Research Article Open Access
Multi-Enzyme Complex for the Management of Delayed Onset Muscle Soreness after Eccentric Exercise: A Randomized, Double Blind, Placebo Controlled Study
Vuppala KK*, Majeed M, Kumar AS, Majeed S and Vaidyanathan P
ClinWorld Private Limited, Peenya Industrial Area, Bangalore, Karnataka
- *Corresponding Author:
- Vuppala KK
M.Ph, ClinWorld Private Limited
Peenya Industrial Area
Bangalore, Karnataka
Tel: +917760956367
E-mail: kiran@clinworld.org
Received Date: September 24, 2016; Accepted Date: October 25, 2016; Published Date: November 01, 2016
Citation: Vuppala KK, Majeed M, Kumar AS, Majeed S, Vaidyanathan P (2016) Multi-Enzyme Complex for the Management of Delayed Onset Muscle Soreness after Eccentric Exercise: A Randomized, Double Blind, Placebo Controlled Study. Sports Nutr Ther 1: 113. doi: 10.4172/2473-6449.1000113
Copyright: © 2016 Vuppala KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Nutrition Science Research
Abstract
Background: Delayed onset muscle soreness (DOMS) results from muscle overload or strenuous exercise that goes beyond the intensity or duration for which the muscle is accustomed to performing. It is accompanied with the sensation of pain, tenderness, deep ache, and stiffness in muscles that usually begins several hours after the unaccustomed exercise. The aim of this study was to compare the efficacy of multi enzyme complex with a matching placebo in reducing pain associated with DOMS induced by standardized eccentric exercise. Methods: Twenty healthy males (10 pairs) were randomized in this double blind, placebo controlled trial to receive a placebo or multi enzyme complex capsule (50 mg) thrice a day for a period of 3 days. Mean differences within the group and between groups were assessed at each data collection time-point using Analysis of Covariance (ANCOVA) and Wilcox on signed rank sum test for all outcome measures. Results: In this controlled clinical study, intake of multi enzyme complex for 3 days resulted in no statistically significant changes in the descriptive statistics and efficacy analysis in muscle power and grip strength measured by hand held dynamometer. Algometer readings of thigh muscle, showed statistical significance (p<0.043). Decrements were observed in McGill Pain Questionnaire showing high statistical significance. Reducing trend was observed in bio markers of muscle damage (creatine kinase and lactate dehydrogenase) as well. Conclusion: The study results suggest that compared to placebo, Multi enzyme complex supplementation improves the outcome measures related to DOMS induced by standardized eccentric exercise.
Keywords
Delayed onset muscle soreness (DOMS); Muscle soreness questionnaire (MSQ); Pressure pain threshold (PPT); Hand held dynamometer; Illinois agility run test; Multi-enzyme complex
Introduction
Delayed onset muscle soreness is related to muscle damage occurring several hours after unaccustomed exercise, particularly when eccentric muscle activity is involved [1,2]. Contracting muscles are forcibly lengthened with eccentric exercise like downhill running which limits physical function for several days [3,4]. This triggers an inflammatory response and the production of reactive oxygen species (ROS) that sustain inflammation and oxidative stress by promoting the activation of transcription factors like the nuclear factor-κβ (NF— κβ), a pro-inflammatory master switch that controls the production of inflammatory markers and mediators [5]. The inflammatory response ensures musculoskeletal injury; uncontrolled inflammation may prolong skeletal muscle recovery [4].
Delayed onset muscle soreness (DOMS) is a well-documented phenomenon, often occurring as the result of the unaccustomed or high intensity eccentric exercise. Associated symptoms include muscle shortening, increased passive stiffness, swelling, decreases in strength and power, localized soreness and disturbed proprioception. Symptoms will often occur within 24 h post-exercise and typically subside after 3-4 days. The severity of damage and soreness varies as a function of several factors [6].
