Na/K-ATPase Oxidant Amplification Loop Regulates Cellular Senescence
Received: 01-Sep-2023 / Manuscript No. bcp-23-99402 / Editor assigned: 04-Sep-2023 / PreQC No. bcp-23-99402 / Reviewed: 18-Dec-2023 / QC No. bcp-23-99402 / Revised: 22-Sep-2023 / Manuscript No. bcp-23-99402 / Published Date: 29-Aug-2023 DOI: 10.4172/2168-9652.1000430
Abstract
As Na/K-ATPase oxidant amplification loop (NKAL) has been implicated in several diseases and oxidant injury plays a pivotal role in aging, we examined the effect of NKAL in the molecular pathogenesis of senescence. First, C57BL6 old mice were supplemented with a western diet to induce oxidant injury and pNaKtide was used to antagonize the NKAL. The western diet-induced functional and morphological changes of aging in adipose tissue and heart were ameliorated by pNaKtide. Next, different in vitro models of oxidative stress-induced aging were employed to study the effect of NKAL in human dermal fibroblasts (HDFs). pNaKtide significantly attenuated the expressions of senescence, cellular injury and apoptosis markers. Further stimulation of the NKAL with glucose oxidase augmented cellular senescence whereas treatment with pNaKtide attenuated it. pNaKtide exhibited a significant protective effect against cellular oxidative stress similar to other antioxidants viz, N-Acetyl Cysteine and Vitamin E. Of note, the attenuation of cellular senescence was greater in pNaKtide treated cells than in the antioxidants treated cells. Hence, the overall findings highlight the potential role of NKAL in the progression of aging, which may help in future antiaging therapeutic interventions.
Introduction
Aging is a dynamic and degenerative process driven by various physiological changes including cell division, oxidative stress, DNA damage and senescence [1-4 ]. It is characterized by a progressive decline of tissue and organ function due to cellular senescence and/or apoptosis [5, 6]. Cumulative evidence suggests that oxidant stress that causes damage to cellular proteins, lipids and DNA plays a key role in the molecular mechanisms of age-related morbidity and mortality [7- 9 ]. The oxidative modification of cellular macromolecules contributes to their functional defects leading to a decline in organ function and thereby greater vulnerability to chronic disease and death [3]. The redox imbalance generated from the accumulation of free radicals or the defective antioxidant mechanisms may pose a risk to cellular homeostasis and lead to cellular death or promote the early onset of degenerative diseases [10-12]. The oxidative stress-induced disruption of cellular signaling pathways can aggravate cellular senescence and apoptosis, the main contributors of age-related pathologies [13-15 ].
The role of Na/K-ATPase – Src – EGFR signaling pathway as a feed-forward amplification loop for oxidants (Na/K-ATPase oxidant amplification loop, NKAL) has been well established in previous studies [16-18]. Na/K-ATPase can act as a specific receptor for cardiotonic steroids (CTS) and as a non-specific receptor for Reactive oxygen species (ROS), inducing conformational changes in Na/K-ATPase α-1 subunit, which in turn, phosphorylates Src, followed by the transactivation of EGFR. This initiates a signaling cascade resulting in additional ROS generation. The prominent role of NKAL in various metabolic and degenerative diseases have been demonstrated previously [16, 17, 19]. The novel peptide derived from the N terminal domain of Na/KATPase α1 subunit, pNaKtide, acts as an antagonist of Na/K-ATPase signaling [20-23]. Given the important role of oxidative stress in the pathogenesis of aging and in the light of the aforementioned previously published articles, in the present study, we aimed to investigate the role of NKAL in the progression of aging and to explore the possible amelioration by its antagonist, pNaKtide.
Materials and Methods
Experimental design for in vivo experiment
All animal studies were approved by the Marshall University Animal Care and Use Committee in accordance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals. C57BL6 young mice (8 weeks old, male) and Old mice (16 months old, male) were purchased from Jackson Laboratory. Upon arrival at the Byrd Biotechnology Center, Animal Research Facility, mice were placed in cages and were fed normal chow diet and had access to water ad libitum or were fed Western Diet (WD) and had ad libitum access to high fructose solution [18]. WD containing fructose is a well-known model of diet-induced metabolic and redox imbalance [24]. WD contained 42% fat, 42.7 % carbohydrate, and 15.2% protein yielding 4.5 KJ/g. Fructose was made at a concentration of 42g/L, yielding 0.168 KJ/ mL. The animals were randomly divided into 7 groups as follows: (1) Young, (2) Old-Baseline, (3) Old, (4) Old+WD, (5) Young+pNaKtide, (6) Old+pNaKtide, and (7) Old+WD+pNaKtide. Old baseline group (16 months) was sacrificed immediately and tissues were saved in liquid nitrogen until assayed. After 4 weeks of control or WD diet respectively, groups 5, 6 and 7 were injected with pNaKtide for 8 weeks (dissolved in saline and injected I.P. at a dose of 25-mg/kg-body weight every 7 days. The body weight was measured every week. Young (4 months) and Old (19 months) mice were sacrificed, followed by heart and adipose tissue collection. The tissues were flash-frozen in liquid nitrogen and maintained at -80°C until assayed.
TBARS measurement in adipose and cardiac tissues and HDFs
Oxidative injury in adipose tissue, cardiac tissues, and human dermal fibroblasts (HDFs) were measured using a Thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol. Data were normalized to total protein and presented as micromoles per milligram of protein.
Histopathological examination of adipose tissue
Visceral fat samples from each group were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned. Formalin-fixed, paraffin-embedded sections were cut (6 μm thick) and mounted on glass slides. The sections were deparaffinized in xylene. Sections of visceral fat were stained with Hematoxylin and eosin (H&E). Preparation, fixation, and data analysis of adipose tissue was conducted as previously described.
Transthoracic Echocardiography
Transthoracic echocardiography (TTE) was performed 24 hours prior to sacrifice as described previously [16]. The following cardiac characteristics were calculated from the data: myocardial performance index = (IVCT + IVRT)/ET, relative wall thickness (RWT) = (PWT + AWT)/EDD, cardiac output (CO) = stroke volume × HR/1000, fractional shortening = (EDD − ESD)/EDD, ejection fraction (EF) = (EDV − ESV)/EDV and the left ventricular mass index (LVMI) = 1.05[(EDD + PWT + AWT)3 − EDD3]/ body weight (g).
Experimental design for in vitro experiments
Primary HDFs were obtained from ATCC (PCS-201-012) and cultured in fibroblast basal media (PCS-201-030) supplemented with low serum growth kit (PCS-201-041). Cultures were maintained at 37°C in a 5% CO2 incubator and medium were replaced every 48 h. At 80% confluence, the cells were detached using trypsin and split in a 1:3 ratio. HDF cells were plated at a density of 10,000 cells/cm2 for subsequent experiments.
Effect of H2O2, UV radiation, Glucose oxidase (GO) treatment, N-acetylcysteine (NAC), and Vitamin E (α-tocopherol) on cellular senescence.
