Research Article Open Access
Nano Scale Potentiometric and Spectrophotometric Assays for 2,4-Dichlorophenoxyacetic Acid
El-Beshlawy MM*
Department of Chemistry, Collage of Girls, Ain Shams University, Egypt
- *Corresponding Author:
- El-Beshlawy MM
Department of Chemistry
Collage of Girls, Ain Shams University, Egypt
Tel: 00966538326107
E-mail: munmun1231@yahoo.com
Received date: June 28, 2015; Accepted date: July 14, 2015; Published date: July 21, 2015
Citation: El-Beshlawy MM (2015) Nano Scale Potentiometric and Spectrophotometric Assays for 2,4-Dichlorophenoxyacetic Acid. J Anal Bioanal Tech 6:257 doi: 10.4172/2155-9872.1000257
Copyright: © 2015 El-Beshlawy MM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The aim of this work was to investigate the ability of silicate wire ion selective electrode to measure 2,4 dichlorophenoxyacetic acid (2,4-D). The slope of the electrode was 56.2 mV decade -1 in the range from 10-6 to 10-2 M with lower limit of detection 8.3 × 10-7 M. The electrodes were successfully applied to determination of 2,4-D by separate and standard addition potentiometry. The electrode was used to detect the effect of pH and temperature on 2,4-D placed on baby watercress plant grown in the clay soil and the residue after five day from addition. Leaf chlorophylls quantity measured utilize spectrophotometric or colorimetric assay following extraction of pigments of watercress by ethanol. This work was carried at 286, 440, 662 nm. Beer’s law was obeyed in the concentration range of 2.21 mg/ml:2.21 μg/ml. The limits of detection were 2.21 μg/ml.
Keywords
2,4-Dichlorophenoxyacetic acid; Potentiometric;Spectrophotometric; Wire ion-selective electrodes, Watercress; Chlorophyll
Introduction
2,4-D is one of the most widely used herbicides in the world andis characterized as low-cost, quite efficient even at low concentrations,and moderately hazardous (class II) according to the World HealthOrganization [1] Although this herbicide easily moves though thesoil, its leaching into the groundwater can be minimized due to thedegradation by microorganisms and also the absorption by plants. Itsprimary route of degradation in the environment is by microorganisms,which increases with temperature, humidity, pH, and organic mattercontent [2,3]. Microbial degradation is a possible route for thebreakdown of 2,4-D, but it is very dependent on the characteristicsof the water. Laboratory studies have shown that in warm, nutrientrich water that has been previously treated with 2,4-D microbialdegradation can be a major factor for dissipation [4]. Residues of 2,4-Dand its salts or esters in water are commonly measure by extraction,chemical derivatization, separation by gas-liquid chromatographyand electron capture detection [4]. Other methods used in thedetermination of 2,4-D residues include high-performance liquid and
thin-layer chromatography [5-7].
Several methods have been reported for the analysis of 2,4-D in food and environmental samples. These methods includegas chromatography (GC) [8-10]. High-Performance LiquidChromatography (HPLC) and UV-spectrophotometry [11,12].However the chromatographic techniques are expensive and notavailable in many quality laboratories worldwide. Spectrophotometry isprobably the most convenient analytical technique for routine analysisbecause of its inherent simplicity, low cost and wide availability in
quality control laboratories [13].
Experimental Section
Materials and methods chemicals
All chemicals used were of analytical-reagent grade. 2,4Dichlorophenoxyacetic acid (Fluka AG) 95%, Sodium silicate (99%),Potassium chloride (99%), Sodium hydroxide (98%), Barium nitrate(98.5%), Ferrous sulphate (98%), L-Glutamic acid (99%), L-Ascorbicacid (99%), Maltose, D-Glucose (extra pure), Acrylamide (99%) and 1,2 Dichloroethane (98%) were provided by (LOBA Chemie) Fluka, Switzerland, Hydrochloric acid (37%) from (Scharau). Ethanol (96%), from HAYMAN and Methanol (99.8%) from AnalaR Distilled water was used to prepare all solutions.
