Mycobacterium tuberculosis HtdY, a Novel Immunostimulatory Antigen, Drives Th1-type T Cell Immunity via TLR4-mediated Activation of Dendritic Cells.
Received: 03-Aug-2021 / Accepted Date: 07-Sep-2021 / Published Date: 14-Sep-2021 DOI: 10.4172/1165-158X.1000205
Abstract
Mycobacterium tuberculosis (MTB), the etiological factor of tuberculosis (TB), is among the most successful of intracellular pathogen that regulates the host immune response. Cellular immune responses perform a pivotal function in host defence against MTB. Thus, it is vital to understand the antigens which drive immune protection, especially Th1-type cellular immunity. The role of 3-hydroxyacyl-l-thioester dehydratase (HtdY, Rv3389c) of MTB in immunological protection was observed in dendritic cell (DC) activation and T cell immunity herein. Recombinant HtdY was applied for inducing maturation and activation of DCs obtained from murine bone marrow. TLR4 and TLR2 knockout mice and pharmacological inhibitors were used to investigate the mechanism by which HtdY activates DCs. MLR assay was performed to characterize HtdY activity with respect to DC activation and T cell polarization. The alteration of cytokines secretion by human PBMCs elicited by HtdY was observed. We found that HtdY prompted DC maturation and activation through augmenting the expression of surface molecule CD80, CD86 and MHC II, and the release of pro-inflammatory cytokines including IL-1β, IL-6, IL-12 and TNF-α from DCs. HtdY-drived DC activation involved TLR4 activation and mitogen-activated protein kinases (MAPKs) signaling pathway. DCs treated with HtdY induced naïve CD4+ T cells to produce IFN-γ. Human PBMCs had higher TNF-α and lower IL-10 response to HtdY in active TB group than that in control group (p<0.01). Our results indicated that HtdY possesses potential to drive Th1-type cellular immunity by TLR4- mediated activation of DCs.
Keywords: Mycobacterium tuberculosis; HtdY; DC maturation; TLR4; Th1 polarization; IL-10
Introduction
Mycobacterium tuberculosis (MTB), the pathogen of tuberculosis (TB), is estimated to infect nearly one-third of the world’s population and accounted for about 1.5 million deaths globally in 2018 [1]. MTB is among the most successful intracellular bacterium which adapt to the immune system of human and cellular immunity have crucial function in host defense against MTB [2]. Dendritic cells (DCs) are the most effective antigen-presenting cells (APCs) to activate naïve T cells [3-5]. As DCs capture and process MTB antigens, they present antigens to naïve CD4+ T cells in an MHC-II dependent manner, which bridges the innate and adaptive immunity [5-8].
Polarization of naïve CD4+ T cells drived by mature DCs relies on the ligation of pattern recognition Receptors (PRRs) during MTB antigens recognition, among which TLR2 and TLR4 play a crucial role [9-12]. Activation of NF-κB and MAPK signal pathways results in the higher expression of the surface molecules including MHCII and CD80, CD86, and various immune-regulatory cytokines, which could polarize the naïve CD4+ T cells for adaptive immunity [13-15]. The polarized Th1, Th2, Th17, Treg and other Th immune responses regulate the anti-MTB immunity cooperatively, among which Th1-biased T cell responses are essential by producing critical cytokines like IFN-γ and TNF-α [16]. In previous work, 3-hydroxyacyl-1-thioester dehydratase Y (HtdY) screened from the cultured filtrate proteins (CFPs) of MTB clinical isolate by dynamic immune-proteomics was identified as a unique immune regulatory protein [17]. HtdY is encoded by Rv3389c and related to the biosynthesis of major and requisite lipids such as cell wall mycolic acids [18-20]. Our results indicated that mycobacterial HtdY stimulated humoral immunity and up-regulated COX-2 expression in mouse macrophages [17]. These results highlighted the potential of HtdY for TB vaccines and novel anti-TB drugs. However, cellular immunity elicited by HtdY has never been reported yet. In this research, we elucidated the mechanism of DC maturation and activation and the polarized T cell responses induced by HtdY.
Materials and Methods
Mice
Female TLR2-/-, TLR4-/- and wild type (WT) C57BL/6 mice of 6-8 weeks age were obtained from the Jackson Laboratory (Bar Harbor, ME) and Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China), respectively. Mice were maintained under specific pathogen-free conditions at Department of Laboratory Animal Science, School of Medicine, Shanghai Jiao Tong University (China). All experimental procedures were approved by the Ethics Committee at School of Medicine, Shanghai Jiao Tong University.
Subjects
All subjects were 21-68 years old from Henan Provincial Chest Hospital. Bacteriological positive TB patients had positive IGRA responses (Beijing Wantai Biological Pharmacy Enterprise CO., LTD., Beijing, China) and positive sputum smear examination or cultures or positive molecular detections by real-time PCR (MeltPro®, Xiamen Zeesan Biotech Co., Ltd., Fujian, China). Bacteriological negative TB patients had positive IGRA responses but negative sputum smear examination or cultures for 3 times or negative molecular detections. All had the history of receiving BCG vaccine in the past. Blood was obtained before or no later than 1 week of chemotherapy of TB. All subjects were free of diabetes, HIV, HBV or HCV infection, hepatic or renal dysfunction. Healthy controls were from Physical Examination Center, Henan Provincial Chest Hospital with normal chest radiography and no TB clinical symptoms or close contact with TB patients. Written informed consents were acquired from all subjects, and the investigation received approval from the Ethics Committee at School of Medicine, Shanghai Jiao Tong University.
