Mycosynthesis of Silver Nanoparticle from Aspergillus niger
Received: 06-Sep-2016 / Accepted Date: 20-Sep-2016 / Published Date: 27-Sep-2016 DOI: 10.4172/2155-952X.1000246
Abstract
Nanotechnology is an emerging field of science which encompasses fabrication of nanoparticles that possess distinct morphological properties with enhanced functionality. Nanobiotechnology plays a fundamental role in the development of a bio-based green approach for their synthesis. This method is not only ecofriendly but provides a clean and nontoxic way for the fabrication and assembly of different nanoparticles. Microorganisms such as fungal strain of Aspergillus niger could be employed to produce silver nanoparticles. By utilizing fungal biomass and its LCF (Live cell filtrate), colloidal AgNPs were synthesized through reduction of silver metal ions by nitrate reductase enzyme present in the fungal filtrate. Color change from yellow to brownish black of the experimental flask incubated with silver nitrate solution at 25°C for 48 h indicates silver nanoparticle formation. UV vis spectrometry results showed that the maximum absorbance was obtained at 420 nm and 400 nm.
Keywords: Nanotechnology, Silver nanoparticles (SNPs), Aspergillus niger
249563Introduction
Green synthesis of nano particles using bio-nano factories such as microorganisms is a novel environmental friendly science for producing nanoparticles of definite size and shape with unique physical and chemical properties [1-3]. Because of these unique properties, nanoparticles have diverse applications in different areas such as chemical industries, textile industries, nano medicine, clinical diagnostics (nanobots), electronics, tissue engineering, artificial implantations [4] IT, biosensors [5], biological imaging [6], biological markers, labeling, etc. [7,8]. Organisms such as bacteria and fungi serves as potential biofactories for the production of variety of nanoparticles, e.g. copper, gold and silver, etc. [9]. Holmes et al. reported that the organism such as bacteria, Klebsiella aerogenes is involved in the intracellular synthesis of cadmium sulphide [10].
Nanoparticle when exposed to cadmium ions. Similarly silver and gold nanoparticles were formed by the reduction of these metal ions by the fungus Verticillium sp. and Fusarium oxysporum [9]. Klaus et al. on the other hand observed the amalgamation of silver nanoparticles with distinct morphology in the periplasmic space of the bacteria, Pseudomonas stutzeri AG259 which was isolated from silver mine [11]. There are numerous examples in the literature depicting the utilization of microorganisms for the production of different nanoparticles. These bio based approaches are not only ecofriendly but provides a clean and nontoxic way for the fabrication of nanocrystals. Therefore the main aim of this research work was to produce nanoparticles of silver using the fungal strain Aspergillus niger and to investigate the reaction conditions required for bioreduction.
Materials and Methods
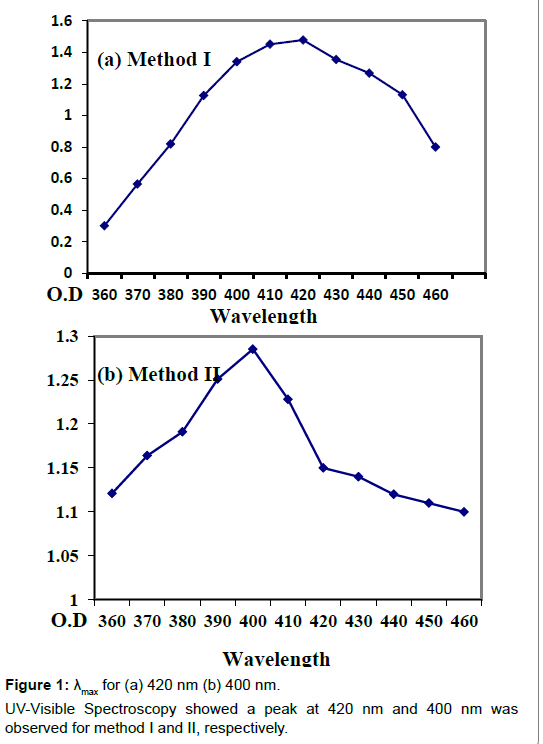
The fungal culture of Aspergillus niger was obtained from Yeast and Fungal Biotechnology lab, BUITEMS. Extracellular mycosynthesis of AgNPs by culturing Aspergillus niger aerobically in 1 L CD (cezapex Dox) liquid broth which consists of glucose (10 g/l), yeast extract (1 g/l), zinc sulphate (0.01 g/l), calcium chloride (0.5 g/l), magnesium sulphate (0.5 g/l), ferrous sulphate (0.01 g/l), Potassium dihydrogen phosphate (1 g/l) and Sodium nitrate (2 g/l) dissolved in one liter of distilled water and autoclaved for 15 min at 121°C at 15 psi (pound/square inches). The cultured flasks were then incubated at room temperature on a rotatory shaker at 140 rpm for 120 h. The fungal mycelia was obtained after five days of growth through Whatman’s filter paper no.1 and washed with distilled water and the fungal LCF (Live cell filtrate) was saved for further use. The filtrate was then centrifuged at 15000 rpm for 15 min. For method I 20 ml of centrifuged filtrate was incubated with 150 ml of silver nitrate solution (1 mM). And the flask was plugged tightly with cotton and further wrapped in aluminum foil to be kept in dark for 48 h in an incubator at 25°C. In this case control was freshly prepared CD broth with silver nitrate solution. For method II 20 g of fungal biomass (wet weight) was placed in 200 ml double distilled water for three days at 27°C and agitated at 140 rpm on a rotatory shaker. After incubation the mycelia was harvested by using filter paper. Obtained filtrate was centrifuged at 5000 rpm for 10 min. 20 ml of supernatant was treated with 150 ml of 1 mM AgNO3 and incubated for reduction at 25°C in dark for 48 h. Control containing double distill water (without the fungal biomass) with 20 ml aqueous silver nitrate was run simultaneously as a control. Silver nanoparticle synthesis was observed by gradual change in the color of the experimental flasks and UV Vis spectrometry analysis was done to determine λmax for both the methods (Figure 1).