After 24 hours of plating, the cells were treated with 200 μM H2O2 for 2 hours. After 2 hours, they were briefly rinsed with PBS and replaced with normal media with or without pNaKtide at different concentrations ranging from 0.5 μM to 4 μM. Following the dose response experiments, the subsequent treatment with pNaKtide was done at a concentration of 1 μM for 72 hours. Similarly for UV radiation treatment, after 24 hours of plating, the HDFs were exposed with UV radiation (KERNEL KN-4003 BL) at a distance of 30 cm to the cells through a thin layer of phosphate buffer saline pH 7.4 at room temperature. The cells were treated twice daily for 5 days with UVB at 311nm for duration of 2 minutes at a dose of 2.5J/cm2. After every radiation, PBS was replaced with normal media with or without pNaKtide at 1 μM concentration. For the GO experiments, HDF cells were plated 24 hours prior to exposure with an increasing dose of GO (Sigma G-2133) ranging from 1, 3, 5 and 10mU/ml for 72 hours treated with or without 1 μM pNaKtide. For the treatment with NAC, the HDFs were treated at different concentrations ranging from 1mM, 5mM and 10mM for 72 hours, to generate a dose concentration curve. The subsequent treatment with NAC was done at a concentration of 5mM. For Vit E treatment, the HDFs were plated for 24 hours and then treated with α-tocopherol at different concentrations ranging from 25 μM to 100 μM for 72 hours. Following the dose response experiments, the subsequent treatment with α-tocopherol was done at a concentration of 50 μM.
Senescence-associated β-galactosidase (SA- β-gal) activity
HDFs were plated in a 6-well plate and exposed to H2O2 or UV radiation with or without subsequent pNaKtide treatment as described above. SA-β-gal activity was detected using a Senescence β-Galactosidase Staining kit (Cell signaling Technology #9860) according to the manufacturer’s protocol. Cells were viewed using light microscopy, and the percentage of cells with SA-β-gal activity was calculated from the number of blue-stained cells divided by total number of cells multiplied by 100.
γ-H2AX immuno-fluorescent staining for DNA Damage
DNA damage was measured in HDFs based on immunofluorescent staining of γ-H2AX. Cells were grown in a six-well plate on coverslips pretreated with poly-lysine. Following exposure to H2O2 with or without subsequent pNaKtide treatment as described above, cells were washed with PBS and fixed with cold 1:1 methanol: acetone. Cells were permeabilized with two washes of 0.025% Triton in PBS. Cells were blocked using 1% BSA in PBS for 30 minutes. The cells were incubated overnight at 4°C with primary antibody, Anti- γ-H2AX antibody (phospho S139; diluted 1:100 in 1% BSA in PBS). Slides were washed in 0.025% Triton in PBS, and the secondary antibody (Alexa Fluor 488 diluted 1:1000 in 1% BSA in PBS) was incubated in the dark at room temperature for 1 hour. Cells were washed three times with PBS. Cells were mounted and counterstained with 50 mg/ml DAPI. Images were collected using a digital inverted microscope that spanned the entire area of the cells; the percentage of γ-H2AX-positive cells was calculated from the number of γ-H2AX-positive cells divided by total number of cells multiplied by 100.
MTT and CyQuant assays for cell proliferation
HDFs were plated in 96-well plates and exposed to GO with or without subsequent pNaKtide treatment as described above. A Vybrant MTT Cell Proliferation Assay Kit (V-13154, Invitrogen) was used according to the manufacturer’s protocol. CyQUANT Cell Proliferation Assay Kit (C7026, Invitrogen) was used for cell count according to the manufacture’s protocol. Fluorescence was measured at excitation/emission wavelengths of 480/520 nm (Spectramax i3x). Fluorescence measurements were converted to cell number based on a standard curve with an r2>0.99.
Measurement of caspase-9 activity
In vitro analysis of caspase 9 activity in HDFs was performed using the Caspase 9 Assay Kit (ab65608, Abcam, US) according to the manufacture’s protocol. Caspase 9 activity was measured based on the absorbance at 400–405 nm, collected using a Spectramax i3x. Data were compared with the absorbance of control samples.
TUNEL assay for DNA damage detection
DNA double-strand breaks were detected in HDFs and frozen mouse tissues, (heart and adipose tissue) using the Click-iT® Plus TUNEL Assay (Thermo Fisher Scientific Inc., US) according to the manufacturer’s protocol. Specimens were then mounted using VECTASHIELD® mounting medium and counterstained with 50 mg/ ml DAPI. Images were collected using a digital inverted microscope that spanned the entire area of the cells and tissues on the cover slip. The percentage of TUNEL-positive cells was calculated based on the number of positively stained cells divided by the total number of cells multiplied by 100.
RNA extraction and real-time PCR
Total RNA was extracted from HDFs, adipose, and heart tissues using RNeasy Protect Mini kit (QIAGEN, Maryland, USA) according to manufacturer’s instructions. Total RNA (1μg) was transcribed into cDNA using GeneAmp kit (Applied Biosystems, Branchburg, NJ, USA) reverse transcription reagents. Total RNA was analyzed by a quantitative real time polymerase chain reaction. Real-time PCR was performed using SYBR Green PCR Master Mix on a 7500 HT Fast Real- Time PCR System (Applied Biosystems). Specific primers used were p21, apolipoprotein J (ApoJ), fibronectin, matrix metalloproteinase 9 (MMP9), ki67, peroxisome proliferator-activated receptor γ (PPARγ), tumor necrosis factor- α (TNF-α), collagenase and GAPDH. Each reaction was performed in triplicate. The comparative threshold cycle method was used to calculate the fold amplification as specified by the manufacturer. All experimental samples were normalized using GAPDH as an internal control.
Statistical analyses
Results presented as bar diagram or box-and-whisker plots. In bar diagram, each bar represents values as means ± standard error of mean (SEM). In box-and-whisker plots, data are presented as the median (central line), upper and lower quartiles and whiskers (extreme values) from independent experiments. All statistical analysis were performed using GraphPad Prism version 9 (GraphPad, San Diego, CA, USA). One-way ANOVA was performed to identify the statistical significance, followed by Tukey’s post hoc test for multiple comparison. Statistical significance was assigned at p<0.05 or p<0.01 for confidence interval of 95% or 99%, respectively.
Results
Effect of pNaKtide on body weight, tissue weight, energy expenditure, locomotor activity, and oxygen consumption in C57BL6 aging mice.
In this study we demonstrated the role of Na/K-ATPase signaling and it is antagonist, pNaKtide, in a murine model of aging. The mice were subjected to WD regimen to induce oxidative stress. Our findings demonstrated that body weight, visceral fat, and subcutaneous fat demonstrated an increase in the old mice, with further escalation observed in the old mice fed a WD (Table 1). Notably, the administration of pNaKtide treatment led to a significant reduction in these aforementioned parameters (Table 1). Conversely, no significant differences were observed in any of these measures between the young mice and the young mice treated with pNaKtide.
| Young (N=6; 2 mths) | Young + pNaKtide (N=6; 4 mths) | Old Baseline (N=8; 16 mths) | Old (N=9; 19 mths) |
Old + pNaKtide (N=15; 19 mths) | Old + WD (N=8; 19 mths) |
Old+WD +pNaKtide (N=8; 19 mths) | |
|---|---|---|---|---|---|---|---|
| Table 1A: Effect of pNaKtide on body weight, visceral fat, subcutaneous fat, and heart weight in C57Bl6 old mice (on the day of sacrifice) | |||||||
| Bodyweight (g) | 29.1 ± 1.1 | 28.3 ± 1.2 | 39.1 ± 1.1 ** | 35.1 ± 0.5 **^ | 33.5 ± 0.4 *^^ | 50.5 ± 1.2**^^## | 44.5 ± 0.8 **^^##&& |
| Visceral Fat (g) | 0.74 ± 0.06 | 0.79 ± 0.08 | 1.95 ± 0.08 ** | 1.97 ± 0.06 ** | 1.16 ± 0.03 **^^## | 4.48 ± 0.11**^^## | 2.56 ± 0.09**^^##&& |
| Subcutaneous Fat (g) | 0.33 ± 0.02 | 0.32 ± 0.01 | 0.72 ± 0.04 ** | 0.71 ± 0.02** | 0.41 ± 0.02 ^^## | 2.74 ± 0.12**^^## | 1.18 ± 0.05**^^##&& |
| Heart Weight (g) | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.25 ± 0.02 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.23 ± 0.01 | 0.21 ± 0.01 |
| Table 1B: Effect of pNaKtide on energy expenditure; locomotor activity and oxygen consumption in C57BL6 old mice | |||||||
| Energy Expenditure (kcal/ kg/hr) | 16.1 ± 0.8 | 17.8 ± 1.2 | 15.9 ± 0.3 | 15.8 ± 0.3 | 16.1 ±0.3 | 12.6 ± 0.2**^^## | 14.9 ± 0.4& |
| Locomotor Activity (Ambulatory Count/ 24 hr) | 23542 ± 599 | 24390 ± 979 | 20027 ± 468* | 19779 ± 491** | 22878± 684^## | 13563 ± 676**^^## | 19948 ± 385*&& |
| Oxygen Consumption (mL/kg/hr) | 3153 ± 47 | 3347 ± 127 | 2899 ± 66 | 2817 ± 311 | 3161 ± 58 | 2444 ± 34* | 3101 ± 76& |
Table 1: Effect of pNaKtide on body weight, tissue weights, and metabolic parameters in C57BL6 old mice.