Instrumentation
The electrochemical measurements were carried out with HANNAinstrument 211 (EZODO) for measuring pH. Sensitive balance modelAG204 (METTLER TOLEDO) was used for measurements. Spectral measurements were carried out by using a single beam UV (OPTIMA)SP-SPECTROPHOTOMETER, with quartz cells of 1 cm optical path length.
Stock standard solution of 2,4-D
An accurately 0.01 M solution was prepared by dissolving 0.212 gof 2,4-D standard in 25% ethanol, transferred into a 100 mL volumetricflask and diluted to the mark with 25% ethanol and mixed well. Thisstock solution was further diluted with 25% ethanol to obtain workingsolutions in the ranges of 10-3:10-11 M, reservation solutions in brown bottles inside the refrigerator.
Construction of wire ion selective electrode
Membrane was prepared by mixing well of 96% sodium silicate,1% active coal, 3% dioctylephethalate with 1 cm of fusible polyethylenetube. The mixture was poured into 3 cm diameter petri dish with 10 mltetrahydrofuran (THF). A pure silver wire electrode was dipped in thecoating membrane solution several time, allow the film to dry for aboutfive min repeat the process until 2.0 mm diameter. Wire electrode wassoaked about 3 h in 1 × 10-3 M of 2,4-D. All potentiometric determination were performed using the analytical cell, Ag /membrane/2,4-D test solution//Reference electrode.
Electrode calibration
Connect the wire electrode to the pH meter in the presence ofreference electrode. The calibration of the electrode was preceded using standard solutions of 2,4-D ranging from 10-7:10-2 M.
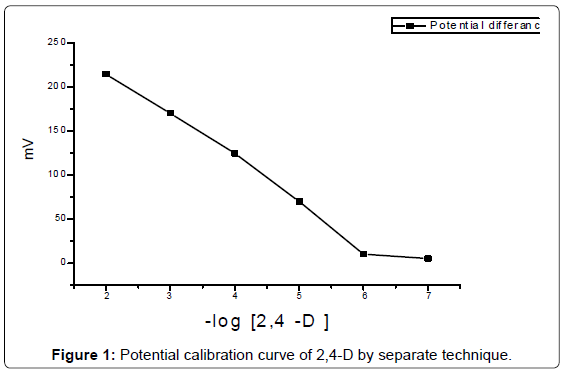
Separate and spiking addition method was used by the sequencefrom low to a higher concentration. The measured potential was plottedagainst logarithm of 2,4-D concentration after one min (Figure 1). Theelectrode was washed with distilled water and dried with tissue paperand back to base line by immersing in water between measurements, stored in a dark place immersed in 10-5 M.
Response times
Calibration curve was measured from the lower (10-7 M 2,4-D) tothe higher concentrated (10-2 M of 2,4-D) solutions the time traces of the calibration curves of silicate electrode was represented in Figure 2.
Selectivity
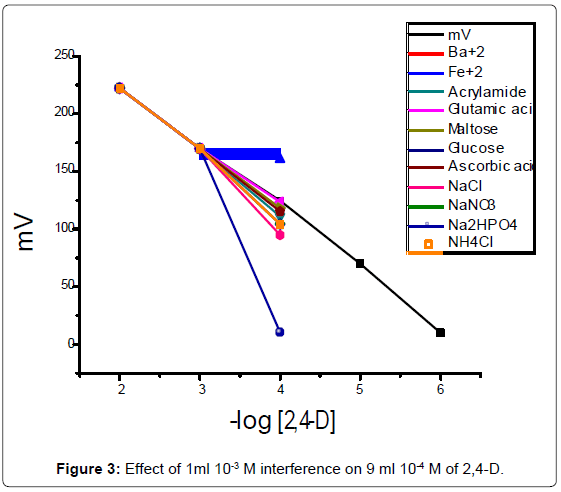
Selectivity coefficients Kpot2,4-D of the electrodes towards differentspecies were determined by the standard addition technique method [14] in which the following equation was applied
Kpot=(a`A - aA)/aB
where a`A known activity of primary ion, aA fixed activity ofprimary ion and aB activity of interfering ions. The results are appear by potting mV in the presence of different interference (Figure 3).