Expression and purification of recombinant HtdY
The recombinant HtdY (accession no. NP_217906) was amplified by PCR from MTB H37Rv genomic DNA using the following primers: forward, 5′-TTATCCATGGCGATTGATCCGAACTCC-3′ (NcoI) and reverse, 5′-TATTAAGCTTCTAACCCGCCACGTACTCCAC-3′ (Hind-III). The product was ligated into the pET32a vector as described [17]. The recombinant clone was identified by DNA sequencing and transformed into E. coli BL21. The recombinant HtdY was purified using Ni-NTA resin after cell disruption by sonication. The endotoxin was removed by phase separation in Triton X-114. LAL assay (Chinese Horseshoe Crab Reagent Manufactory, Xiamen, China) was performed to determine the endotoxin residual in the HtdY preparation. BCA kit (Pierce, Rockford, IL, USA) was used to analyze the protein concentration. The purity of HtdY was evaluated by SDS-PAGE.
Generation and culture of murine bone marrow-derived DCs
Murine bone marrow was obtained from the tibiaes and femurs of WT, TLR2-/- and TLR4 -/- C57BL/6 mice. Bone marrow cells were treated with red blood cells lysis buffer and washed with RPMI1640. The cells were plated in 6-well culture plates (107 cells/ml; 3 ml/well) in RPMI1640 (Life Technologies, Grand Island, USA) supplemented with 10% heat-inactivated fetal bovine serum (FCS, Life Technologies, Grand Island, USA), 100 U/ml penicillin, 100μg/ml streptomycin, 50M β-mercaptoethanol, 50 ng/ml GM-CSF (R&D systems) and 50 ng/ml IL-4 (R&D systems) at 37°C in the presence of 5% CO2. Culture medium was changed on day 3. On day 6, the suspended and loosely adherent DC clusters were collected. The DCs underwent positive selection on paramagnetic columns (LS columns; Miltenyi Biotec) after marked with bead-conjugated anti-CD11c mAb (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions.
Treatment of DCs with recombinant HtdY and flow cytometry analysis of surface molecule expression
DCs were cultured in 10 μg/mL of HtdY for 24 h with 100 ng/ml of LPS serving as a positive control. Cells were collected, rinsed with fluorescence activated cell sorting (FACS) solution buffer and stained with FITC-conjugated anti-CD11c, PE-conjugated anti-MHC II, PE-Vio770-conjugated anti-CD80 and APC-conjugated anti-CD 86 (Biolegend, San Diego, CA, USA) at 4°C for 20 min. Cells were rinsed once with 2 ml FACS solution buffer and resuspended in 100 μl FACS solution buffer. The fluorescence was detected by flow cytometry (FACS Calibur, Becton Dickinson, San Jose, CA, USA) and the data were analyzed using BD Cell-Quest Pro data analysis software.
Measurement of cytokines
The cytokine concentrations in culture supernatants were analyzed by ELISA kits (mouse IFN-γ; human IL-2, IL-4, IL-10, IL-17, TNF-α and IFN-γ, eBioscience, San Diego, CA, USA; mouse IL-6, IL-12p70, IL- 1β, IL-10 and TNF-α, R&D Systems), according to the manufacturer’s instructions.
Treatment of DCs with pharmacological inhibitors of signaling pathways
All pharmacological inhibitors were obtained from Cayman. The cultures contained 0.1% (v/v) dimethyl sulfoxide (Millipore, Sigma, St Louis, MO, USA) as a solvent control. Prior to incubation with HtdY (10 μg/ml) for 24 h, DCs were rinsed with PBS and pre-incubated with inhibitors in RPMI 1640 medium for 1h. The concentrations of inhibitors after careful titration were as follows: U0126, 10 μM; SB203580, 20 μM; SP600125, 10 μM; and Bay11-7082, 20 μM.
Mixed lymphocyte reaction
Murine lymphocytes were prepared from spleen utilizing EZSep™ mouse 1× Lymphocyte Separation Medium (Dakewe Biotech, Shenzhen, China) according to manufacturer’s instructions. Naïve CD4+ T cells were marked with both CD4 and CD62L Micro Beads and separated by MACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany) from entire lymphocytes. DCs were incubated with 10 μg/ml HtdY for 24 h before washing with PBS and co-cultured with naïve CD4+ T cells (2×106) at a DC: T cell ratio of 1: 10. The cells and supernatants were collected respectively after 48 h of co-culturing.