The heart weight was also evaluated, and no significant differences were observed among any of the experimental groups (Table 1). Energy expenditure was analyzed as heat production in Kcal/Kg/hr [18]. Our findings demonstrated a noteworthy rise in energy expenditure in pNaKtide-treated old mice subjected to a WD regimen, in comparison to old mice subjected to a WD alone (Table 1). In addition, the locomotor activity was evaluated, and the ambulatory counts over a 24-hour period were analyzed. Old mice exhibited reduced locomotor activity compared to young mice, while old mice fed a WD showed decreased activity levels when compared to young mice, old baseline mice, and old mice. Notably, the administration of pNaKtide to old mice resulted in an improvement in locomotor activity compared to both the old baseline mice and old mice (Table 1). Oxygen consumption was analyzed in terms of mL/kg/hr. Our results indicated a significant improvement in oxygen consumption in pNaKtide-treated old mice subjected to a WD regimen, in comparison to old mice subjected to a WD alone (Table 1).
pNaKtide treatment improves the adipocyte phenotype and apoptosis in C57BL6 aging mice.
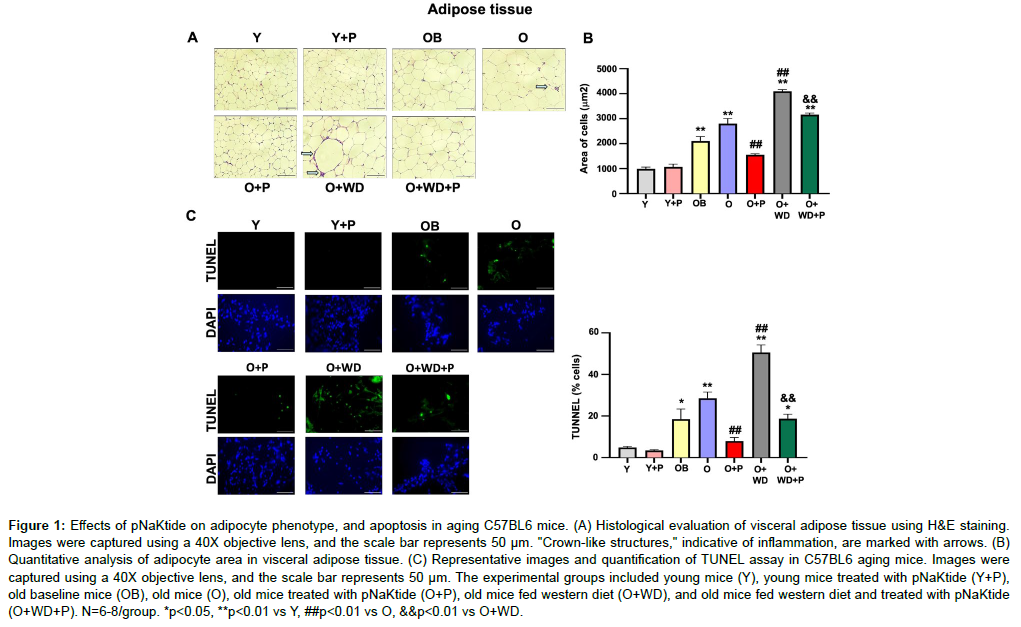
Studies have shown that during the aging process, adipose tissue leads to increase levels of oxidative stress [26, 27]. We evaluated the histology of adipocytes in the visceral adipose tissue by hematoxylin and eosin staining. Our results showed an increase in adipocyte size in the adipose tissue of old mice, with further increases observed in old mice subjected to a WD regimen. (Figure 1A and B). Notably, in the adipose tissue of the old mice, crown-like structures were identified, indicative of macrophage accumulation surrounding adipocytes (shown by arrows), with a further increase noted in old mice subjected to a WD regimen. (Figure 1A). The pNaKtide treatment significantly attenuated these increases in both groups. The TUNEL assay showed significant increase in apoptosis in the adipose tissue of old mice, which was further increased in old mice subjected to a WD regimen. However, the administration of pNaKtide effectively attenuated these increases (Figure 1C).
Figure 1: Effects of pNaKtide on adipocyte phenotype, and apoptosis in aging C57BL6 mice. (A) Histological evaluation of visceral adipose tissue using H &E staining. Images were captured using a 40X objective lens, and the scale bar represents 50 μm. "Crown-like structures," indicative of inflammation, are marked with arrows. (B) Quantitative analysis of adipocyte area in visceral adipose tissue. (C) Representative images and quantification of TUNEL assay in C57BL6 aging mice. Images were captured using a 40X objective lens, and the scale bar represents 50 μm. The experimental groups included young mice (Y), young mice treated with pNaKtide (Y+P), old baseline mice (OB), old mice (O), old mice treated with pNaKtide (O+P), old mice fed western diet (O+WD), and old mice fed western diet and treated with pNaKtide (O+WD+P). N=6-8/group. *p<0.05, **p<0.01 vs Y, ##p<0.01 vs O, &&p<0.01 vs O+WD.
pNaKtide treatment improves the oxidative stress, inflammation, and senescence in C57BL6 aging mice.