Effect of pH
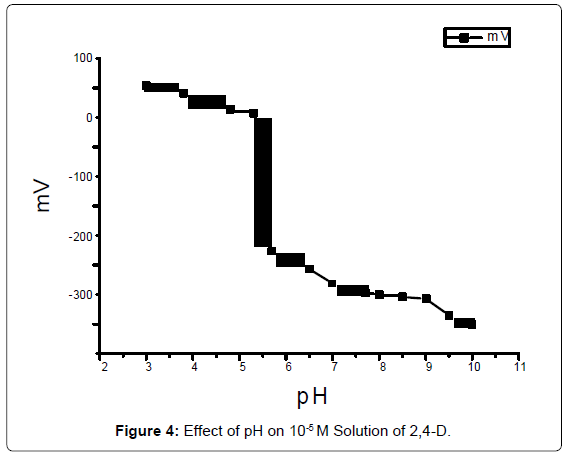
The effect of pH of the potential of electrode was investigated. Thepotential was measured at a 2,4-D concentration solution (10-5 M) fromthe pH value of 3 up to 9. The solution was acidified by the additionof very small volumes of 0.2 N HCl then the pH value was increased gradually using 0.2 N NaOH for each pH value, two pH/mV meterswere used to measure the potential. The potential was recorded and plotted against different pH values (Figure 4).
Analytical application
Growing watercress: Watercress seeds were germinated in 250gm clay dry soil with about 3-10 seeds per pot, after two weak. The temperature of the plants was maintained between 20-25°C.
Watercress treatment: A total of 20 pots were used to grow the watercress plants, two pots didn't contain 2,4-D. Stock solution of 2.21μg /ml of 2,4-D was prepared in 25% ethanol as solvent eighteen potswere treated by 50 ml of the previous stock solution for each pot afteradjust number of watercress plants (1, 2, 3, 5, 8,..) and, the pH (2.5-8)and temperature of solutions (-5, 15, 27, 43), plants are irrigated daily with distilled water.
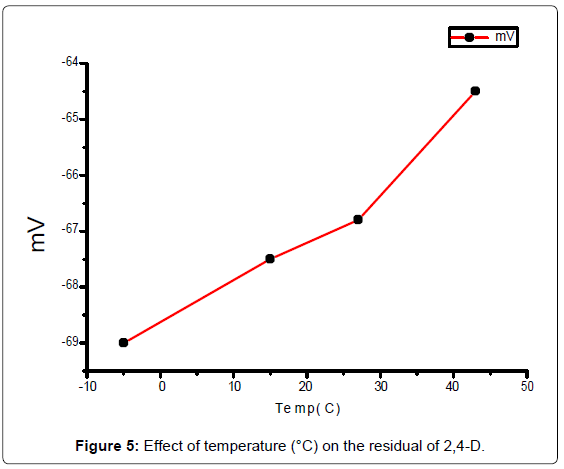
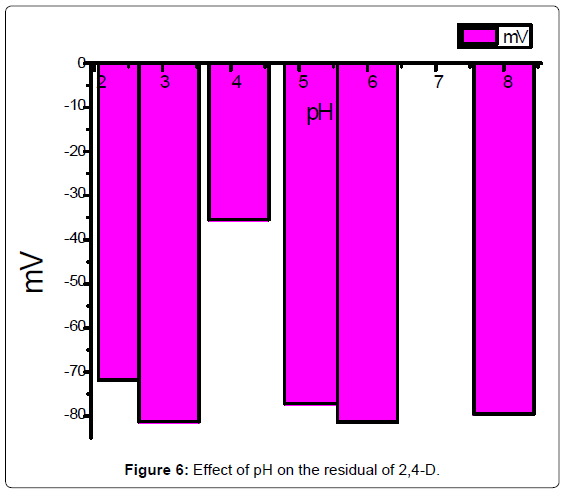
Residual of 2,4-D: After two, three and five days from adding pesticides on the pot of watercress plants. 40 ml distilled waterare added to each pot. The calibration curve was used to determinethe concentration of 2,4-D in each pot plant solution above, allmeasurements was recorded after one minute of electrode immersing.Effect of pH and temperature on the residual was drawing in Figures 5 and 6.
