Nail Involvement as the Only Onset Manifestation of Pemphigus Vulgaris
Received: 23-Jul-2020 / Accepted Date: 06-Aug-2020 / Published Date: 13-Aug-2020 DOI: 10.4172/2476-2024.1000167
Abstract
Pemphigus vulgaris (PV) is a blistering autoimmune, chronic and uncommon disease that affects the skin, mucous membranes and skin appendages. The onset of the disease usually consists of mucosal lesions (50%-70%), skin manifestations (10%-15%), or both. Nail involvement usually develops in association with the previously mentioned manifestations, being exceptionally the only affected area at the onset of the disease.
A 52 year old patient with no important medical history was evaluated after two weeks of antibiotic treatment for painful, swollen, erythematous-violaceous, poorly delimited lesions, with subungueal haemorrhage, affecting the nails and periungueal tissue of the first and third right toes and the third left finger; the nail plate presented hyperkeratosis, yellowish discoloration and distal onycholysis. Complementary blood analysis and skin cultures did not reveal positive results. A skin biopsy showed suprabasal acantholytic dermatosis with no inflammatory components, and immunofluorescence manifested IgG intercellular deposits, with no evidence of IgA, IgM, C3 or fibrinogen deposits. Autoimmune serologic studies were positive for anti-intercellular substance antibody (1/80) and negative for anti-basal membrane and anti-nuclear antibodies. With these findings the patient was diagnosed with Pemphigus Vulgaris, being nail lesions the exclusive onset manifestation.
Keywords: Pemphigus vulgaris; Nails
Introduction
Pemphigus vulgaris (PV) is a blistering autoimmune, chronic and uncommon disease that affects the skin, mucous membranes and skin appendages. The onset of the disease usually consists of mucosal lesions (50%-70%), skin manifestations (10%-15%), or both. Nail involvement usually develops in association with the previously mentioned manifestations, being exceptionally the only affected area at the onset of the disease.
Case Presentation
52-year old male, with no previous illness and no toxic or epidemiologic background, with no personal or familial history of autoimmune disease, and employed as a clerk; presents to the emergency department complaining of over a month with painful nail lesions that affected the first and third right toes. During this visit he was erroneously diagnosed with whitlow, and prescribed antibiotic oral therapy (ciprofloxacin 500 mg. every 12 hours) and anti-inflammatory medication (metamizole 500 mg. every 8 hours). After two weeks of treatment, the patient develops oral aphtas and a solitary aphta in genitalia; all these lesions were painful, recurrent and self-limited.
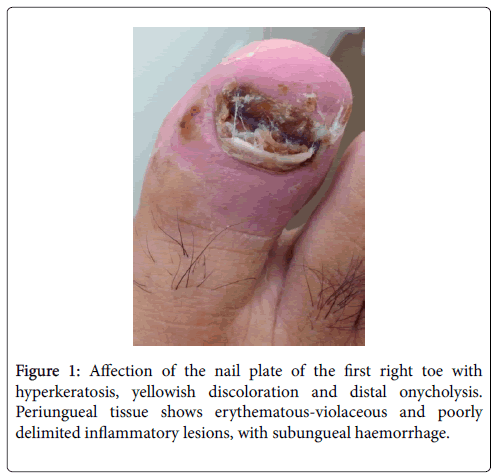
The patient was evaluated at the Internal Medicine consult due to persistence of nail lesions and fever. Physical exploration revealed painful, swollen, erythematous-violaceous, poorly delimited lesions, with subungueal haemorrhage, affecting the nails and periungueal tissue of the first and third right toes and the third left finger, compatible with acute paronychia; the nail plate presented hyperkeratosis, yellowish discoloration and distal onycholysis (Figure 1). The rest of the exploration was normal; there was no evidence of red eye, cardio-respiratory auscultation with no murmurs, no palpable masses in the abdomen, peripheral pulses were palpable and no other cutaneous lesions were recorded. In oral cavity he presented a solitary aphta localized on the jugal mucosa. Alternative diagnosis was considered, including infectious diseases (endocarditis), inflammatory illnesses (vasculitis or Behcet disease) and thromboembolic complications. A series of paraclinical analysis were requested.
Biochemical blood analysis reported a slight augmentation of liver enzymes (GOT 44 IU/L, GPT 81 IU/L), serologic expression of hepatotropic viruses and C. burnetti , C. jejuni and Brucella spp , were negative. CBC showed no leukocytosis or neutrophilia and minimum eosinophilia (1190 per mm3) without complement consumption and normal levels of acute phase proteins (CRP 1,2 mg/d, ESR 4 mm/s). One of the skin cultures isolated Pseudomonas aeruginosa, sensible to ceftazidime and quinolones; the rest of skin and blood cultures were negative. Screening for thrombophilia, urine sediment analysis, electrocardiogram and chest X-rays were also negative.
A skin biopsy showed suprabasal acantholytic dermatosis with no inflammatory components (Figure 2). Direct immunofluorescence manifested IgG intercellular deposits, with no evidence of IgA, IgM, C3 or fibrinogen deposits. Autoimmune serologic studies were positive for anti-intercellular substance antibody (1/80) and negative for anti-basal membrane and anti-nuclear antibodies. With these findings the patient was diagnosed with Pemphigus Vulgaris, being nail lesions the exclusive onset manifestation.