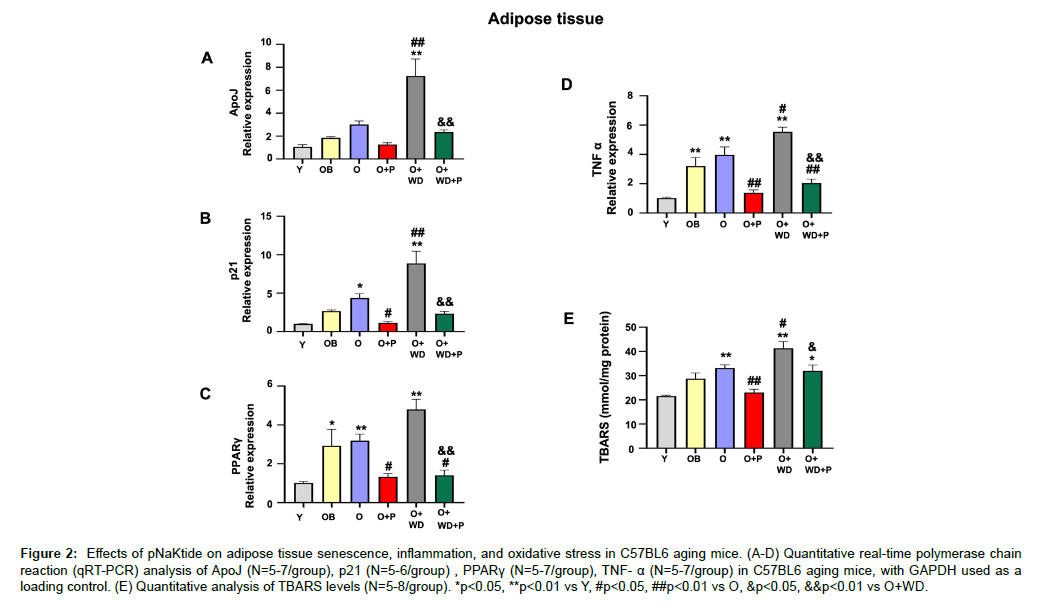
It is shown that the expression levels of “PPAR-γ”, an indicator of adipocyte hypertrophy and physiological dysregulation of adipose tissue is upregulated in diet-induced obesity [28]. Similarly, the levels of ApoJ and p21 are known to increase during stress-induced cellular senescence [29, 30]. Our findings demonstrated that ApoJ, p21, and PPARγ levels were increased in the adipose tissue of old mice, with further increase observed in old mice subjected to a WD regimen (Figure 2A, B, and C). Notably, the administration of pNaKtide treatment led to a significant reduction in these markers. Levels of TNFα, a pro-inflammatory cytokine, and TBARS, another marker of oxidative stress, exhibited significant increase in the adipose tissue of old mice and old mice subjected to WD regimen when compared to young mice (Figure 2D and E).
Figure 2: Effects of pNaKtide on adipose tissue senescence, inflammation, and oxidative stress in C57BL6 aging mice. (A-D) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of ApoJ (N=5-7/group), p21 (N=5-6/group) , PPARγ (N=5-7/group), TNF- α (N=5-7/group) in C57BL6 aging mice, with GAPDH used as a loading control. (E) Quantitative analysis of TBARS levels (N=5-8/group). *p<0.05, **p<0.01 vs Y, #p<0.05, ##p<0.01 vs O, &p<0.05, &&p<0.01 vs O+WD.
pNaKtide treatment improves heart function, senescence and oxidative stress in C57BL6 aging mice.
Cardiac hypertrophy and diastolic dysfunction were associated with aging as assessed using echocardiographic methods (Table 2). Old mice and old mice subjected to a WD regimen showed an increase of the ventricular wall thickness (anterior wall thickness (AWT), posterior wall thickness (PWT), relative wall thickness (RWT)) as well as an increased in left ventricular mass index (LVMI Moreover, impaired cardiac function was observed in these groups, as indicated by the myocardial performance index (MPI). The administration of pNaKtide attenuated these changes, as shown in Table 2. We further observed a significant increase in fibronectin, p21 and ApoJ levels in the heart of groups, old mice and old mice subjected to a WD regimen (Figure 3A, B and C). The administration of pNaKtide led to a significant reduction in these increases. The TUNEL assay showed significant increase in apoptosis in the hearts of old mice, which was further increased in old mice subjected to a WD regimen (Figure 3D and E). Levels of TBARS were also measured, showing a significant increase in old mice and old mice subjected to a WD regimen when compared to young mice (Figure 3F). These changes were significantly attenuated by pNaKtide treatment.
| Variable | Young (n=10) | Old Baseline (n=11) | Old (n=8) | Old + pNaKtide (n=16) | Old + WD (n=8) | Old+WD+ pNaKtide (n=8) |
|---|---|---|---|---|---|---|
| HR, beat/min | 436±7 | 455±10 | 409±18 | 436±12 | 445±16 | 445±13 |
| EDA, mm2 | 25.9±0.4 | 29.5±0.5** | 31.6±0.7** | 29.4±0.5** | 30.1±0.6** | 28.3±0.7## |
| ESA, mm2 | 16.6±0.6 | 19.5±0.5* | 23.4±0.9**^^ | 19.6±0.6**## | 21.6±0.3** | 19.8±0.7*## |
| EDD, mm | 4.28±0.05 | 4.74±0.06** | 4.83±0.12** | 4.82±0.06** | 4.60±0.07 | 4.71±0.10** |
| ESD, mm | 3.14±0.08 | 3.45±0.07 | 3.68±0.14** | 3.60±0.07** | 3.70±0.07** | 3.55±0.07* |
| PWT, mm | 0.54±0.01 | 0.72±0.01** | 0.77±0.03** | 0.66±0.01**## | 0.65±0.02**## | 0.62±0.02*^^## |
| AWT, mm | 0.64±0.01 | 0.72±0.01** | 0.82±0.02**^^ | 0.71±0.01**## | 0.79±0.02**^ | 0.70±0.01##&& |
| ET, msec | 47.2±1.7 | 45.8±1.5 | 49.0±2.2 | 46.8±0.7 | 41.6±1.6# | 45.8±1.5 |
| IVCT+IVRT, msec | 19.6±0.7 | 20.1±0.6 | 24.5±1.3**^ | 20.6±0.6# | 26.8±1.2**^^ | 21.6±0.8&& |
| PaVTI, mm | 28.2±0.7 | 27.5±0.5 | 25.6±1.0 | 27.3±0.5 | 22.2±1.2**^^ | 22.9±1.2**^^ |
| PaD, mm | 1.04±0.01 | 1.09±0.02 | 1.13±0.02* | 1.06±0.01 | 1.06±0.02 | 1.10±0.02 |
| RWT | 0.275±0.005 | 0.304±0.003** | 0.332±0.009**^^ | 0.284±0.002^## | 0.316±0.007** | 0.279±0.004^^##&& |
| MPI | 0.42±0.02 | 0.44±0.01 | 0.50±0.02** | 0.44±0.01 | 0.64±0.02**^^## | 0.47±0.02&& |
| FS, % | 26.5±1.1 | 27.2±1.4 | 23.9±1.4 | 25.4±0.7 | 19.5±1.0**^^ | 24.6±0.9& |
| EF, % | 60.2±1.7 | 61.1±2.2 | 55.6±2.4 | 58.3±1.1 | 47.7±1.9**^^ | 56.9±1.5& |
| CO, ml/min | 10.3±0.2 | 11.7±0.6 | 10.5±0.8 | 10.5±0.4 | 8.7±0.5^^ | 9.6±0.5 |
| LVM, mg | 88±3 | 137±5** | 161±8** | 132±5**## | 130±5**# | 120±6**## |
| LVMI | 3.49±0.16 | 3.73±0.13 | 4.62±0.27**^^ | 3.82±0.13## | 2.67±0.10*^^## | 2.58±0.14**^^## |
Table 2: Summary of transthoracic echocardiograph results.
Figure 3: Effects of pNaKtide on heart senescence, apoptosis, and oxidative stress in C57BL6 aging mice. (A-C) qRT-PCR analysis of fibronectin (N=4-7/group), p21 (N=5-7/group), and ApoJ (N=5-7/group) in C57BL6 aging mice, with GAPDH used as a loading control. (D-E) Qualitative image and quantitative analysis of TUNEL assay. Images were captured using a 40X objective lens and the scale bar represents 50 μm (N=6-8/group). (F) Quantitative analysis of TBARS levels (N=6-7/group). **p<0.01 vs Y, #p<0.05, ##p<0.01 vs O, &p<0.05, &&p<0.01 vs O+WD.