Human peripheral blood mononuclear cells (PBMCs) isolation and activation
Heparinized venous blood samples suspended in PBS were isolated by Ficoll-Hypaque (Dakewe, Beijing, China) density gradient centrifugation at 2000 rpm for 20 min. PBMCs were collected, washed twice with PBS and re-suspended at a concentration of 5×105 cells/ml in complete RPMI 1640 medium added with 10% heated-inactivation FCS (both obtained from Life Technologies, Grand Island, USA), 100 U/ml penicillin and 100 μg /ml streptomycin. PBMCs plated into 12- well plates were cultured for 1-2 h, and stimulated with 10 μl HtdY at a final concentration of 10 μg/ml for 48 h in 5% CO2 at 37ºC. The supernatant was harvested for cytokines assay by ELISA.
Statistical analysis
Each experiment was repeated three times and the data were presented as mean ± SEM. Statistical analyses were performed using Student’s t test and one-way ANOVA followed by multiple comparisons among the groups. GraphPad Prism software (Version 8.01; GraphPad software, San Diego, CA, USA) was used for all statistical analyses. Differences were considered to be significant at p< 0.05.
Results
The recombinant HtdY protein induces maturation and activation of mouse DCs
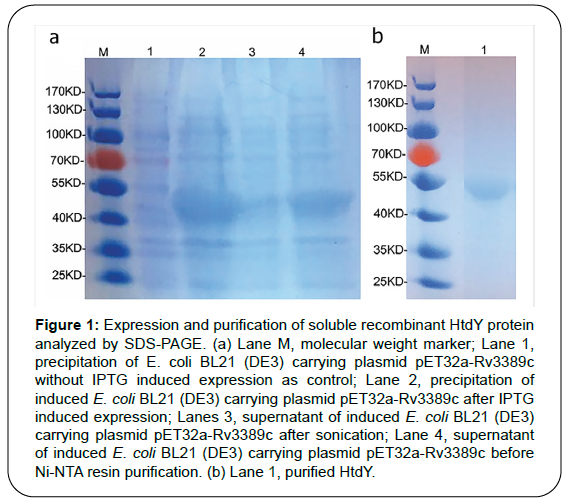
Recombinant HtdY was confirmed by SDS-PAGE with a molecular mass approximating 47 KDa (Figure 1). HtdY preparation contained typically less than 6 pg/mL (Figure 2a. In addition, 10 μg/mL HtdY had similar effect to 100 ng/mL LPS on up-regulation of these surface markers on DCs.
Figure 1: Expression and purification of soluble recombinant HtdY protein analyzed by SDS-PAGE. (a) Lane M, molecular weight marker; Lane 1, precipitation of E. coli BL21 (DE3) carrying plasmid pET32a-Rv3389c without IPTG induced expression as control; Lane 2, precipitation of induced E. coli BL21 (DE3) carrying plasmid pET32a-Rv3389c after IPTG induced expression; Lanes 3, supernatant of induced E. coli BL21 (DE3) carrying plasmid pET32a-Rv3389c after sonication; Lane 4, supernatant of induced E. coli BL21 (DE3) carrying plasmid pET32a-Rv3389c before Ni-NTA resin purification. (b) Lane 1, purified HtdY.
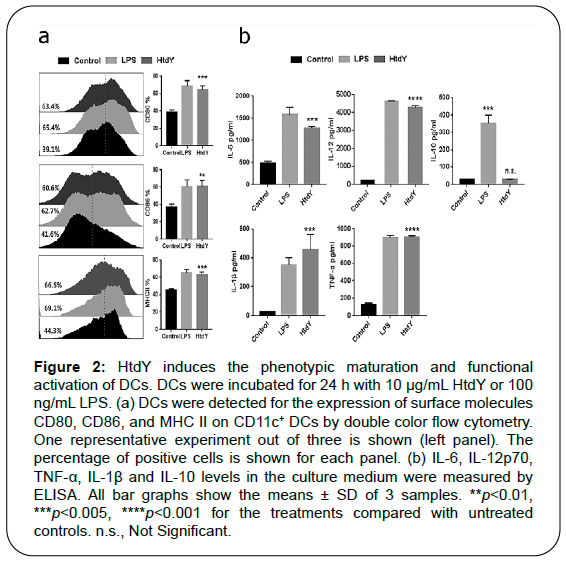
Figure 2: HtdY induces the phenotypic maturation and functional activation of DCs. DCs were incubated for 24 h with 10 μg/mL HtdY or 100 ng/mL LPS. (a) DCs were detected for the expression of surface molecules CD80, CD86, and MHC II on CD11c+ DCs by double color flow cytometry. One representative experiment out of three is shown (left panel). The percentage of positive cells is shown for each panel. (b) IL-6, IL-12p70, TNF-α, IL-1β and IL-10 levels in the culture medium were measured by ELISA. All bar graphs show the means ± SD of 3 samples. **p<0.01, ***p<0.005, ****p
To examine whether HtdY-mediated DC phenotypic maturation was associated with functional activation, we then analyzed the release of pro- and anti-inflammatory cytokines (Figure 2b). As expected, we found that HtdY-stimulated DCs secreted extensive amount of pro-inflammatory cytokines including TNF-α, IL-1β and IL-6, while these cytokines secreted by unstimulated DCs was negligible. We next examined the release of IL-12p70 and IL-10, which prompt the proliferation and differentiation of Th1 and Th2 cells separately. Data showed that HtdY significantly raised the secretion of IL-12p70 without affecting that of IL-10. These results revealed that HtdY mediates the functional maturation of DCs and DCs matured by HtdY might drive Th1-type immunity.