Considerable amount of research on the treatment of DOMS has been carried out till date but no single treatment has been proven successful in consistently preventing or treating DOMS. Treatment strategies have often integrated multiple therapeutic approaches such as cryo therapy, ultrasound, compression therapy, stretching and deep tissue massage [7-11]. There is some evidence that ibuprofen, naproxen, and massage may accelerate the resolution of DOMS [12]. In addition, several dietary supplements have been tested in the treatment of DOMS including protein, vitamin C, proteases (enzymes), phosphatidylserine, chondroitin sulphate, and fish oil, all with variable success [4,12-18].
Non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen are used widely as anti-DOMS recourse. NSAIDs are known to interfere with chemo taxis of monocytes as well as inhibit neutrophil aggregation [19]. Monocytes produce cytokines, which are responsible for most of the physiological responses accompanying injury, and neutrophils produce elastase and collagenase, which increase vascular permeability via degradation of the vasculature and healthy tissue near the injury site [20]. It is possible that the use of NSAIDs may impair and lengthen the healing process.
In spite of inconsistencies, dose and timing of various NSAIDs also in different studies there are side effects such as gastrointestinal distress and hypertension. Hence NSAIDs are not an optimal choice for treating DOMS [12]. Using enzymes to combat DOMS is also well established.
A study by inner field in 1957 examined the anti-inflammatory effects of protease enzyme therapy and showed that protease enzyme supplementation may have anti-inflammatory effects [21].
Commercially, digestive enzymes are isolated from various sources such as the pancreas of higher animals (swine and cattle), higher vegetables (barley), and microorganisms including bacteria and fungi. The multi enzyme complex (DigeZyme®) consists of MO free broad acting enzymes obtained from the fermentation process with Aspergillusoryzae, including amylase, protease and lipase; this group of enzymes breaks carbohydrates, proteins and fats and all three groups of enzymes are resistant to the action of gastric juices, while retaining their digestive activity. DigeZyme® was also clinically evaluated for enhanced absorption of minerals and vitamins.
Absorption issues and the destruction of enzymes in the gut have severely limited the effectiveness of traditional anti-DOMS enzyme therapy. Sitosterols -- plant sterols and protease enzymes can help reduce the inflammation associated with DOMS. Earlier research has suggested protease oral supplementation to shorten recovery time post injury through the inhibition of arachidonic cascade. Protease is also believed to inhibit the biosynthesis of pro-inflammatory agents [22], increase tissue permeability, facilitate resorption of oedema and accelerate the restructuring of damaged tissue [23]. Protease utilization is generally considered safe.
The current clinical study aims at investigating the efficacy of multienzyme complex, a multi-enzyme complex which is a combination of five digestive enzymes (alpha-amylase, neutral protease, cellulase, lactase and lipase), as a dietary supplement in the management of DOMS.
Therefore, the current randomized, double blind placebo controlled trial was conducted to evaluate the effect of multi-enzyme complex (amylase, lipase, lactase, cellulose and neutral protease) for the management of delayed onset muscle soreness after eccentric exercise.
Materials and Methods
Product description
DigeZyme® is an “off - white to creamy white powder of multienzyme complex”. This multi-enzyme complex consists of amylase, protease, lipase, cellulase and lactase. Placebo capsules containing equivalent weight of maltodextrin. No differences in colour, taste, texture or packaging were detectable between the two products. Capsules were sealed in identically-appearing, high-density polyethylene bottles with desiccant. The enzyme activity of multi-enzyme complex was determined by following standard methods as per Food Chemicals Codex guidelines (Table 1).
| S. No. | Enzyme | Enzyme activity (Units/g) |
|---|---|---|
| 1 | Alpha-amylase | 24000 DU/g (Dextrinizing Unit/gram) |
| 2 | Cellulase | 1100 CU/g (Cellulase Unit/gram) |
| 3 | Lipase | 200 FIP/g (FédérationInternationalePharmaceutiqueUnit/gram) |
| 4 | Lactase | 4000 ALU/g (Acid Lactase Unit/gram) |
| 5 | Neutral Protease | 6000 PC/g (Protease Unit on L-tyrosine basis/gram) |
Table 1: Composition detail of multi-enzyme complex (DigeZyme) formulation.
Enzyme units were defined as per Food Chemical Codex (FCC), 5th ed. 2004. The National Academy Press Washington DC.
