Research Article Open Access
Mycoremediation of Imidaclopridin the Presence of Different Soil Amendments using Trichoderma_longibrachiatum and Aspergillusoryzae Isolated from Pesticide Contaminated Agricultural fields of Uttarakhand
| Saurabh Gangola*, Pankaj, Priyanka Khati and Anita Sharma | |
| Department of Microbiology, GBPUAT, Pantnagar, Uttarakhand | |
| Corresponding Author : | Saurabh Gangola Department of Microbiology GBPUAT Pantnagar, Uttarakhand Tel: 094583 22830 E-mail: saindsaurabh@gmail.com |
| Received July 02, 2015; Accepted August 07, 2015; Published August 09, 2015 | |
| Citation: Gangola S, Pankaj, Khati P, Sharma A (2015) Mycoremediation of Imidaclopridin the Presence of Different Soil Amendments using Trichoderma_longibrachiatum and Aspergillusoryzae Isolated from Pesticide Contaminated Agricultural fields of Uttarakhand. J Bioremed Biodeg 6:310. doi:10.4172/2155-6199.1000310 | |
| Copyright: © 2015 Gangola S, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Imidacloprid (I-[(6-chloro-3-pyridinyl)-methyl]-N-nitro-2-imidazolidinimine), is a chloronicotinyl insecticide and used to control biting and sucking insects, It is very persistent in the soil with a half-life of more than 100 days. Biodegradation of imidacloprid (20) ppm was checked at 0, 10 and 15 day interval by two fungal isolates, from contaminated soil of agricultural field. The isoates were characterized as Aspergillusoryzae and Trichodermalongibrachiatumon the basis of morphological and molecular techniques. Highest degradation of imidacloprid was reported in consortium (92%) followed by FII(85%), FIII (81%) and control(11%)after 15 days.Immobilization of fungal isolates in sodium alginate and agar disc enhanced biodegradation of imidacloprid. After 15 days, maximum biodegradation of imidacloprid was (95%) and (97%) in consortium immobilized in sodium alginate, and agar discs respectively. Effect of organic amendment on rate of biodegradation was also observed. The maximum biodegradation was observed in consortium amended with bagasse (99%) followed by consortium amended with Hen Manure (94%) and consortium amended with Farm Yard Manure (91%). ITS regions of the two fungal isolates i.e FII and FIII showed 100% similarity with Aspergillusoryzae and Trichodermalongibrachiatum respectively.
| Keywords |
| Biodegradation; Imidacloprid; Mycoremediation; Amendments |
| Introduction |
| A pesticide is a chemical substance, biological agent (such as a virus or bacteria), antimicrobial, disinfectant or device to control any pest [1]. Pesticides are generally used against insect and nematodes infestation in plants for the improvement of food production, inspite of their benefits, the continuous use of pesticides cause severe effect on the health of human, animal and environment [2]. Different types of insecticides(organochlorine,organophosphorus,carb amates,pyrethroids and neonicotinoids) were synthesized and put into application,have gained increasing interest in the agricultural sector. Imidacloprid, a member of chloronicotinylneonicotinoid compounds is most commonly used on rice, cereal, maize, sunflower, potatoes and vegetables [3]. Imidaclopridinterfers with the transmission of stimuli in the insect’s nervous system. Half-life of imidaclopridranges from 42 to 129 days. Depending on soil type, fertilizers use and presence or absence of ground cover [4]. |
| Bioremediation can be aneffective solution for reducing pollution level in the environment by reducing the concentrations and/or the toxicity of chemical compounds and restoring natural conditions [5]. Bioremediation where fungi are employed is called Mycoremediation [6]. Fungi are reported to utilize hazardous chemical for their own growth. Production of extracellular enzymes help them to utilize different carbon sources. Fungal hyphae are also able to penetrate solids and reach microhabitats like water-filled micropores in soil [7]. In this way fungi may gain a much better access to the nutrients and contaminants in environments where the compounds are heterogeneously distributed and inaccessible to bacteria [8]. |
| Materials and Methods |
| Sample collection |
| Soil samples were collected in sterile autoclave bags from the contaminated sites of agricultural fields of Udham Singh Nagar, Uttarakhand and used for the isolation of pesticide degrading microorganisms (fungi). |