Effect of pNaKtide on H2O2-induced senescence in HDFs
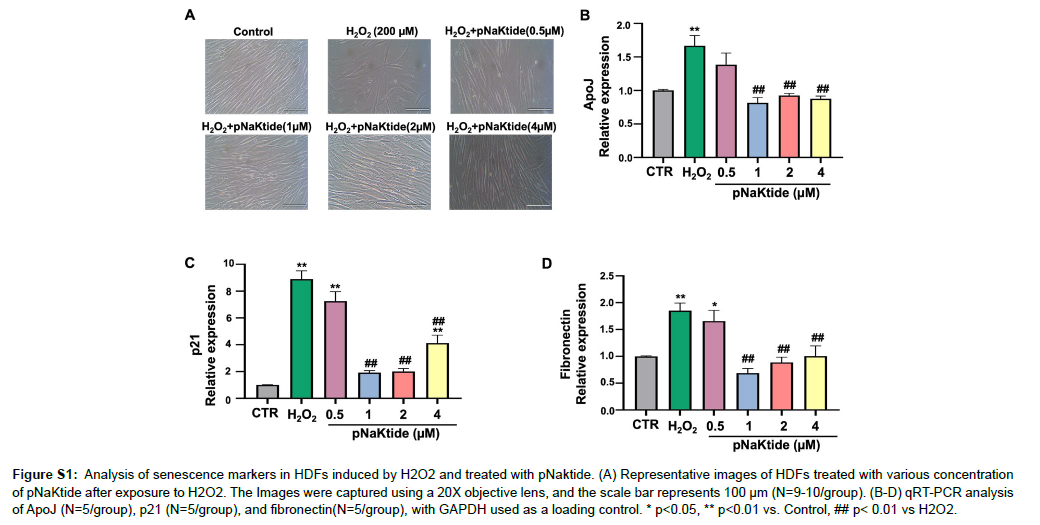
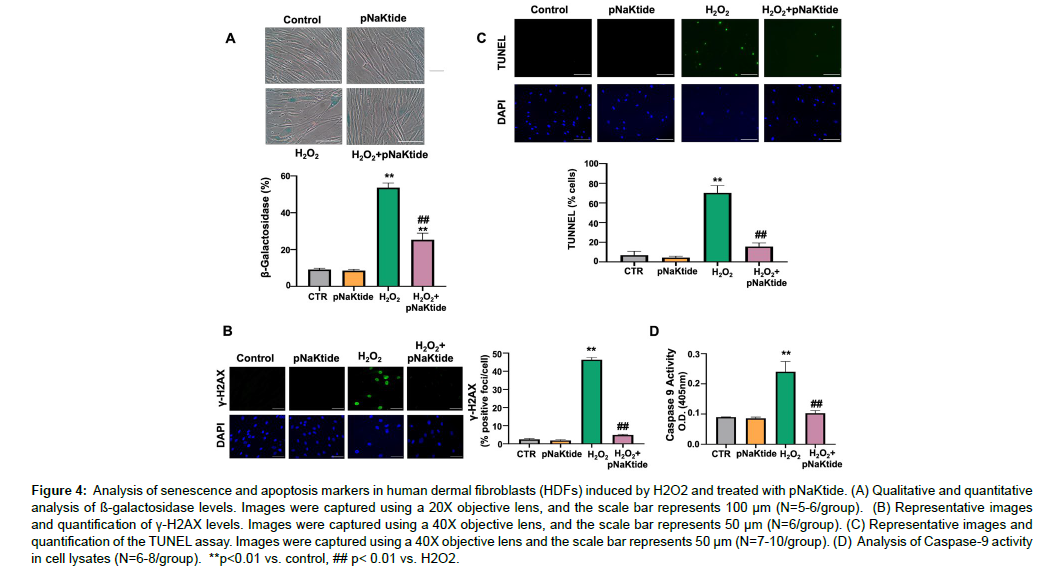
Studies have shown that H2O2 induces oxidative stress and DNA damage, which can result in cellular senescence [31 , 32]. HDFs cells were exposed to varying concentration of pNaKtide to determine the optimum dose that effectively attenuates senescence (Figure S1A). Our findings demonstrated that HDFs treated with H2O2 exhibited morphological alterations characteristic of cellular senescence, including cellular enlargement, flattening, elongation and reduced cell number (Figure S1A). In addition, we observed a significant upregulation in the expression of senescence genes ApoJ, p21, and fibronectin [33] in H2O2-treated cells compared to untreated control cells (Fig. S1B, C and D). Treatment with 1μM pNaKtide significantly attenuated this effect when administered to H2O2 treated cells. Increased levels of beta-galactosidase a marker associated with senescence [34], and phosphorylated histone H2AX (γ-H2AX), a marker of double-stranded DNA breaks [35], were observed in the H2O2 group, and the administration of pNaKtide led to a significant reduction in these changes (Figure 4A and B). The TUNEL assay was used to detect apoptotic cells that undergo extensive fragmented and degraded DNA [36]. Our results showed that apoptosis levels were increased in cells treated with H2O2 and this increase was attenuated by pNaKtide administration (Figure 4C). Caspase-9, a key protein involved in apoptosis signaling [37 ], exhibited increased activity in H2O2 -treated cells, which was reduced by pNaKtide administration (Figure 4D).
Figure S1: Analysis of senescence markers in HDFs induced by H2O2 and treated with pNaktide. (A) Representative images of HDFs treated with various concentration of pNaKtide after exposure to H2O2. The Images were captured using a 20X objective lens, and the scale bar represents 100 μm (N=9-10/group). (B-D) qRT-PCR analysis of ApoJ (N=5/group), p21 (N=5/group), and fibronectin(N=5/group), with GAPDH used as a loading control. * p<0.05, ** p<0.01 vs. Control, ## p< 0.01 vs H2O2.
Figure 4: Analysis of senescence and apoptosis markers in human dermal fibroblasts (HDFs) induced by H2O2 and treated with pNaKtide. (A) Qualitative and quantitative analysis of ß-galactosidase levels. Images were captured using a 20X objective lens, and the scale bar represents 100 μm (N=5-6/group). (B) Representative images and quantification of γ-H2AX levels. Images were captured using a 40X objective lens, and the scale bar represents 50 μm (N=6/group). (C) Representative images and quantification of the TUNEL assay. Images were captured using a 40X objective lens and the scale bar represents 50 μm (N=7-10/group). (D) Analysis of Caspase-9 activity in cell lysates (N=6-8/group). **p<0.01 vs. control, ## p< 0.01 vs. H2O2.
Effect of pNaKtide on senescence genes in H2O2-treated HDFs
Studies have shown that Ki-67 is a well-established marker of cell proliferation [38, 39]. Our findings demonstrated significantly lower mRNA expression in the H2O2-treated group, which was restored in the pNaKtide-treated group (Table 3). Moreover, mRNA levels of other senescence markers, including ApoJ, MMP9, and collagenase, were measured and found to be significantly decreased in the pNaKtidetreated group compared to the H2O2 -treated group (Table 3).
| mRNA Relative expression |
Control (n=5-6) | Control + pNaKtide (n=6) | H2O2 (n=5-6) | H2O2 + pNaKtide (n=5-6) |
|---|---|---|---|---|
| RT-PCR genes | ||||
| ApoJ | 1.0± 0.02 | 0.89 ± 0.18 | 1.66 ± 0.15* | 0.81 ± 0.07## |
| MMP9 | 1.0± 0.01 | 0.91 ± 0.20 | 3.42± 0.42** | 1.72 ± 0.33## |
| Collagenase | 1.0± 0.03 | 0.99 ± 0.26 | 2.16 ± 0.35** | 0.54 ± 0.06## |
| Ki-67 | 1.0± 0.02 | 1.19 ± 0.15 | 0.39 ± 0.07** | 1.02 ± 0.08## |
Table 3: Effects of pNaKtide on senescence genes in H2O2-induced senescence in HDF.