HtdY protein induced DC maturation via TLR4
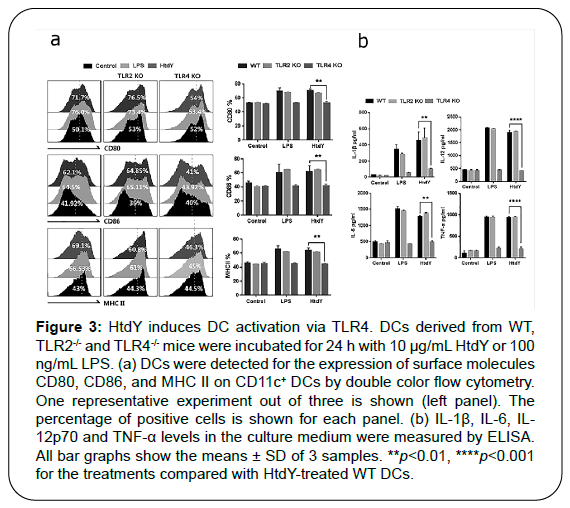
DCs can be activated by the interplays between the pattern recognition receptors (PPRs), including Toll-like receptors (TLRs), and pathogen-associated molecular patterns (PAMPs) of MTB secretory antigens and cell-wall compositions. Various MTB proteins that activate DCs via TLR2 or TLR4 have been identified [13-16]. To investigate whether HtdY could be recognized by TLR2 or TLR4 on DCs, we determined the surface molecules expression and pro-inflammatory cytokines production of WT, TLR2–/–, and TLR4–/– DCs treated by HtdY. The expression of surface molecules including CD80, CD86 and MHC class II (Figure 3a) as well as pro-inflammatory cytokines IL-1β, IL-6, IL-12p70 and TNF-α release (Figure 3b) were raised in WT and TLR2–/– DCs while abrogated in TLR4–/– DCs, demonstrating that HtdY might be a TLR4 activator in mouse DCs.
Figure 3: HtdY induces DC activation via TLR4. DCs derived from WT, TLR2-/- and TLR4-/- mice were incubated for 24 h with 10 μg/mL HtdY or 100 ng/mL LPS. (a) DCs were detected for the expression of surface molecules CD80, CD86, and MHC II on CD11c+ DCs by double color flow cytometry. One representative experiment out of three is shown (left panel). The percentage of positive cells is shown for each panel. (b) IL-1β, IL-6, IL- 12p70 and TNF-α levels in the culture medium were measured by ELISA. All bar graphs show the means ± SD of 3 samples. **p<0.01, ****p
Activation of the MAPK pathway mediates HtdY-induced DC maturation
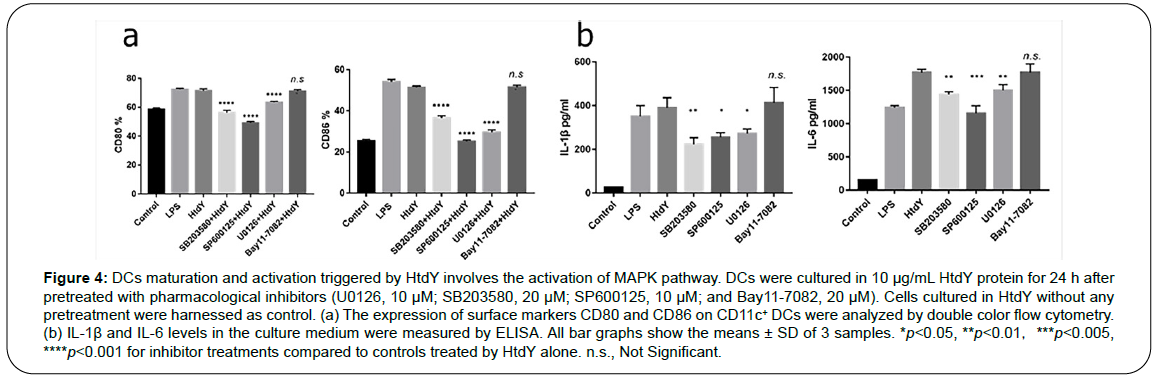
MAPK and NF-κB signaling pathways play critical roles in mediating the DCs maturation. To identify the involvement of these kinases in HtdY-drived maturation of DCs, we utilized extremely selective pharmacological inhibitors to analyze HtdY-induced expression of co-stimulatory molecules. In particular, Cells were pre-incubated with a p38 inhibitor (SB203580), an ERK1/2 inhibitor (U0126), a JNK inhibitor (SP600125), or an NF-κB inhibitor (Bay11-7082) for 1 h ahead of exposure to HtdY. Cells stimulated with HtdY without pretreatment by any inhibitors were harnessed as control. All the pharmacological inhibitors other than the NF-κB inhibitor significantly depressed the HtdY-drived expression of surface co-stimulatory molecules CD80 and CD86 (Figure 4a) and HtdY-drived release of IL-1β and IL-6 (Figure 4b) from DCs. These data preliminarily demonstrated that the MAPK signaling pathways play a significant role in optimal HtdY-induced DC maturation and activation.