| Screening of imidacloprid degrading fungal strains |
| Microbial inocula were prepared by shaking 20 g soil in 100 ml potato dextrose broth for overnight at 28ºC at 150 rev min–1. After filtration soil suspension was used to inoculate potato dextrose agar plates supplemented with 10 ppm imidacloprid. Inoculated plates were incubated for 5 to 10 days at 280C. Obtained fungal colonies were enriched by transferring to higher concentration of imidacloprid in the same medium. Recovered fungal isolates were purified by growing them on Czapek-Dox medium. The medium was adjusted to pH 6.0 and autoclaved to sterilize at 121ºC for 20min. |
| The fungal isolates with maximum tolerance level of the imidacloprid, were grown in minimal medium supplemented with imidacloprid. The MIC was checked at different concentration (30 ppm to 350 ppm) of the imidacloprid. |
| Biodegradation of imidacloprid in minimal medium |
| Biodegradation of imidacloprid was studied using selected fungal isolates (FII and FIII) in liquid medium. The experiment was performed in duplicate. Czapek-dox broth (50 ml) was taken in a 100 ml flask. Imidacloprid (20ppm) was added to all the sets, to which 1 ml of 4 day old fungal culture was also added. Degradation of imidacloprid was analyzed in the presence of individual fungal isolate and in the presence of their consortium (FII+FIII). Inoculated flasks were incubated at 30°C at 150 rpm. One ml aliquot of the broth was taken from all flasks separately on 0, 10, and 15th day for extraction of the imidacloprid. Uninoculated flasks spiked with imidacloprid acted as control. Quantification of imidacloprid was done by High performance liquid chromatography (HPLC). |
| Biodegradation of imidacloprid by immobilized fungal culturesin sodium alginate beads and agar discs |
| Biodegradation of imidacloprid was tested using immobilized fungal isolates insodium alginate beads and agar discs. Sodium alginate beadswere prepared by using 4% sodium alginate and mixed with homogenized fungal culture(s). Mixture was poured drop by drop into pre chilled 0.4M CaCl2 (autoclaved) through syringe to make fine beads in the laminar air flow. Beads were stored at 4oC for overnight. To prepare agar disc, agar solution (4%) was autoclaved and allowed to cool up to 44oC.Homogenized fungal cultures were mixed with molten agar and poured in plate aseptically in a laminar air flow. Agar discs of equal size were cut by using cork borer. |
| For biodegradation experiment, 50 ml of Czapek-dox mediumwas taken in a 100 ml flask in two sets, for sodium alginate beads and for agar discs each. Imidacloprid (20ppm) was added to all the sets with 10 sodium alginate beads and agar discs separately (having equal fresh weight of fungal culture). One ml aliquot of the broth was taken from all the flasks separately on 5, 10, and 15th day for extraction and analysis. Uninoculated flasks spiked with imidacloprid acted as control. Quantification of pesticide was done by HPLC using FID detector. |
| Biodegradation of pesticides in soil amended with different waste material |
| For this experiment, soil was collected from Breeder Seed Production Centre (BSPC) of GBPUAT Pantnagar and autoclaved 3 times. Amendments used for biodegradation study were Farm Yard Manure, Hen Manure and Bagasse which were collected from Kichha, Pantnagar and Nagla respectively. Biodegradation experiment was conducted with soil in the presence of three amendments in different sets. Fifty gm soil was taken in 16 sets of the flasks with control (Table-1). Each flask was supplemented with one type of amendment (1.25 gm). Imidacloprid (20 ppm) was mixed properly with soil and amendment in each flask. After mixing, 2 ml of 48 hr old homogenized culture of each fungal isolate was inoculated in each flask except control. In one set, mixture of both the fungi FII and FIII (1 ml of each culture) was added. Ingredients of the flask were mixed properly and autoclaved water (13%) was added in each flask. Flasks were plugged with cotton plugs for aseptic condition and aeration. Samples were extracted after 3, 6 and 15th day of incubation and residual pesticide was quantified by HPLC. (Table 1) |
| Extraction and cleanup of imidaclopridfrom soil |