Effect of antioxidants on H2O2-induced senescence in HDFs
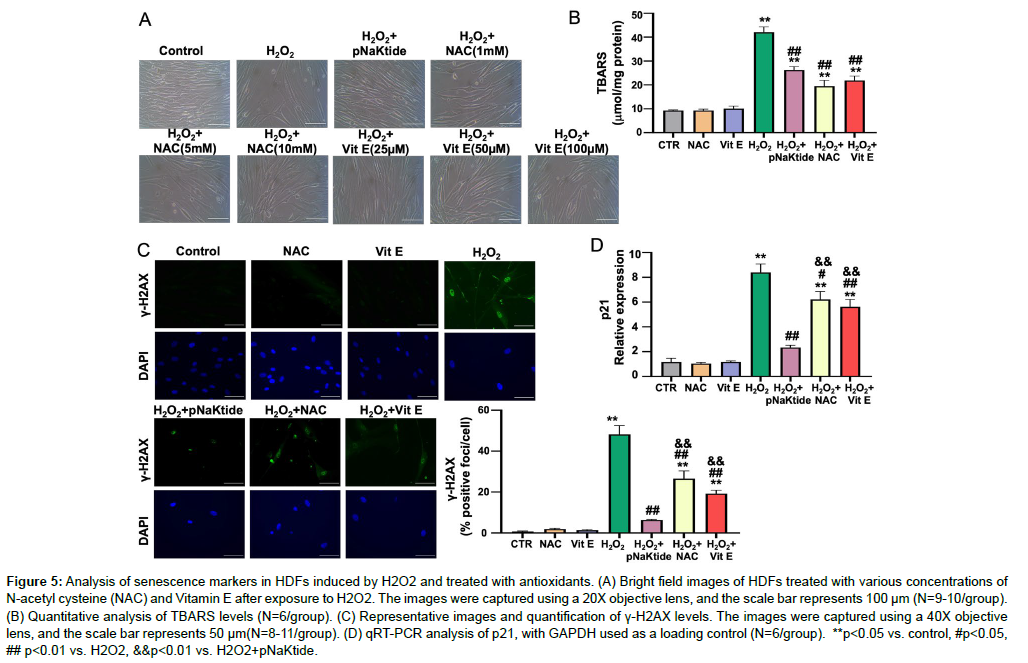
Studies have shown that NAC and Vitamin E, both known antioxidants, have demonstrated positive effects in attenuating the cellular senescence [40-42]. HDFs cells were exposed to varying concentration of NAC and Vitamin E to determine the optimum dose that effectively attenuates senescence in our H2O2 model (Figure 5A). Our results showed that 5mM concentration of NAC or 50uM concentration of Vitamin E were deemed to be the optimal concentration to attenuate the morphological or biochemical changes of senescence markers (γ-H2AX and p21), but less so than pNaKtide (Figure 5A, C and D). Levels of TBARS were also measured, showing that the reductions in cellular oxidative stress achieved for NAC and Vitamin E were comparable to those observed with pNaKtide (Figure 5B).
Figure 5: Analysis of senescence markers in HDFs induced by H2O2 and treated with antioxidants. (A) Bright field images of HDFs treated with various concentrations of N-acetyl cysteine (NAC) and Vitamin E after exposure to H2O2. The images were captured using a 20X objective lens, and the scale bar represents 100 μm (N=9-10/group). (B) Quantitative analysis of TBARS levels (N=6/group). (C) Representative images and quantification of γ-H2AX levels. The images were captured using a 40X objective lens, and the scale bar represents 50 μm(N=8-11/group). (D) qRT-PCR analysis of p21, with GAPDH used as a loading control (N=6/group). **p<0.05 vs. control, #p<0.05, ## p<0.01 vs. H2O2, &&p<0.01 vs. H2O2+pNaKtide.
Effect of GO and pNaKtide on oxidative stress, cell proliferation, and apoptosis in HDFs
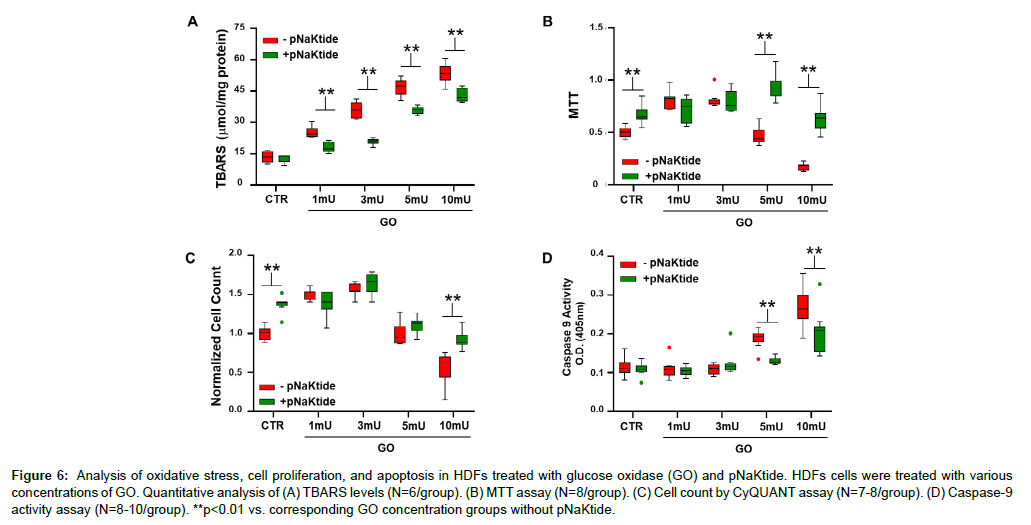
GO generates moderate amounts of H2O2 which results in a steady increase in intracellular ROS [43]. Concentrations of GO ranging from 0 to 10 mU/ml were administered to HDFs. Our result showed that TBARS level increased with increasing concentrations of GO (Figure 6A). Treatment with pNaKtide attenuated this increase; however it is important to note that TBARS concentration in the pNaKtide treated cells was still demonstrably elevated with the high concentrations of GO. To determine the role of Na/K-ATPase signaling in maintaining cell function and proliferation, MTT and CyQUANT assays were performed. Based on the MTT assay, 1 and 3 mU/ml of GO increased cell proliferation compared with control group, whereas 5 and 10 mU/ ml of GO decreased cell count (Figure 6B). Based on these observations, we hypothesized that some activation of the NKAL, demonstrated with 1 and 3 mU/ml GO may actually result in enhanced cell proliferation. Along these lines, we saw that concomitant treatment with pNaKtide allowed for enhanced cell proliferation in the groups exposed to 5 and 10 mU/ml of GO. The CyQUANT assay showed that compared with controls, the number of cells increased with GO 1 mU/ml and GO 3 mU/ml respectively; whereas 5 and 10 mU/ml of GO decreased cell count (Figure 6C). Our results further showed that pNaKtide treatment did not increase cell proliferation in the groups exposed to 1, 3, and 5 mU/ml of GO. However, cell counts were increased in cells treated with 10 mU/ml GO with pNaKtide compared with 10 mU/ml GO alone. Based on caspase 9 activity, 5 and 10 mU/ml of GO increased apoptosis significantly compared with the control group. Treatment with pNaKtide negated this effect (Figure 6D). These results support the concept that oxidants at low concentrations can stimulate cell proliferation whereas at higher concentrations, cell apoptosis results [44]. Interestingly, pNaKtide not only decreased the net amount of oxidant stress as assessed by the accumulation of TBARS but had more profound effects on cell proliferation and apoptosis than one would anticipate from the antioxidant effect alone.