Treatment was initiated with oral steroids at 0.5 mg/kg/day in descending dosage, until a month of treatment was completed. The patient was also prescribed with topic mupurocin every 8 hours during the first 7 days, combined with azathioprine 50 mg per day for 15 days, ascending to 100 mg per day for the next 15 days. The patients’ clinical and analytical condition improved after completing treatment, the skin and nail lesions nearly disappeared and analytical parameters normalized within the first month, there was no relapse in a 6 month follow-up.
Discussion
We present a case of PV with nail lesions as the onset manifestation for the diagnosis. When evaluating nail lesions as described in our clinical case, it is crucial to consider pemphigus vulgaris as a differential diagnosis, especially in the Emergency Department, where the assistance pressure limits the time of evaluation for each patient. At first our patient was misdiagnosed with low and prescribed ineffective antibiotic therapy, however, this diagnosis was excluded due to the persistence of symptoms in spite of antibiotic administration.
The onset manifestations in our patient match the ones described in other articles, including paronychia (especially affecting the first three fingers) and onychomadesis; also the less frequent onycholisis, onychodistrophia, discoloration of the nail plate and the presence of Beau lines [1,2].
The temporal relationship between nail involvement and the other manifestations of PV is variable; it can take part of the preceding signs, present during the course of the disease, or be the only manifestation of it [3]. In our patient nail lesions manifested 8 weeks prior to the onset of oral afthas.
Extensive nail lesions can correlate with the severity of the disease, the prognosis worsens when haemorrhagic nail lesions are associated [4,5]. However, in our patient the prognosis was not modified, he did not develop other cutaneous lesions and the nail and oral manifestations resolved within the month of appropriate treatment, maintaining an adequate clinical response during the follow-up consults.
There is limited information on nail and periungueal changes in patients with PV. It is hypothesised that the periungueal blisters could be responsible for the nail matrix malfunction [6,7]. Characteristic immunofluorescence patterns have also been published, which include epidermic intercellular deposits of IgG and C3 [8]. Nail involvement in PV tends to be considered as uncommon, nonetheless, a prospective study recently published, evaluated symptoms in 79 patients with this disease, and reported nail affection in 34.2% of the cases [4]. In another study of 64 patients, 47% manifested nail lesions during the course of the disease and 14 of them (22%) directly caused by Pemphigus vulgaris [9].
Some authors have postulated the existence of other antigenic factors that would determine nail involvement; this thesis is supported by the different distribution of injuries in pemphigus vulgaris and pemphigus foliaceus [10].
Topical treatment is usually not effective. It is necessary to treat with systemic immunosuppressive drugs, generally accomplishing adequate control of the disease [2,11]. Different associations of steroid and steroid-sparing-immunosuppressors have been described [12]; in our case, once the aetiology was defined, treatment was initiated with steroids in descending dosage in combination with azathioprine, since this association is highly effective and safe in pemphigus vulgaris and leads to long-term remission in most patients [13].
Conclusion
This case shows nail lesions as the onset manifestation of pemphigus vulgaris. More investigations are needed to clarify the aetiopatogenesis of this presentation.
References
- Rivera Diaz R, Alonso Llamazares J, Rodriguez Peralto JL, Sebastian Vanaclocha F, Iglesias Diez L (1996) Nail involvement in pemphigus vulgaris. Int J Dermatol 35: 581-582.
- Tosti A, André M, Murrell DF (2011) Nail involvement un autoimmune bullous disorders. Dermatol Clin 29: 511-513.
- Carducci M, Calcaterra R, Franco G, Mussi A, Bonifati C, et al. (2008) Nail involvement in pemphigus vulgaris. Acta Derm Venereol 88: 58-60.
- Lee H, Wong WR, Lee MC, Hong HS (2004) Acute paronychia heralding the exacerbation of pemphigus. Int J Clin Pract 58: 1174-1176.
- Reich A, Wisnicka B, Szepietowski JC (2008) Haemorrhagic nails in pemphigus vulgaris. Acta Derm Venereol 88: 542.
- Pardo-Castello V (1960) Diseases of the Nails, 3rd edn. Springï¬eld, IL 177-178.
- Parameswara YR, Chinnappiah N (1981) Onychomadesis associated with pemphigus vulgaris. Arch Dermatol 107: 759-760.
- Fulton RA, Campbell I, Caryland D, Simpson NB (1983) Nail bed immunofluorescence in pemphigus vulgaris. Acta Derm Venereol 63: 170-172.
- Schlesinger N, Katz M, Ingber A (2002) Nail involvement in pemphigus vulgaris. Br J Dermatol 146: 836-839.
- Sison-Fronacier L, Bystryn JL (1986) Regional variations in antigenic properties of the skin. A possible cause for disease-specific distribution of skin lesions. J Exp Med 164: 2125-2130.
- Engineer L, Norton LA, Ahmed AR (2000) Nail involvement in pemphigus vulgaris. J Am Acad Dermatol 43: 529-535.
- Mascarenhas R, Fernandes B, Reis JP, Tellechea O, Figueiredo A (2003) Pemphigus vulgaris with nail involvement presenting with vegetating and verrucous lesions. Dermatol Online J 9: 14.
- Hertl M, Jedlickova H, Karpati S, Marinovic B, Uzun S, et al. (2015) Pemphigus. S2 Guideline for diagnosis and treatment-guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol 29: 405-414.
Citation: Morillo JSG, Garcia FJN, Román AR, Prieto AO (2020) Nail Involvement as the Only Onset Manifestation of Pemphigus Vulgaris. Diagn Pathol Open 5: 167. DOI: 10.4172/2476-2024.1000167
Copyright: © 2020 Morillo JSG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2105
- [From(publication date): 0-2020 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1394
- PDF downloads: 711