Figure 4: DCs maturation and activation triggered by HtdY involves the activation of MAPK pathway. DCs were cultured in 10 μg/mL HtdY protein for 24 h after pretreated with pharmacological inhibitors (U0126, 10 μM; SB203580, 20 μM; SP600125, 10 μM; and Bay11-7082, 20 μM). Cells cultured in HtdY without any pretreatment were harnessed as control. (a) The expression of surface markers CD80 and CD86 on CD11c+ DCs were analyzed by double color flow cytometry. (b) IL-1β and IL-6 levels in the culture medium were measured by ELISA. All bar graphs show the means ± SD of 3 samples. *p<0.05, **p<0.01,***p<0.005, ****p
HtdY-stimulated DCs induce Th1 immune responses
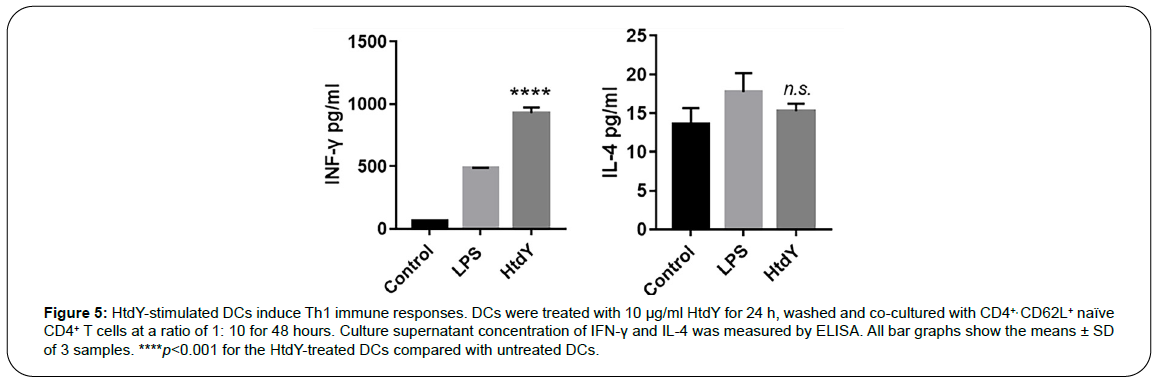
The cytokines secreted from specific antigen elicited maturation and activation of DCs facilitate the polarization of naive CD4+ T cells towards different Th responses. Therefore, we performed MLR assay to characterize HtdY activity with respect to DCs and T-cell interactions. Naive CD4+ T cells modulated by HtdY-treated DCs produced larger amounts of IFN-γ than did those modulated by untreated DCs, while IL-4 production had no difference. (Figure 5), suggesting that HtdYinduced maturation and activation of DCs contribute to naive CD4+ T cells polarization towards Th1 immune reactions.
Figure 5: HtdY-stimulated DCs induce Th1 immune responses. DCs were treated with 10 μg/ml HtdY for 24 h, washed and co-cultured with CD4+, CD62L+ naïve CD4+ T cells at a ratio of 1: 10 for 48 hours. Culture supernatant concentration of IFN-γ and IL-4 was measured by ELISA. All bar graphs show the means ± SD of 3 samples. ****p
The response of cytokines in HtdY-stimulated human PBMCs
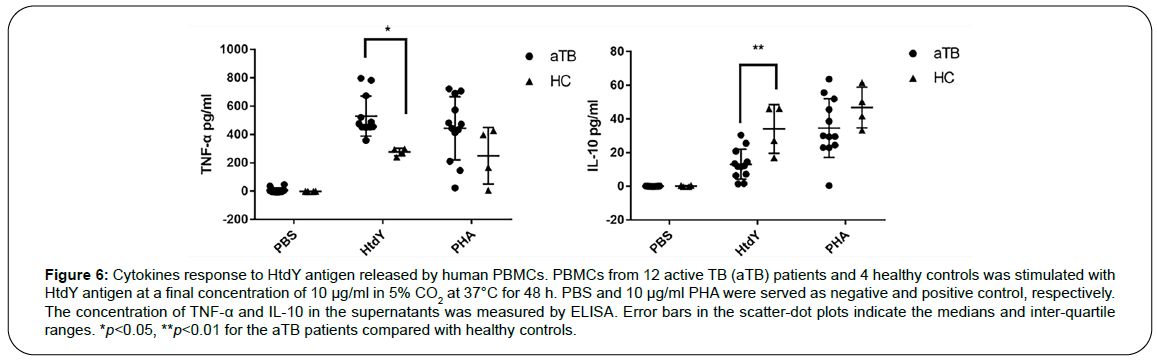
To further analyze whether HtdY could alter specific cellular immune response in PBMCs from TB patients, cytokines associated with TB were screened with active TB patients and healthy controls. The level of IFN-γ, IL-2, IL-4, IL-12 and IL-17 in TB group did not change significantly after HtdY stimulation (data not shown), and TNF-α had a higher response while IL-10 had a lower response to HtdY in TB group compared to healthy control group (Figure 6). These data suggested that HtdY might trigger Th1 cellular immunity in human PBMCs.