Figure 6: Analysis of oxidative stress, cell proliferation, and apoptosis in HDFs treated with glucose oxidase (GO) and pNaKtide. HDFs cells were treated with various concentrations of GO. Quantitative analysis of (A) TBARS levels (N=6/group). (B) MTT assay (N=8/group). (C) Cell count by CyQUANT assay (N=7-8/group). (D) Caspase-9 activity assay (N=8-10/group). **p<0.01 vs. corresponding GO concentration groups without pNaKtide.
pNaKtide improves UV radiation-induced senescence in HDFs
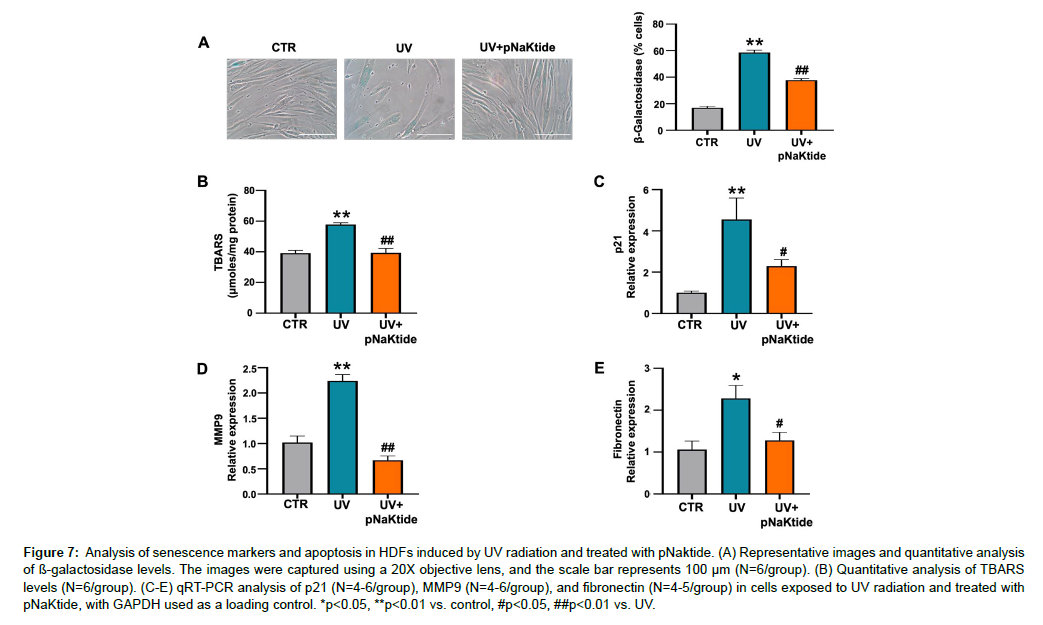
HDFs were subjected to UV radiation, a known inducer of senescence mediated by oxidative stress [45]. Analysis of senescenceassociated SA β-Gal levels and TBARS showed significantly increased levels in the UV-treated group compared to the control group (Figure 7A and B). These increases were attenuated in the pNaKtide-treated group. Furthermore, mRNA expression levels of key senescencerelated genes, including p21, MMP9, and fibronectin, were significantly upregulated in the UV-treated group, however their expression were attenuated in the presence of pNaKtide treatment (Figure 7C, D, and E). These findings suggest that pNaKtide may serve as a promising intervention to counteract UV radiation-induced senescence in HDFs.
Figure 7: Analysis of senescence markers and apoptosis in HDFs induced by UV radiation and treated with pNaktide. (A) Representative images and quantitative analysis of ß-galactosidase levels. The images were captured using a 20X objective lens, and the scale bar represents 100 μm (N=6/group). (B) Quantitative analysis of TBARS levels (N=6/group). (C-E) qRT-PCR analysis of p21 (N=4-6/group), MMP9 (N=4-6/group), and fibronectin (N=4-5/group) in cells exposed to UV radiation and treated with pNaKtide, with GAPDH used as a loading control. *p<0.05, **p<0.01 vs. control, #p<0.05, ##p<0.01 vs. UV.
Discussion
The present study demonstrated that NKAL exhibits a significant role in regulating the aging process. Our previous study has shown that supplementation of western diet can activate Na/K-ATPase signaling that accelerates cellular oxidative stress [18]. Hence, in the present study, we studied the effect of age and oxidative stress in adipose tissue, and heart by employing western diet as a dietary intervention. We observed that old mice and old mice fed a WD had significant organ damage in both adipose tissue and heart due to excessive oxidative stress generated by the activation of NKAL with WD. pNaKtide could significantly ameliorate these processes by antagonizing Na/ K-ATPase signaling. Adipose tissue dysfunction and subsequent systemic inflammation are often associated with aging similar to the pathogenesis of obesity [27]. Similarly, old mice exhibited excessive fat accumulation and enlarged adipocytes along with dysregulation in inflammation, apoptosis and senescence pathways in the adipose tissue. These changes were worsened by WD and reversed by pNaKtide. Aging of heart tissues is associated with impaired cardiac function detectable with echocardiography and fibrosis measurable with histology [46f]. Echocardiography findings in our study supported the functional changes associated with the aging heart. Furthermore, old mice supplemented with WD showed significantly higher damage to cardiac tissue, which was effectively attenuated by pNaKtide.
Senescence represents an array of progressive and phenotypically diverse cellular states, which underlie diverse biological processes that regulate aging and age-related diseases [47]. In aging, senescence contributes to an overall decline in the regenerative potential of tissues that will be intensified in a paracrine manner to spread the senescence phenotype to healthy cells through ROS, inflammatory cytokines and chemokines [48, 49]. A senescent phenotype, which is evident in aging, was observed in HDF cells treated with sub-lethal doses of H2O2, UV radiation, and GO. However, pNaKtide treatment could protect the cells from oxidant-induced senescent damage. Interestingly, we observed a significant downregulation in the expression of prominent molecular makers of senescence [34, 50-54] in pNaKtide-treated HDFs exposed to H2O2. Even though pNaKtide and the antioxidants, N-Acetyl Cysteine and Vitamin E, showed similar protection against oxidative stress, the attenuation of oxidation-induced senescence was more prominent in pNaKtide treated cells, which was executed by the inhibition of NKAL. Given that Na/K-ATPase signaling is known to amplify oxidative stress [17], it seems likely that it is inducing senescence through that mechanism.
Cell injury, necrosis, and apoptosis are all markers of oxidative stress [31]. In our study, H2O2, UV radiation, and GO treatment increased levels of cell injury and apoptosis markers. Subsequent pNaKtide treatment decreased these factors, supporting the hypothesis that Na/K-ATPase signaling affects the overall oxidation levels in cells. A balance of antioxidant and prooxidant activities in response to oxidative stresses is important for longevity by suppressing the accumulation of oxidative stresses and DNA damage [55]. Our oxidation titration experiments using GO suggest that the Na/KATPase oxidant amplification loop preferentially “feeds” oxidants into cellular locations where oxidant stress effectively accomplishes the damage to cellular components involved in the aging process. In HDF cells, lower levels of oxidant stress stimulate proliferation whereas higher degrees of such stress produces programmed cell death. We noted that antagonism of the Na/K-ATPase oxidant amplification loop preferentially blocked the cell death, allowing ongoing stimulation of cell proliferation to occur at levels of oxidant stress usually associated with net decreases in cell number. These data indicate that the feedforward oxidative mechanism of NKAL may reduce the beneficialto- detrimental oxidation threshold, encouraging the transition to senescence. We therefore emphasize that interference with the NKAL with pNaKtide achieves a greater anti-aging effect than would be achieved by simple anti-oxidant administration.