Results
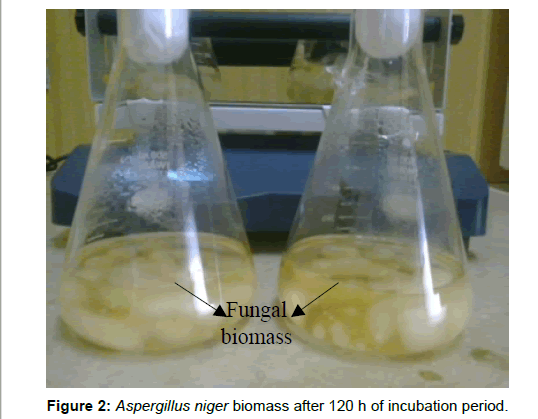
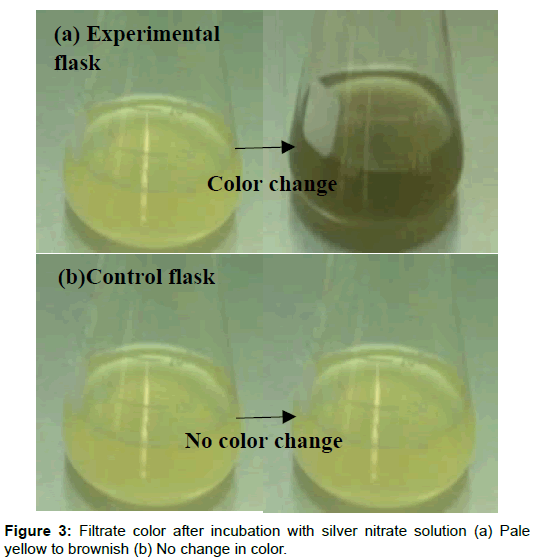
After incubation of cultured flasks at room temperature on a rotatory shaker at 140 rpm for 120 h Aspergillus niger was grown successfully in CD (Czapex Dox) liquid broth and sufficient biomass was obtained (Figure 2). After 48 h of incubation of the experimental flasks with 1 mM of AgNO3 (for both method I and method II), color change was seen from yellow to brownish black only in the experimental flask. No change of color was seen in the controls (Figure 3). The intensity of the color increases as the time of incubation increases.
Discussion
Enough reaction time to the fungus to utilize all the nutrients available in the CD media and continuous agitation on a rotatory shaker provides uniform distribution of all the media nutrients as a result sufficient fungal biomass was obtained. The incubated fungal filtrate resulted in the mycosynthesis of silver nanoparticles. Harvested mycelia of Aspergillus niger can also be incubated in distill water to obtain fungal live cell filtrate for silver nanoparticle production. Thus two approaches could be employed for the biosynthesis of silver nano crystals in in vitro. The experimental flask (method I) containing live cell filtrate (LCF) of Aspergillus niger was yellowish before the addition of silver nitrate solution however due to the addition of silver nitrate color gradually changes from yellow to brownish black indicating the reduction of silver nitrate to silver nanoparticles [12-14]. However no color change was seen in the control flasks after incubation with silver nitrate solution. In case of the experimental flask (method II) containing LCF of Aspergillus niger was colorless but the color gradually changes from colorless to light brown/black after the addition of silver nitrate solution. The possible reason for this change in color is the presence of extra cellular protein reductase in the aqueous fungal filtrate which upon contact with silver nitrate solution results in silver ions reduction forming silver nanoparticles [15,16]. However absence of nitrate reductase in the control flasks does not result in silver ion reduction therefore no color change was seen.