Spectrophotometric determination of 2,4-D
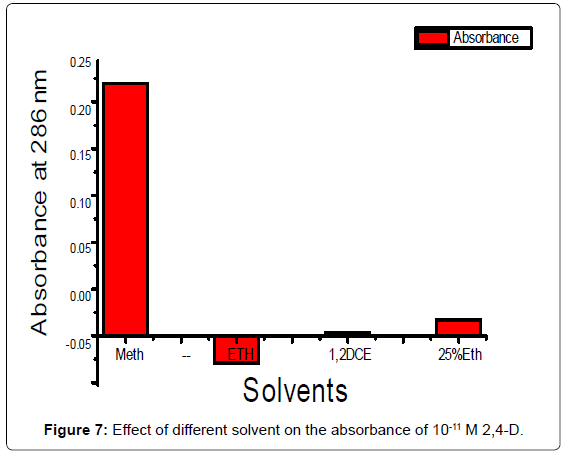
Selection of solvent: It was important to see the effects of varioussolvents on the photodegradation of 2,4-D. After assessing the solubility of 2,4-D in different solvent as methanol, ethanol, 25% ethanol and 2,4 dichloroethane. 25% ethanol has been selected as common solvent for developing spectral characteristics, 0.00221 ng /ml solution of 2,4-D present in each solution. The absorbencies are plotted against the different solvent at 286 nm (Figure 7). It has been observed the absorption within half an hour of the beginning of the preparations in each solvent. Blank solution was the solvent.
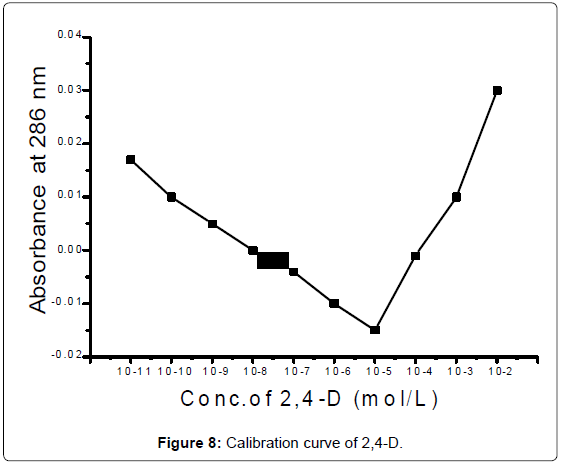
Beer's Law concentration range: The stock solution were suitable diluted with 25% ethanol to get concentration range from 0.00221 gml-1-0.0221 ng/ml for 2,4-D. The absorbance was measured at 286 nm.Standards of the compound of interest are made, and their absorbanceand concentration values are used to create a standardization graph(Figure 8). From the calibration curve 2,4-D obey Beer's law between 2.21 mg/ml to 2.21 μg/ml.
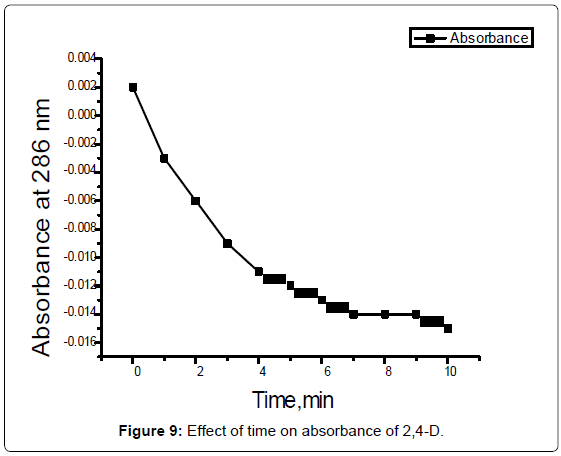
Photo degradation of 2,4-D by UV Light: The cuvet was filledwithin about 1.5 ml of the top with the blank and 10-3 M of 2,4-Dsolution. Wipe the outside of each cuvet gently with a lint-free tissueto remove any fingerprints or solution that may be on the surface.Adjust the wavelength to 286 nm, the absorbance of 10-3 M of 2,4-D was determined and plotted against time (Figure 9).