Numerous anti-aging pharmacological interventions have been shown to modulate proliferative, apoptotic and cell survival signaling pathways associated with cellular senescence [56, 57]. Our study demonstrates that Na/K-ATPase signaling accelerated the development of senescence markers, whereas signaling antagonism attenuated it. The effects of Na/K-ATPase observed on the molecular, cellular, and tissue levels, emphasize the importance of this process in normal and oxidative stress-induced aging. Notably, pNaKtide dramatically attenuated markers of aging at all levels. Overall the study targets a novel pathway, NKAL, with the goal of developing therapeutic options for aging in humans by transitioning pNaKtide toward clinical use.
Acknowledgements
This work was supported by National Institutes of Health Grants HL109015 (to J.I.S. and Z.X.), HL071556 and HL105649 (to J.I.S.), HL55601, and HL34300 (to N.G.A.), by the Brickstreet Foundation (to J.I.S. and N.G.A.) and by the Huntington Foundation, Inc.
Authors Contributions
Komal Sodhi: Designed the experiments, analyzed data and participated in the writing of the manuscript.
Krithika Srikanthan: Performed experiments and analyzed data
Xiaoliang Wang: Performed experiments and analyzed data.
Perrine Goguet-Rubio: Performed experiments and analyzed data
Athar Nawab: Performed experiments and analyzed data.
Juan R. Sanabria: Edited the manuscript.
Zijian Xie: Participated in the design of experiments.
Joseph I. Shapiro: Conceived and designed the overall project as well as individual experiments, analyzed data and participated in the writing of the manuscript.
Competing Interests
The authors do not have any competing interest
References
- Davidovic M (1999) Genetic stability: the key to longevity? Med Hypotheses 53(4): 329-32.
- Risques RA (2008) Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology 135(2): 410-8.
- Liguori I (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13: 757-772.
- McHugh D, Gil J (2018) Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol 217(1): 65-77.
- Cerella C (2016) Roles of Apoptosis and Cellular Senescence in Cancer and Aging. Curr Drug Targets 17(4): 405-15.
- Lopez-Otin C (2013) The hallmarks of aging. Cell 153(6): 1194-217.
- Yao H, Rahman I (2012) Role of histone deacetylase 2 in epigenetics and cellular senescence: implications in lung inflammation and COPD. Am J Physiol Lung Cell Mol Physiol 303(7): L557-66.
- Jones DP (2008) Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295(4): C849-68.
- Liang FQ, Godley BF (2003) Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res 76(4): 397-403.
- Iakovou E, Kourti M (2022) A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front Aging Neurosci 14: 827-900.
- Abdollahi M (2003) Oxidative stress in aging. Oxid Med Cell Longev 2014: 876-834.
- Hagen TM (2003) Oxidative stress, redox imbalance, and the aging process. Antioxid Redox Signal 5(5): 503-6.
- Jones DP (2006) Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res 9(2): 169-81.
- Kregel KC, Zhang HJ (2007) An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol 292(1): 18-36.
- Faraonio R (2022) Oxidative Stress and Cell Senescence Process. Antioxidants (Basel) 11(9).
- Liu J (2016) Attenuation of Na/K-ATPase Mediated Oxidant Amplification with pNaKtide Ameliorates Experimental Uremic Cardiomyopathy. Sci Rep 6: 34592.
- Yan Y (2013) Involvement of reactive oxygen species in a feed-forward mechanism of Na/K-ATPase-mediated signaling transduction. J Biol Chem 288(47): 34249-34258.
- Sodhi K (2023) Inhibition of Na/K-ATPase signaling Attenuates Steatohepatitis and Atherosclerosis in Mice Fed a Western Diet. Cell Mol Biol (Noisy-le-grand) 69(2): 162-171.
- Liu J, et al. (2002) Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney Int 62(6): 2118-25.
- Li Z (2011) Na/K-ATPase mimetic pNaKtide peptide inhibits the growth of human cancer cells. J Biol Chem 286(37): 32394-403.
- Li Z (2009) NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem 284(31): 21066-76.
- Liu J (2012) Reactive Oxygen Species Modulation of Na/K-ATPase Regulates Fibrosis and Renal Proximal Tubular Sodium Handling. Int J Nephrol 2012: 381320.
- Lai F (2013) Identification of a mutant alpha1 Na/K-ATPase that pumps but is defective in signal transduction. J Biol Chem 288(19): 13295-304.
- Kim IH (2016) Aging increases the susceptibility of hepatic inflammation, liver fibrosis and aging in response to high-fat diet in mice. Age (Dordr) 38(4): 291-302.
- Parlee SD (2014) Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol 537: 93-122.
- Van der Heijden RA (2015) High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging (Albany NY) 7(4): 256-68.
- Tchkonia T (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9(5): 667-84.
- Vidal-Puig A (1996) Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97(11): 2553-61.
- Matos L, Gouveia A, Almeida H (2012) Copper ability to induce premature senescence in human fibroblasts. Age (Dordr) 34(4): 783-94.
- Gire V (2004) DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J, 23(13): 2554-63.
- Chen Q, Ames BN (1994) Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci USA 91(10): 4130-4.
- Chen H, Li Y, Tollefsbol TO (2013) Cell senescence culturing methods. Methods Mol Biol 1048: 1-10.
- Kumazaki T (1991) Fibronectin expression increases during in vitro cellular senescence: correlation with increased cell area. Exp Cell Res 195(1): 13-9.
- Chen QM (2000) Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann N Y Acad Sci 908: 111-25.
- Kuo LJ, Yang LX (2008) Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo 22(3): 305-9.
- Kyrylkova K (2012) Detection of apoptosis by TUNEL assay. Methods Mol Biol 887: 41-7.
- Wurstle ML, Laussmann MA, Rehm M (2012) The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res 318(11): 1213-20.
- Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182(3): 311-22.
- Bruey JM (2010) Circulating Ki-67 index in plasma as a biomarker and prognostic indicator in chronic lymphocytic leukemia. Leuk Res 34(10): 1320-4.
- Marthandan S (2015) Effects of ebselen and N-acetyl cysteine on replicative aging of primary human fibroblast strains. Immun Ageing 12: 8.
- La Fata G, et al. (2015) Vitamin E Supplementation Delays Cellular Senescence In Vitro. Biomed Res Int 2015: 563247.
- Butt H, et al. (2017) Protective role of vitamin E preconditioning of human dermal fibroblasts against thermal stress in vitro. Life Sci 184: 1-9.
- Liu J. et al. (2017) Na/K-ATPase Signaling and Salt Sensitivity: The Role of Oxidative Stress. Antioxidants (Basel) 6(1).
- Foyouzi N, et al. (2004) Effects of oxidants and antioxidants on proliferation of endometrial stromal cells. Fertil Steril 82 Suppl 3: 1019-22.
- Zeng JP (2014) Repeated exposure of mouse dermal fibroblasts at a sub-cytotoxic dose of UVB leads to premature senescence: a robust model of cellular photoaging. J Dermatol Sci 73(1): 49-56.
- Alcendor RR, et al. (2007) Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100(10): 1512-21.
- Van Deursen JM (2014) The role of senescent cells in ageing. Nature 509(7501): 439-46.
- Acosta JC, et al. (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15(8): 978-90.
- Nelson G, et al. (2012)
Citation: Sodhi K, Srikanthan K, Wang X, Goguet-Rubio P, Nawab A, et al.(2023) Na/K-ATPase Oxidant Amplification Loop Regulates Cellular Senescence.Biochem Physiol 12: 430. DOI: 10.4172/2168-9652.1000430
Copyright: © 2023 Sodhi K, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar , Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2647
- [From(publication date): 0-2023 - Nov 24, 2025]
- Breakdown by view type
- HTML page views: 2301
- PDF downloads: 346