Ethics approval
Ethics approval was obtained through the Sparsh Hospital for Advanced Surgeries, #146, Infantry Road, Bangalore prior to the initiation of the study. The study documents reviewed were (protocol, informed consent form, investigator brochure, and case record form). The aforesaid Ethics Committee was registered under central drugs standard control organization as per the gazette notification number F.28-10/45-H(1), dated 21 Dec 1945 and last amended vide notification number G.S.R. 76(E) dated 08 Feb 2012.
Informed consent
This study included a total of 20 healthy volunteers. An initial screening visit was scheduled during which all subjects first signed written informed consent. Before the signing of the informed consent adequate oral and written information concerning the study was provided to the subjects and subjects were provided with ample opportunity to consider their participation in the study. After obtaining a signed informed consent subjects were screened for the study.
Study design
For the DOMS model under study, we selected the following design features: prospective, double blind, randomized, and placebo controlled. The analgesic efficacy of DigeZyme® was compared with a matching placebo. The pre specified efficacy end points of the study were assessed on Day 0 (pre exercise) and 72 hours post exercise.
Participants
Twenty healthy males with no known musculoskeletal pathology participated in the study. Subjects were excluded if they met one or more of the following exclusion criteria: treatment with antiinflammatory/ analgesic/antioxidant drugs in the previous month, abnormal liver or renal function tests, laboratory findings suggestive of an active inflammatory or infectious process and presence of any known disease.
The participants also completed a health history questionnaire designed to identify the degree of risk for cardiovascular or orthopaedic complications during exercise.
Supplementation with multi enzyme complex
All participants in the experimental group received multi enzyme complex supplementation over a 3 day period. During this period, they consumed one capsule of multi enzyme complex thrice a day. The participants in the placebo group received capsules of similar size and colour. They were given identical instructions on dosage of study supplement to be followed. Study supplements were prepared and provided by Sami Labs Limited, Bangalore. The administration of treatment and placebo was blinded for both the participants and the investigator. There were no side effects reported by the participants as a result of the supplementation.
Data collection protocol
Subjects visited the clinic on day 0 (baseline visit) and subsequent visits on day 1, day 2 and day 3. After recording vital signs (blood pressure, pulse rate, heart rate and respiratory rate), medical and medication history, physical examination was conducted.
Randomisation and allocation concealment
The randomisation sequence was prepared by an independent statistician, independent of the sponsoring organization and not involved in conduct or reporting of the study. An alpha numeric code was generated for both the active and placebo to improve the blindness of the study and concealment of allocations. Computer generated random allocation software (version 2.0) was used for the allocation of concealment. Block randomization (only one block) was followed wherein the subjects were randomized to receive either active or placebo. The randomization codes were kept strictly confidential and were accessible only to authorized persons on an emergency basis as per the Sponsor standard operating procedures until the time of unblinding.
Blinding
The study was double blinded wherein neither the Investigator nor the trial participants knew whether they would receive the active or the placebo. The investigational products were provided in pre labelled containers to avoid bias.
Procedures Followed
Protocol for inducing DOMS
Subjects were instructed to ingest the study supplement and report to the site after 24-hours for the baseline readings. After 10 hours fasting, heart rate and ratings of perceived exertion were monitored at rest, every 5 minutes during exercise and 10 minutes into recovery. The participants mounted a level motorized treadmill and warmed up for 5 minutes at a self-selected pace. Post 5-min warm up, treadmill speed was increased until a heart rate of 80% of predicted maximal heart rate was achieved and were instructed to maintain this pace for 5 min. The treadmill grade at this time was adjusted to 10% and was maintained for 30 minutes. Subjects then completed a 5 minutes active cool-down at a self-selected pace and a 5 minutes seated passive recovery period. Subjects were restricted from indulging in any other physical activity 24 hours prior and 72 hours after the exercise session [24].