The color change was observed within 48hrs of incubation which arises due to excitation of surface plasmon resonance [17-19]. The morphology of the synthesized nanoparticles reflects the absorbance peak and in the present research the surface plasmon band occurs in the visible region of the light spectrum with broad absorbance peak at 420 nm for method I and 400 nm for method II indicating a wide distribution of the silver nanoparticles. The intensity of the peak correlates the amount of nanoparticles produced during the reaction as narrow distribution of the size of the nanoparticles is indicated by a sharp peak whereas a broad peak indicates a wide distribution. The increase in intensity of the color occurred due to gradual increase in the amount of silver nanoparticles produced from silver ions reduction. The mycosynthesized silver nanoparticles were found to be well distributed in the solution with not much aggregation and were found to remain stable for several weeks which may be due to the interaction between the silver nanoparticles and proteins present in the Aspergillus niger fungal filtrate [16].
Conclusion
Mycosynthesis of silver nanoparticles can easily be achieved in in vitro using fungi as the biological system which provides a simple and ecofriendly way to produce colloidal nanoparticles of silver. Aspergillus niger can easily be exploited and maintained under controlled conditions, utilizing its biomass and biomass extract for the production of silver nanoparticles.
References
- Ahmed M, Karns M, Goodson M, Rowe J, Hussain SM, et al. (2008) DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicology and Applied Pharmacology 233:404-410.
- Rai M, Yadav A, Gade A (2008) Currenttrends in phytosynthesis of metal nanoparticles. Crit Rev Biotechnol 28:277-284
- Huang J, Li Q, Sun D, Lu Y, Su Y, et al.(2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomumcamphora leaf. Nanotechnology 18.
- Santhosh KT, Abdul RA, Rajakumar G, Marimuthu S, Bagavan A, et al.(2011) Synthesis of silver nanoparticles using Nelumbonuciferaleaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res 108:693-702
- Probin P, Elizabeth ZD, Jahirul M, Moumita S, Sukanya BB (2012) Green synthesis of silver nanoparticles using leaf extract of Myricaesculenta. Int J of Nano ScandNanotechnol 3:73-79.
- Nagaraj B, Divya TK, Malakar B, Krishnamurthy NB, Dinesh R, et al. (2012) Phytosynthesis of gold nanoparticles using Caesalpiniapulcherrima (Peacock flower) flower extract and evaluation of their antimicrobial activities. Dig J of Nanomat and Biostru 7:899-905.
- Le AT, Huy PT, Tam LT, Tam PD, Hieu N, et al.(2011)Novel silver nanoparticles: Synthesis, properties and applications. Int J of Nanotechnol 8: 278-290
- Singh R, Singh NH (2011) Medical application of nanoparticles in biological imaging, cell labelling, antimicrobial agents and anticancer nano drugs. American Scientific Publishers7: 489-503
- Sastry M, Ahmad A, Khan MI, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and Actinomycetes. CurrSci 85: 162-70
- Holmes JD, Smith PR, EvansGowing R, Richardson DJ, Russel DA, et al. (1995) Energy-dispersive-X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiellaaerogenes. Arch Microbiol 163:143-147
- Jeorger K, Jeorger R, Olsson E, Granqvist C (2001) Bacteria as workers in the living factory: Metal accumulating bacteria and their potential for material science. Trends Biotechnol 19: 15-20.
- Jae YS, Beom SK (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. bioprocessBiosystEng 32: 79-84.
- Balaji DS, Basavaraja S, Deshpande R, Bedre D, Prabhakar BK, et al. (2009) Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporiumcladosporioidesfungus. Colloids and Surfaces BBiointerfaces 68: 88-92.
- Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, et al. (2001) Bioreduction of AuCl4ions by the fungus Verticilliumsp and surface trapping of the gold nanoparticles formed. AngewanteChemie International Edition 40: 3585-3588
- Anil KS, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, et al. (2007) Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett 29: 439-445.
- Durán N, Marcato PD, Alves OL, de Souza GIH, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusariumoxysporum strains. J Nanobiotechnology 3: 1-8.
- Henglein A (1993) Physicochemical properties of small metal particles in solution: ‘Microelectrode’ reactions, chemisorption, composite metal particles and the atom-to-metal transition. PhysChem B 97: 5457-5471
- SastryM, Mayya KS,Bandyopadhyay K (1997) pH dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surf A 127: 221-228.
- Sastry M, Patil V,SainkarSR(1998) Electrostatically controlled diffusion of carboxylic acid derivatized Silver colloidal particles in thermally evaporated fatty amine film s. PhysChem B 102: 1404-1410.
Citation: Nida TK, Namra J (2016) Mycosynthesis of Silver Nanoparticle from Aspergillus niger. J Biotechnol Biomater 6:246. DOI: 10.4172/2155-952X.1000246
Copyright: © 2016 Nida TK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15198
- [From(publication date): 9-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 14139
- PDF downloads: 1059