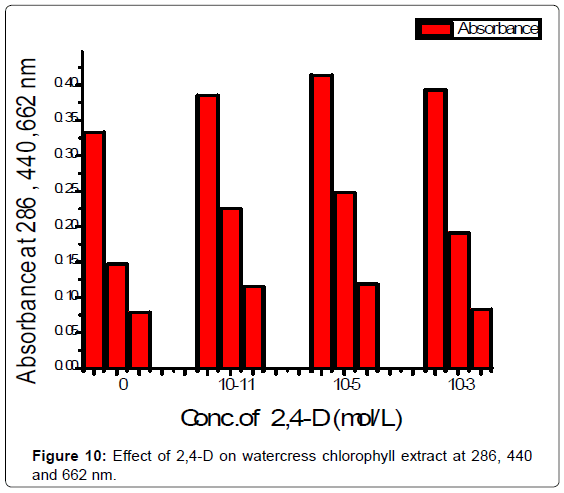
Effect of 2,4-D on watercress leaf chlorophyll Extract: Youngbaby-leaf watercress were acquired directly from organic local farmersin region of South Saudi Arabia. Cut of 7 g of watercress leaf, rinse the leaves with distilled water and pat dry. Tear up the leaves and place them in a 50 ml ethanol half an hour. Ethanol is a water-miscible solvent, removes all pigments from soft-leafed. The blank solution was prepared by 1 ml of chlorophyll extract and 11 ml distilled water and sample stock solutions were prepared by mixing aliquots of different concentration (10-11, 10-5 and 10-3) of 2,4-D were precisely pipette into 10 ml distelled water and 1 ml chlorophyll extract. The absorbance of solutions was measured at 286, 440, 662 nm and plotted against different concentration of 2,4-D in sample solutions (Figure 10).
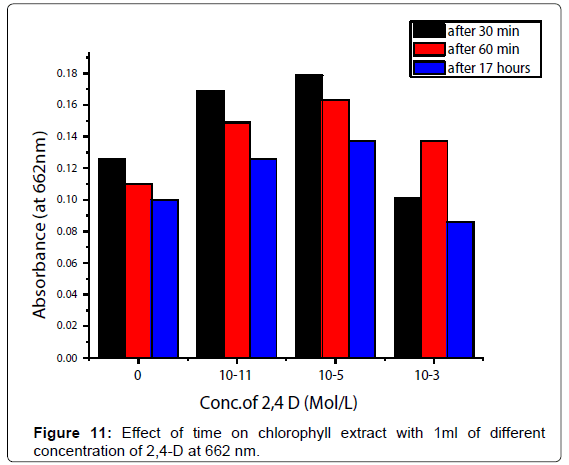
Time considerations: Effect of time on the absorbance of watercressleaf chlorophyll extract with 1 ml different concentration of (10-11, 10-5 and 10-3 M) of 2,4-D solutions was determined and plotted (Figure 11).
Result and Discussion
2,4-D was classified among the phenoxy acid herbicides MCPA asa class-2B carcinogen possibly carcinogenic to humans. The movementof auxin, (2,4-D) is a common herbicide,. Denmark, Norway, Kuwaitand the Canadian provinces of Québec and Ontario, 2,4-D use is severely restricted in the country of Belize [15].
