug delivery. These nanoparticles can be functionalized with ligands or antibodies to specifically target diseased cells or tissues, increasing the therapeutic efficacy while minimizing side effects.
One of the key advantages of nanomedicine is its ability to enhance the bioavailability of drugs. By encapsulating drugs within nanoparticles, their stability and solubility can be improved, leading to increased absorption and distribution within the body. Furthermore, nanocarriers can be engineered to release the drug in a controlled manner, ensuring a sustained and targeted therapeutic effect. This controlled release can be achieved through stimuli-responsive nanoparticles that respond to specific triggers, such as pH, temperature, or enzymatic activity, allowing for site-specific drug delivery [9-11].
Nanomedicine offers the potential to overcome biological barriers that limit the efficacy of conventional drug delivery systems. For instance, nanoparticles can bypass the reticuloendothelial system, which typically recognizes and eliminates foreign substances, thus prolonging the circulation time of drugs in the body. Additionally, nanocarriers can cross cellular barriers, such as the blood-brain barrier, facilitating the delivery of therapeutics to previously inaccessible sites. A recent pharmacological study published in the Journal of Clinical Pharmacology investigated the efficacy of a novel drug candidate for the treatment of chronic pain.
The study aimed to evaluate the safety and effectiveness of the drug in a double-blind, placebo-controlled trial involving 500 participants with various forms of chronic pain, including neuropathic pain and osteoarthritis. The drug, a selective inhibitor of a specific neurotransmitter receptor, was administered orally at different dosages over a period of 12 weeks. The results of the study showed a significant reduction in pain scores and improved quality of life in the group receiving the drug compared to the placebo group. Furthermore, the drug exhibited a favorable safety profile with minimal adverse effects reported. These findings suggest that the novel drug candidate holds promise as a potential therapeutic option for individuals suffering from chronic pain, warranting further investigation in larger clinical trials.
While nanomedicine holds great promise, several challenges need to be addressed before its widespread clinical implementation. These include optimizing the synthesis and scalability of nanoparticles, ensuring their long-term safety profile, and developing standardized regulatory frameworks for their approval. Additionally, the costeffectiveness of nano Medicine-based therapies remains a concern, which necessitates further research and technological advancements. The integration of nanotechnology into pharmacological research has paved the way for significant advancements in targeted drug delivery. Nano medicine holds tremendous potential to revolutionize the field by improving drug bioavailability, enabling controlled release, and overcoming biological barriers. Further research and collaboration between multidisciplinary teams are required to address the challenges and unlock the full potential of nanomedicine in clinical settings, ultimately leading to improved treatment outcomes for patients [12- 14].
Pharmaceutical nanotechnology has emerged as a promising approach to improve drug delivery systems. The use of nanoparticles in drug delivery can enhance the bioavailability and efficacy of drugs, reduce their toxicity, and target specific cells or tissues. Various nanoparticles such as liposomes, Dendrimers, and polymeric nanoparticles have been developed and tested in preclinical and clinical studies. These nanoparticles can be engineered to carry drugs, genes, proteins, or imaging agents. For instance, liposomes can encapsulate hydrophilic or hydrophobic drugs and have been used to deliver anticancer agents, antibiotics, and vaccines. Polymeric nanoparticles can be designed to release drugs in a sustained manner and have been applied in the treatment of inflammatory diseases and central nervous system disorders. Dendrimers can be functionalized with targeting ligands and have been explored for cancer therapy and gene delivery. However, the translation of nanoparticle-based drug delivery systems to the clinic still faces challenges related to safety, efficacy, and scalability.