Figure 6: Cytokines response to HtdY antigen released by human PBMCs. PBMCs from 12 active TB (aTB) patients and 4 healthy controls was stimulated with HtdY antigen at a final concentration of 10 μg/ml in 5% CO2 at 37°C for 48 h. PBS and 10 μg/ml PHA were served as negative and positive control, respectively. The concentration of TNF-α and IL-10 in the supernatants was measured by ELISA. Error bars in the scatter-dot plots indicate the medians and inter-quartile ranges. *p<0.05, **p
Discussion
The potential of the host to restrain MTB infection relies on the ability of the innate immune cells, especially professional antigenpresenting cells like DCs, and macrophages to initiate a promptly and efficient adaptive T cell immunity [2,21]. There are considerable concerns that mycobacterium components such as Rv0577 and Rv0315 played vital parts in driving cell-mediated immune reaction against MTB infection [22,23]. Based on our research, HtdY could prompt DC maturation and activate DCs by reinforcing the expression of MHC II, CD 80 and CD 86 surface markers. DCs undergoing maturation can differentiate into exceptionally antigen presenting cells with the ability to activate naïve T cells. Moreover, HtdY augmented the release of the pro-inflammatory cytokines including IL-1β, IL-6, IL-12 and TNF-α by DCs.
Further, we found that HtdY treated DCs were activated through TLR4 by experiment on TLR4 knock-out mice. The presence of numerous PRRs, such as Toll like receptors on DCs generally promotes host immunity and allow specialized APCs to promptly recognize encroaching pathogens and increasing the expression of surface costimulatory molecules and inflammatory and regulatory cytokines as well [24], both of which influence significantly on the consecutive progress of T cell immune responses.
We crudely investigated that the MAPK signaling pathways are critical for HtdY-mediated DC activation, arousing the expression of phenotypic markers for DC maturation as well as production of proinflammatory cytokines. Further work needs to be done to observe the phosphorylation of MAPKs induced by HtdY. Defensive immunity against intracellular pathogens such as MTB relies particularly on cellular immunity implemented by powerful anti-infectious performance of Th1 subset of CD4+ T cells [25-27]. Th1 cell are featured by the secretion of their signature cytokine, IFN-γ, and this cytokine is an important mediator for sequestering MTB growth, intra-endosomally achieving microbial killing, and perhaps more importantly, aiding the formation and maturation of the granuloma [28]. In addition, IL-6 and IL-12 are demanded for an effective priming of an IFN-γ response, accelerating the proliferation of naïve T cells with increased level of IFN-γ. Thus, HtdY is a novel DC agonist as well as for the occurrence of protective immunity against MTB, and IL-6 facilitates cytokine generation in differentiated Th1 cells [29]. In this study, we revealed that HtdY treated DCs released high level of IL-12, which is the major factor driving Th1 reactions; while the release of IL-10, a common inhibitor of IL-12 [30], was not raised. Furthermore, DCs maturation and activation induced by HtdY antigen drived T cell immune responses toward Th1 polarization.
TNF-α was higher in TB group than that in control after PBMCs stimulated by HtdY. TNF-α is a critical Th1 pro-inflammatory cytokine governing TB pathogenesis and induced in the early MTB infection phase [31]. The early production of this cytokine is required to modulate granuloma biogenesis and integrity [32,33]. IL-10 expression is elevated in TB patients and is secreted by almost all lymphocytes as an anti-inflammatory and immune-suppressive cytokine [34,35]. It inhibits the production of NO and other bactericidal substances as well as pro-inflammatory cytokines and suppresses the expression of co-stimulatory factors by macrophages [36,37]. IL-10 can prevent the production of IL-12 by DCs and inhibit Th1 polarization [30]. It can also regulate the cytokines involved in the induction of CD4+ T cell differentiation in vivo and disrupts the ratio of Th1 and Th2 cells, increasing MTB susceptibility, immune escape and persistence [38]. In our study, IL-10 response of PBMCs was lower after stimulated by HtdY in TB group, indicating that HtdY might also be immune-protective by inhibiting Th2 immune response to boost Th1 immunity during MTB infection. However, cytokine responses of PBMCs to HtdY stimulation needs to be further observed taking inadequate samples and the inherent variability of human immune responses into account.
Conclusion
This research demonstrated that HtdY has important functions in the activation and maturation of DCs and commencement of adaptive immune responses by polarizing the T cells developing to a Th1 response via TLR4 activation of dendritic cells, among which MAPK signaling pathways might play a significant role. It provides a reference for further investigation of MTB pathogenesis as well as the development of preventive strategies or diagnostic reagent for MTB infection.
Funding
This project was funded by the Thirteen-Fifth Mega Scientific Project “Prevention and treatment of AIDS, viral hepatitis and other infectious diseases” of China [2017ZX10201301-003-003], Application Basis and Frontier Project 2020 in Wuhan, China [2020.2.6.1.1223] and National Natural Science Foundation of China [81871613].
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Availability of Data and Materials
The data and material used to support the findings of this study are available from the corresponding authors upon request.