2,4-D concentration is one of the affecting factor to the rate ofreaction, so that affects to the performance of silicate ion selectiveelectrode. It has been found that separate technique is the best wayto apply Nernst equation. Response characteristics of the silicate ionselective electrode appear in Table 1, the results showed that the highest sensitivity is 42.1 ng/ml.
| Parameter | Value* |
|---|---|
| Slope,(mV/decade) | 56.2 ±2 |
| Intercep , mV | 260 ±5 |
| Correlation coefficient between mVand-log[H+], (r) | -0.014 |
| Detection limit, M | 8.3× 10-7 |
| Response time for10-4 M solution,s | 15 |
| WorkingpHrange | 2.5-5.5 |
| Standard Deviation (SD) | 2.61 |
| Relative Standard Deviation(RSD) | 4.48 |
*Average of five replicate
Table 1: Response characteristics of the silicate wire ion selective electrode.
The purpose of this study was to investigate the working electrode conditions to obtain the maximum performance. The research studiedof the electrode character was pH and response time. The pH optimumof 2,4-D at a range from 2.5 to 5.5. Response time is a measure ofthe rate of degradation reaction of 2,4-D. The response time withconcentration higher than 10-4 M were of the order of 15 seconds.The silicate electrode has good selectivity to 2,4-D, in the presence ofdouble-charged metal ions, as Fe+2 and Glutamic acid, and has poor sensitivity in the presence of Na2HPO4.
The residual pesticide was decreases to 1.005, 0.663, 0.221 μg ml-1,after two, three, and five days respectively from the original solution(2.21 μg ml-1) added on the pots of watercress. The number of plants didnot effect on the residual of the pesticide concentration. The residualof 2,4-D degradation of (10.6 μg ml-1) by the temperature effect wasvaried from -5 to 43°C (Figure 5), the large amount of residual of 2,4-D was at 43°C, for a period of time following treatment. It is thereforeto be expected that temperature may influence the rate of degradation response and govern to some extent, Plants that put out the pesticideat low temperature longer than the age of the plants that put them ata temperature of exterminator other heat. This research study reportsthe concentrations of residual 2,4-D (pH 2.5-9) taken up by watercressgrown. This study showed that the pH 4 of 2,4-D solution is the lower pesticide absorption by soil and watercress plants [16,17].
It is very important to see the effect of different solvent as methanol,ethanol, 1,2-dichloroethane and 25% ethanol on the photodegradationof 10-11 M of 2,4-D (Figure 1). It is observed that the degradation of 2,4-D in the ethanol was the maximum and the degradation in methanolwas the minimum. The velocity of photodegradation of 10-3 M of 2,4-D in 25% ethanol is very high (Figure 3).
2,4-D was studied in the concentration range 10-11:10-2 M .The absorbance was decreased from 10-11:10-5 M the reason is thephotodegradation of 2,4-D in this region. Microbial degradation isconsidered and breakdown of 2,4-D . The most important mechanism of microbial degradation involves the removal of the acetic acid side chain. This is followed by ring cleavage and degradation to produce aliphatic acids such as succinic acid. The rate of microbial degradation is dependent upon the water potential 17, the absorbance increased and obeyed Beer's law from 10-5:10-2 M. The molar absorptivity coefficient equal to 1000 l mol-1 cm-1. Pigment content of chlorophyll were increased after adding , the reason is photodegradation of 2,4-D in this region.10-3 M < 10-5 M < 10-11 M of 2,4-D (Figure 10) at 286,440 and 662 nm. The absorption decreasing with the effect of time (Figure 11).
Conclusion
Silicate wire electrodes was suitable for the determination of 2,4-Dwith regard to working concentration range, slope, pH range, responsetime, and selectivity . The electrodes exhibited good reproducibilityover a useful lifetime of 2 months. Spectrophotometric study allowsunderstand 2,4-D toxicity and it’s excellent deleterious effect. Thereproducibility, recovery, and operational simplicity of the methodsmake them suitable to determined 2,4-D. The detection limit was 2.21 μg ml-1.