| Five gram soil was mixed with 20 ml acetone in a 50 ml flask and shaken for 1 hr and then 2 ml of sample was centrifuged at 10000 rpm for 10 min. Supernatant (1 ml) was transferred to Buchner funnel and mixed with 1gm of sodium sulfate and 1 ml of hexane. After the formation of two separate layers, bottom layer wayts discarded and remaining part was collected in a round bottom flask and left overnight for drying. To the mixture, 2 ml acetonitrile was added and mixed properly. Mixture was filtered through microbial filter. The extracted solution was collected in the eppendorf tube and analyzed by HPLC. |
| Molecular characterization of pesticide degrading fungal isolates |
| Extraction of genomic DNA of selected fungal isolates was done by the methods of Samarrai (1999). Extracted genomic DNA was electrophoreses on 0.8% agarose (W/V) and further amplified in a thermal cycler using universal ITS primer. NCBI BLAST was performed to find out homology and phylogenetic position of the fungi. |
| Results and Discussion |
| Ten fungal isolates were recovered from the pesticide contaminated soil. Among the recovered fungi, FII and FIII were potent pesticide degraders. Both the isolates grew on Imidacloprid (upto 340 ppm for FII and 350 ppm for FIII, whereas rests were unable to grow even up to 50 ppm of imidacloprid. |
| Biodegradation of pesticides in minimal medium |
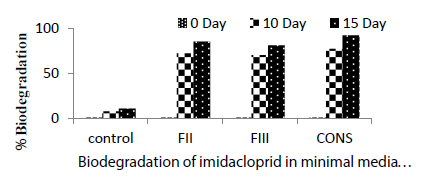
| Degradation of the pesticide at 0 day was 1% in all the treatments including control. Control showed abiotic degradation of the pesticide, which was substracted from the total degradation. Highest degradation of imidacloprid was observed on 10th day in consortium treatment (77%). FII showed 72% degradation followed by FIII (70%) and control (8%) (Table2). On 15th day highest degradation of imidacloprid was reported in consortium (92%) followed by FII (85%), FIII (81%) and control (11%). Maximum degradation (92%) of imidacloprid was observed in liquid medium in the presence of consortium of FII and FIII. All the treatments performed significantly better than the control. Negi et al. [9] reported that microbial consortium showed maximum biodegradation of endosulfan, imidacloprid and carbendazim. Negi et al. [9] also reported that the microbial consortium performed better biodegradation as compared to individual strain. |
| Highest degradation of the pesticide in fungal consortium shows the cometabolism where two or more individual utilize a compound in a sequential way. Mohapatra [10] have done similar type of biodegradation study with Imidacloprid in soil by using the fungal strain Calocybeindica. They observed that the fungus accelerated degradation of imidacloprid in soil to a great extent and at the lower concentration it was more pronounced. After 40 days of incubation 54.7, 46.2 and 34.0% imidacloprid was degraded by C. indica, inoculated in soil as compared to 27, 24.5 and 23% in uninoculated soil at 0.2, 2 and 20μg/ gm of imidacloprid respectively. (Figure 1) (Table 2) |
| Autolysis/Hydrolysis could be one of factors responsible for abiotic degradation as observed in control. Maximum biotic degradation was observed in the consortium,the pattern was: consortium( 81% )>FII( 74%)>FIII( 70% ). (Figure 1) |
| Biodegradation of imidacloprid by immobilized fungal cultures in alginate bead and agar discs |
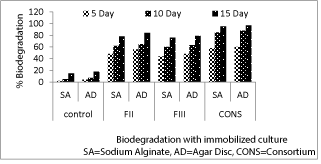
| After 5 days of incubation, highest degradation (60%) of imidacloprid was observed in the consortium immobilized in agar disc followed by sodium alginate bead (55%). Similarly on 10th day, maximum degradation (88%) for the same pesticide was in the consortium treatment immobilized in agar discs. After 15 days, maximum degradation of imidacloprid was (95%) and (97%) in consortium immobilized in sodium alginate and agar discs respectively. After 15 days pesticide degradation in sodium alginate bead was highest in consortium (95%) followed by FII(78%), FIII(76%) and control (15%). In case of agar discs after 15 days maximum degradation was observed in consortium (97%) followed by FII (84%), FIII(79%) and control(18%).(Figure 2) |
| Effect of amendment of organic wastes on biodegradation of imidacloprid in soil. |
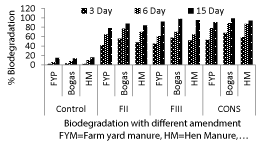