Ethics Statement
The animal experimental procedures were approved by the Ethics Committee in School of Medicine, Shanghai Jiao Tong University. Written informed consents were acquired from all subjects, and the investigation received approval from the Ethics Committee in Henan Provincial Chest Hospital (Permit number: 20190915004).
References
- WHO.nWHO Global Tuberculosis Report.nWorld Health Organization Press., 2019.
- Cooper, A. M.nCell-mediated immune responses in tuberculosis.nAnnu Rev Immunol., 2009;27(1): 393-422.
- Cohn, Z. A., Lustig, D. S., & Steinman, R. M.nIdentification of a novel cell type in peripheral lymphoid organs of mice,nFunctional properties in vivo.nJ Exp Med., 1974;139(6): 1431-1445.
- Belz, G. T., & Nutt, S. L.nTranscriptional programming of the dendritic cell network.nNat Rev Immunol., 2012;12(2): 101-113.
- Reis, E., & Sousa, C.nDendritic cells as sensors of infection.nImmunity., 2001;14(5): 495-498.
- Liu, Y. J., & Shortman, K.nMouse and human dendritic cell subtypes.nNat Rev Immunol., 2002;2(3): 151-161.
- Borel, S., Gutierrez, M. G. & Lerner, T. R.nThe innate immune response in human tuberculosis.nCell Microbiol., 2015;17(9): 1277-1285.
- Lei, H., Sun, Z., Wu, Q., Yu, X., Zheng, H., Zhou, F., et al.nMannosylated structures of mycobacterial lipoarabinomannans facilitate thenmaturation and activation of dendritic cells. CellnImmunol., 2019;335: 85-92.
- Boom, W. H., & Harding, C. V.nRegulation of antigen presentation by Mycobacterium tuberculosis: A role fornToll-like receptors.nNat Rev Microbiol., 2010;8(4): 296-307.
- Chung, Y., Dong, C., Martinez, G. J., & Reynolds, J. M.nToll-like receptor 4 signaling in T cells promotes autoimmune inflammation.nProc Natl Acad Sci USA., 2012;109: 13064-13069.
- Dziarski, R., Golenbock, D., Ingalls, R. R., Lien, E., Tuomanen, E., &nYoshimura, A.nCutting edge: Recognition of Gram-Positive bacterial cell wall components bynthe innate immune system occurs via Toll-like receptor 2.nJ Immunol., 1999;163(1): 1-5.
- Cha, S. B., Choi, S. Y., Cho, S. N., Han, S. J., Kim, W. S., Kim, J. S., et al. Mycobacterium tuberculosis Rv3628 drives Th1-type T cell immunity vianTLR2-mediated activation of dendritic cells and displays vaccine potentialnagainst the hyper-virulent Beijing K strain.nOncotarget., 2016;7(18): 24962-24982.
- Back, Y. W., Choi, H. G., Choi, S., Cha, S. B., Choi, C. H., Kim, W. S., et al.nRv2299c, a novel dendritic cell-activating antigen of Mycobacteriumntuberculosis, fused-ESAT-6 subunit vaccine confers improved and durablenprotection against the hyper-virulent strain HN878 in mice.nOncotarget., 2017;8(12): 19947-19967.
- Bao, Y., Burton, J., Bao, L., Chen, W., Chen, X., Gong, X., Gu, D., & Mi, Y. Mycobacterium tuberculosis PE25/PPE41 protein complex induces activationnand maturation of dendritic cells and drives Th2-biased immune responses.nMed Microbiol Immunol., 2016;205(2): 119-131.
- Chen, H., Cui, Z., Ge, B., Liu, S., Liu, H., Wang, L., et al. Mycobacterium tuberculosis lipoprotein MPT83 induces apoptosis of infectednmacrophages by activating the TLR2/p38/COX-2 signaling pathway.nJ Immunol., 2017;198(12): 4772-4780.
- Mihret, A.nThe role of dendritic cells in Mycobacterium tuberculosis infection.nVirulence., 2012;3: 654-659.
- Chen, C. C., Guo, X. K., Liu, J., Sun, Z. Q., Samten, B., Wang, H. H., et al.nMycobacterial 3-hydroxyacyl-l-thioester dehydratase Y derived from Mycobacterium tuberculosis induces COX-2 expression in mousenmacrophages through MAPK-NF-κB pathway.nImmunol Lett., 2014;161(1): 125-132.
- Gurvitz, A., Hiltunen, J. K., & Kastaniotis, A. J.nHeterologous expression of mycobacterial proteins in Saccharomycesncerevisiae reveals two physiologically functional 3-hydroxyacyl-thioesterndehydratases, HtdX and HtdY, in addition to HadABC and HtdZ.nJ Bacteriol., 2009;191(8): 2683-2690.
- Daffé, M., Eynard, N., Guilhot, C., Legendre, V., Laval, F., Montrozier, H., et al.nRv3389C from Mycobacterium tuberculosis, a member of the (R)-speciï¬cnhydratase/dehydratase family.nBiochem Biophys Acta., 2007;1774(2): 303-311.