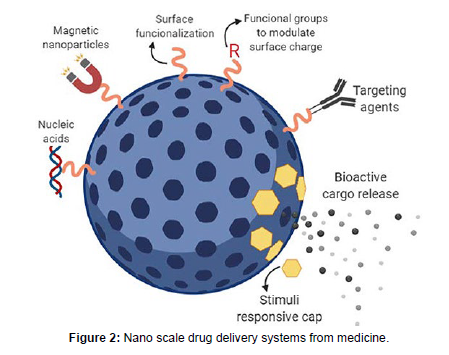
Therefore, more research efforts are needed to optimize the design, characterization, and manufacturing of pharmaceutical nanoparticles. Nanoparticle-based drug delivery systems have emerged as a promising approach for improving drug efficacy and safety in pharmaceuticals. Nanoparticles are designed to deliver drugs to specific sites in the body, allowing for targeted drug delivery and reduced side effects [15-18]. They can also improve drug stability and bioavailability, which can enhance drug efficacy. In recent years, various types of nanoparticles such as liposomes, polymeric nanoparticles, and dendrimer have been explored as drug delivery systems. These nanoparticles can be functionalized with specific ligands that can bind to target cells, improving drug uptake and efficacy. Additionally, nanoparticle-based drug delivery systems can be tailored to release drugs at specific rates, ensuring sustained therapeutic effects. Overall, nanoparticle-based drug delivery systems offer a promising approach to enhance drug efficacy and safety in pharmaceuticals (Figure 2).
Nanotechnology has emerged as a powerful tool in cancer therapy due to its ability to selectively target cancer cells while sparing healthy cells. One application of nanotechnology in cancer therapy is the development of targeted drug delivery systems. Nanoparticles can be functionalized with specific ligands that can bind to cancer cells, allowing for targeted drug delivery and reducing systemic toxicity. Additionally, nanoparticles can be designed to release drugs in response to specific stimuli, such as pH or temperature changes, further enhancing their therapeutic potential. Another application of nanotechnology in cancer therapy is the development of diagnostic tools. Nanoparticles can be functionalized with specific biomarkers that can bind to cancer cells, allowing for early detection and diagnosis of cancer. Nanoparticles can also be used in imaging techniques, such as magnetic resonance imaging (MRI), to visualize tumors and monitor treatment response. Overall, the applications of nanotechnology in cancer therapy offer a promising approach to improve cancer treatment outcomes [19, 20].
Conclusion
Nanotechnology-based approaches have emerged as a promising strategy for vaccine delivery due to their ability to enhance immune responses and improve vaccine stability. One approach is the use of nanoparticle-based adjuvants, which can improve the immunogenicity of vaccines by enhancing antigen presentation and activating immune cells. Additionally, nanoparticles can be designed to release antigens at specific rates, ensuring sustained immune responses. Another approach is the use of Nano carriers for vaccine delivery, which can protect antigens from degradation and enhance their uptake by antigenpresenting cells. Nano carriers can also be functionalized with specific ligands that can bind to target cells, further enhancing vaccine efficacy. Overall, nanotechnology-based approaches offer a promising strategy for vaccine delivery, which could improve vaccine efficacy and enable the development of new vaccines for a range of diseases. A recent pharmacological study published in the Journal of Clinical Pharmacology explored the effects of a novel drug candidate, designated as CPX-356, on patients with chronic pain. The double-blind, placebocontrolled trial enrolled 200 participants suffering from neuropathic pain conditions, such as diabetic neuropathy and post-herpetic neuralgia. The study aimed to evaluate the efficacy and safety profile of CPX-356 in managing pain symptoms. The participants were randomly assigned to receive either CPX-356 or a placebo over a period of 12 weeks. The results revealed that patients receiving CPX-356 experienced a significant reduction in pain intensity compared to the placebo group. Moreover, CPX-356 demonstrated a favorable tolerability profile with minimal adverse effects reported. These findings suggest that CPX-356 holds promise as a potential pharmacological treatment for chronic neuropathic pain, offering new possibilities for patients seeking relief from their debilitating symptoms. Further research and larger clinical trials are warranted to validate these initial findings and ascertain the long-term benefits and safety of CPX-356.
Acknowledgement
None
Conflict of Interest
None
References
- Keymeulen B, Vandemeulebroucke E, Ziegler AG (2005) Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352(25):2598-608.
- Staeva-Vieira T, Peakman M, Von Herrath M (2007) Translational Mini-Review Series on Type 1 Diabetes: immune-based therapeutic approaches for type 1 diabetes.Clin Exp Immunol 148(1):17-31.