| On 3rd day highest degradation (68%) was observed with consortium, amended with bagasse followed by consortium amended with Hen Manure (59%) and consortium amended with Farm Yard Manure(53%) (Figure 3). After 6 day of incubation highest degradation was observed in consortium amended with bagasse (89%) followed by consortium amended with Hen Manure (87%) and consortium amended with Farm Yard Manure (78%). Similar trend but higher degradation of imidacloprid was observed in 15th days. Highest degradation was found in consortium amended with bagasse (99%) followed by consortium amended with Hen Manure (94%) and consortium amended with Farm Yard Manure (91%). (Figure 3) (Table 3) |
| Molecular characterization of pesticide degrading fungal isolates |
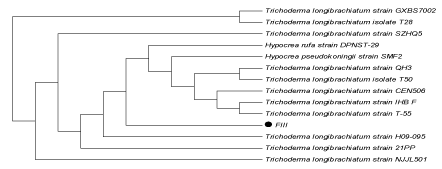
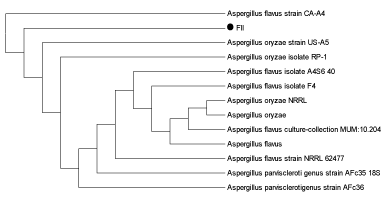
| DNA (ITS region) of both the fungal isolates was amplified using universal primers (T1 and T4) and a distinct band of approximately 750 bp was observed, confirming the preserved ITS region in the fungal strain.On the basis of sequencing of amplified products ITS region of the two fungal isolates FII and FIII showed 100% similarity with Aspergillusoryzae and Trichodermalongibrachiatum respectively. (Figures 4a and 4b) |
| The results showed degradation of imidacloprid was even better than the report by Mohapatra et al. [10] which was 54.7% in 40 days, while in the present study the isolates degraded 92% imidacloprid within 15 days including abiotic degradation. The prolific growth during incubation period (10 to 15 days) could be responsible for higher degradation. Degradation by FII and FIII was comparable and was 9 times than the control. The greatest degradation was observed in consortia (77%) followed by FII (72%), FIII (70%) and control (8%). A minor change in percent degradation of the pesticide in the presence of agar disc and sodium alginate was due to the better interaction and compactness of the material used for immobilization as differernt materials have different interaction with microbial cells. Present experiments address the bioremediation of these herbicides present relatively at high concentrations with simple, low-cost techniques that would be suitable for on-site use. The amendments used in this study were chosen because of their low cost and wide availability. Bayo et al. [11] reported imidacloprid degradation with vermi compost and found that around 78% of imidacloprid remained in the soil. This study suggested that combination of fungal isolates may increase the biodegradation of imidacloprid. Similarly Cox et al. [12] reported the influence of organic amendments on sorption and dissipation of imidacloprid in soil. Increased imidacloprid sorption by the soil was observed when amended with organic matter. Moorman et al. [13] used organic amendments to enhance herbicide degradation in contaminated soils. The use of available organic amendments, such as compost, plant residues, green manure and fertilizers, may be an effective, non-chemical way of improving the pesticidal efficacy of solarization. Combining solarization with organic amendments has a significant potential for improving pathogen control and crop production, especially when solarization alone cannot provide adequate control of the target pathogens [14]. |
| Interest in the microbial biodegradation of pollutants has intensified in recent years as mankind strives to find sustainable ways to clean up contaminated environments and the present study is significant in relation to reduce environmental pollution. The elimination of a wide range of pollutants and wastes from the environment is an absolute requirement to promote a sustainable development of our society with low environmental impact. Biological processes play a major role in the removal of contaminants but even among them fungus is most efficient as they take advantage of the astonishing catabolic versatility of microorganisms to degrade/convert such compounds. The recovered fungal strains and their consortium appear to be suitable candidates for use in the bioremediation of pesticides contaminated sites along with plant growth promotion. The present study can be further extended to functional genomics and metabolomics which can provide the clues about the metabolic pathways and regulatory networks. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4a | Figure 4b |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14354
- [From(publication date):
September-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9837
- PDF downloads : 4517