- Bhargavi, G., Balaji, S., Hassan, S., Palaniyandi, K., & Tripathy, S. P.nProtein-protein interaction of Rv0148 with Htdy and its predicted role towardsndrug resistance in Mycobacterium tuberculosis.nBMC Microbiol., 2020;20(1): 93-107.
- Cooper, A. M., & Torrado, E.nWhat do we really know about how CD4 T cells control Mycobacteriumntuberculosis?nPLoS Pathog., 2011;7(7): e1002196.
- Byun, E. H., Cho, S. N., Jung, I. D., Kim, W. S., Kim, J. S., Kim, H. J., et al. Mycobacterium tuberculosis Rv0577, a novel TLR2 agonist, inducesnmaturation of dendritic cells and drive Th1 immune response.nFASEB J., 2012;26(6): 2695-2711.
- Byun, E. H., Choi, Y., Kim, W. S., Kim, J. S., Kim, S. Y., Koh, W. J., et al.nRv0315, a novel immunostimulatory antigen of Mycobacterium tuberculosis,nactivates dendritic cells and drives Th1 immune responses.nJ Mol Med (Berl)., 2012;90: 285-298.
- Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y. J., et al.nImmunobiology of dendritic cells.nAnnu Rev Immunol., 2000;18: 767-811.
- Bean, A. G., Briscoe, H., Britton, W. J., Feng, C. G., & Hooi, H.nIncrease in gamma interferon-secreting CD8(+), as well as CD4(+), T cells in lungs following aerosol infection with Mycobacterium tuberculosis.nInfect Immun., 1999;67: 3242-3247.
- Chan, J., & Flynn, J. L.nImmunology of tuberculosis.nAnnu Rev Immunol., 2001;19: 93-129.
- Behboudi, S., Brookes, R., Goonetilleke, N., Hill, A. V., & McShane, H.nProtective immunity against Mycobacterium tuberculosis induced by dendriticncells pulsed with both CD8(+)- and CD4(+)-T-cell epitopes from antigen 85A.nInfect Immun., 2002;70(3): 1623-1626.
- Gallegos, A. M., Glickman, M. S., Pamer, E. G., Samstein, M., Su, X., & Heijst,nJ. W.nA gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo.nPLoS Pathog., 2011;7(5): e1002052.
- Andersen, P., Appelberg, R., Leal, I. S., & Smedegârd, B.nInterleukin-6 and interleukin-12 participate in induction of a type 1 protectivenT-cell response during vaccination with a tuberculosis subunit vaccine.nInfect Immun., 1999;67(11): 5747-5754.
- Bertolino, P., Britton, W. J., & Demangel, C.nAutocrine IL-10 impairs dendritic cell (DC)-derived immune responses tonmycobacterial infection by suppressing DC trafï¬cking to draining lymph nodesnand local IL-12 production.nEur J Immunol., 2002;32: 994-1002.
- Coccia, E. M., Etna, M. P., Giacomini, E., & Severa, M.nPro- and anti-inflammatory cytokines in tuberculosis: a two-edged sword in TBnpathogenesis.nSemin Immunol., 2014;26(6): 543-551.
- Dorhoi, A., & Kaufmann, S. H.nTumor necrosis factor alpha in mycobacterial infection.nSemin Immunol., 2014;26(3): 203-209.
- Jung, J. Y., Nau, G. J., & Robinson, C. M.nInterferon-γ, tumor necrosis factor, and interleukin-18 cooperate to controlngrowth of Mycobacterium tuberculosis in human macrophages.nCytokine., 2012;60(1): 233-241.
- Juffermans, N., Speelman, P., Verbon, A., Van, Deventer, S. J., Van, Deutekom,nH., & Van, Der, P.T.nSerum concentrations of cytokines in patients with active tuberculosis (TB)nand after treatment.nClin Exp Immunol., 1999;115(1): 110-113.
- Coffman, R. L., de Waal, M. R., Moore, K. W., & O’Garra, A.nInterleukin-10 and the interleukin-10 receptor.nAnnu Rev Immunol., 2001;19: 683-765.
- Lang, R.nTuning of macrophage responses by Stat3-inducing cytokines: molecularnmechanisms and consequences in infection.nImmunobiology., 2005;210: 63-76.
- Murray, P. J.nUnderstanding and exploiting the endogenous interleukin-10/STAT3-mediatednanti-inflammatory response.nCurr Opin Pharmacol., 2006;6(4): 379-386.
- Ghosh, S., Hasnain, S. E., Joshi, D. C., Mande, S. C., Mukhopadhyay, S., Nair,nS., et al.nThe PPE18 of mycobacterium tuberculosis interacts with TLR2 and activatesnIL-10 induction in macrophage.nJ Immunol., 2009;183(10): 6269-6281.
Citation: Yu X, Li X, Hu P, Li Y, Zhou F, et al. (2021) Mycobacterium tuberculosis HtdY, a Novel Immunostimulatory Antigen, Drives Th1-type T Cell Immunity via TLR4-mediated Activation of Dendritic Cells. Cell Mol Biol 67: 205. DOI: 10.4172/1165-158X.1000205
Copyright: © 2021 Yu X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3718
- [From(publication date): 0-2021 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 2843
- PDF downloads: 875