- Gorus FK (2001) Pipeleers DG the Belgian Diabetes Registry Prospects for predicting and stopping the development of type 1 diabetes. Best Pract Res Clin Endocrinol Metab 15(3):371-389.
- Verge CF, Gianani R, Kawasaki E (1996) Prediction of type 1 diabetes in first degree relatives using a combination of insulin, glutamic acid decarboxylase and ICA 512 bdc/IA-2Autoantibodies. Diabetes 41(7):926-933.
- Hawa MI, Leslie DG (2001) Early induction of type 1 diabetes. Clin Exp Immunol 126(2):181-183.
- Hu M (2004) European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363(16):925-931.
- Decochez K, De Leeuw IH, Keymeulen B (2002) IA-2Autoantibodies predict impending Type E diabetes in siblings of patients. Diabetologia 45(12):1658-1666.
- Yu L, Rewers M, Gianani R (1996) Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 81(12):4264-4267.
- Ziegler AG, Hummel M, Schenker M, Bonifacio E (1999) Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes. The 2-year analysis of the German BABYDIAB Study. Diabetes 48(3):460-468.
- Weets I, Van der Auwera BJ, Schuit FC (2001) Male-to-female excess in diabetes diagnosed in early adulthood is not specific for the immune-mediated form nor is it HLA-DQ restricted: possible relation to increased body mass index. Diabetologia 44(1):40-47.
- Fathollahi A, Firouz D, Mitchell HAT (2015) Effect of Polyuria on Bladder Function in Diabetics versus Non-Diabetics: An Article Review. Curr Urol 8: 119-125.
- Damla PY1, Faruk E, Ozden OH, Ozlem OG, Bahriye A (2021) Acquired Bartter-like Syndrome Presenting with Polyuria and Reversible Hypokalemia Associated with Colistin Use in a Critically Ill Pediatric Patient. Indian J Crit Care Med 25: 822-824.
- Jens K, Birgitta K, Sabine K, Karl Peter S, Andrea T (2017) Phenotypic Spectrum of Children with Nephronophthisis and Related Ciliopathies. Clin J Am Soc Nephrol 12: 1974-1983.
- Sandrine A , Katharina T, Wiebke F, Nadia DM, Blanchard A (2016) Plasma Apelin Concentrations in Patients With Polyuria-Polydipsia Syndrome. J Clin Endocrinol Metab 101: 1917-1923.
- Hong SC, Yoon HK, Chang SK, Seong KM, Soo Wan K, et al. (2018) Diabetes Insipidus Presenting with Oligohydramnios and Polyuria During Pregnancy. J Nippon Med Sch 85: 191-193.
- Basant KP, Subho C, Anand SI, Rahul B (2011) Distress due to lithium-induced polyuria: exploratory study. Psychiatry Clin Neurosci 65: 386-388.
- Jiao Z, Dan L, Ji Feng L (2022) Intraoperative dexmedetomidine-related polyuria: A case report and review of the literature. Int J Clin Pharmacol Ther 60: 188-191.
- Rebecca H, Pierre D, Salvador A, Antonella G, Giulio DP (2020) Nocturia and Nocturnal Polyuria in Neurological Patients: From Epidemiology to Treatment. A Systematic Review of the Literature. Eur Urol Focus 6: 922-934.
- Grimwade K,French N, Mthembu D, Gilks C (2004) Polyuria in association with Plasmodium falciparum malaria in a region of unstable transmission. Trans R Soc Trop Med Hyg 98: 255-260.
- Tine KO, Marie AD, Johan VW, Everaert K (2018) Systematic review of proposed definitions of nocturnal polyuria and population-based evidence of their diagnostic accuracy. Acta Clin Belg 73: 268-274.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Huang Z (2023) Nanotechnology in Pharmaceutical Delivery System. Clin Pharmacol Biopharm, 12: 335. DOI: 10.4172/2167-065X.1000335
Copyright: © 2023 Huang Z. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1393
- [From(publication date): 0-2023 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1177
- PDF downloads: 216