Acknowledgements
The author would like to thank the publishers for their interest in reviewing find.
References
- WHO (2013)The WHOrecommended classification of pesticides by hazard.
- MalekiN,SafaviA, Shahbaazi HR (2005)Electrochemical determination of 2,4-D at a mercury electrode.AnalyticaChimicaActa530: 69-74.
- GaultierJ,FarenhorstA, CathcartJ, GoddardT(2008) Degradation of [carboxyl-14C] 2,4-D and ring-U-14C] 2,4-D in 114 agricultural soils as affected by soil organic carbon content. Soil BiolBiochem 40: 217-227.
- Halter MT (1980) 2,4-D in the Aquatic Environment: Section II in Literature Reviews of Four Selected Herbicides: 2,4-D, Dichlobenil, Diquat, and Endothall. Municipality of Metropolitan Seattle
- World Health Organization (1984) 2,4-Dichlorophenoxyacetic acid (2,4-D). Environmental Health Criteria 29. International Programme on Chemical Safety, Geneva.
- Frank R, Logan L, Clegg BS (1991) Pesticide and polychlorinated biphenyl residues in waters at the mouth of the Grand, Saugeen, and Thames Rivers, Ontario, Canada, 1986-1990. Arch Environ ContamToxicol 21: 585-595.
- Shane S, QueHee, Ronald G, Sutherland (1981) The phenoxyalkanoic herbicides: Chemistry, analysis, and environmental pollution. Chemical Rubber Company Series in Pesticide Chemistry. CRC Press, Boca Raton, USA.
- Tucker SP, Reynolds JM, Wickman DC, Hines CJ, Perkins JB (2001) Development of sampling and analytical methods for concerted determination of commonly used chloroacetanilide, chlorotriazine, and 2,4-D herbicides in hand-wash, dermal-patch, and air samples. ApplOccup Environ Hyg 16: 698-707.
- Cook LW, Zach FW, Klosterman HJ, Bristol DW (1983) Comparison of free and total residues of (2,4-dichlorophenoxy)acetic acid and 2,4-dichlorophenol in millet resulting from postemergence and preharvest treatment. J Agric Food Chem 31: 268-271.
- WilliamsKJ, James CR, Thorpe SA, ReynoldsSL (1997) Two analytical methods for the measurement of 2,4-D in oranges: An ELISA screening procedure and a GC-MS confirmatory procedure. Pesticide Science 50: 135-140.
- Kashyap SM, Pandya GH, Kondawar VK, Gabhane SS (2005) Rapid analysis of 2,4-D in soil samples by modified Soxhlet apparatus using HPLC with UV detection. J ChromatogrSci 43: 81-86.
- Park JY, Choi JH, Abd El-Aty AM, Kim BM, Park JH, et al. (2011) Development and validation of an analytical method for determination of endocrine disruptor, 2,4-D, in paddy field water. Biomed Chromatogr 25: 1018-1024.
- JanMR,ShahJ,Bashir N(2006) Flow injection spectrophotometric determination of 2,4-D herbicide Journal of the Chinese Chemical Society53: 845-850.
- Mostafa GA,Hefnawy MM, Al-Majed A (2007) Sensors 7: 3272-3286.
- IRAC(1987) Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC MonogrEvalCarcinog Risks Hum Suppl 7: 1-440.
- Zetterberg G, Busk L, Elovson R, Starec-Nordenhammar I, Ryttman H (1977) The influence of pH on the effects of 2,4-D (2,4-dichlorophenoxyacetic acid, Na salt) on Saccharomyces cerevisiae and Salmonella typhimurium. Mutat Res 42: 3-17.
- Ghassemi M, FargoL, PainterP,QuinlivanS,ScofieldR,et al. (1981) Environmental Fates and Impacts of Major Forest Use Pesticides, Office of Pesticides and Toxic Substances, WashingtonDC, USA.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14922
- [From(publication date):
August-2015 - Dec 30, 2024] - Breakdown by view type
- HTML page views : 10453
- PDF downloads : 4469