Quantifying muscle soreness using algometer
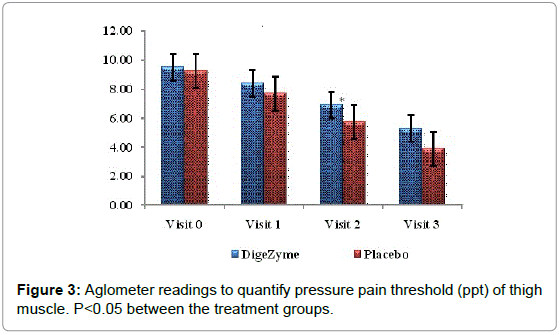
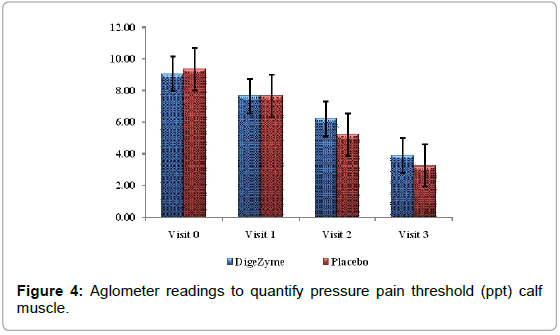
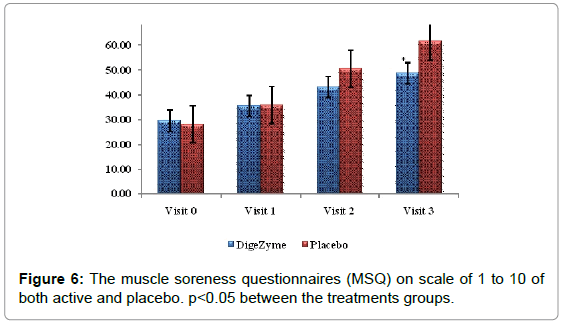
The Muscle Soreness Questionnaires (MSQ) required participants to rate their general soreness on a scale of 1 (normal) to 10 (very, very sore) for the right front thigh and right back thigh. An algometer was used to quantify muscle pain by applying direct pressure over the muscle. It involves documenting the threshold at which the applied pressure over the muscle is perceived as a sensation of pain rather than a pressure; this is referred to as the pressure pain threshold (PPT). The PPT has been demonstrated to be reliable for measuring pain threshold. The algometer standard is to increase pressure linearly to 5 kg/cm2 over 5 seconds according to the method recommended by Fischer. The instrument has a 1 cm2 rubber footplate and a scale marked from 2 to 20 kg/cm2, in increments of 0.2 kg/cm2. Subjects were instructed to report as soon as the sensation of pressure changes to pain by saying ‘pain’, and ‘I will stop’. The footplate of the algometer was held perpendicular to the muscle belly with the gauge turned away from the subject and the examiner. Pressure was increased at a rate of approximately 1 kg/ cm2/s until the subject reported ‘pain’. The examiner then released the pressure and lifted the algometer off the muscle to read the gauge and record the measurement. The needle on the gauge was returned to baseline before each trial using the pressure release button on the algometer [24].
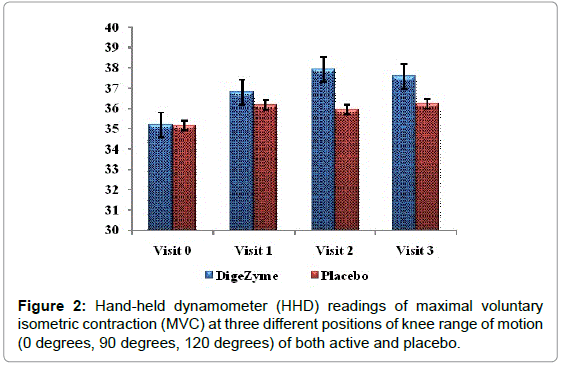
For muscular strength and power using hand held dynamometer
A Hand-Held Dynamometer (HHD) was used to quantify changes in muscle strength by measuring strength during maximal voluntary isometric contraction (MVC) of the muscle. Each participant was asked to perform three repetitions of maximal knee extension and flexion contractions (MVC) at three different positions of knee range of motion (0 degrees, 90 degrees, 120 degrees). The test angles were chosen to account for changes in muscle strength that could occur due to alteration in the length of the muscle at different positions of the knee [25].
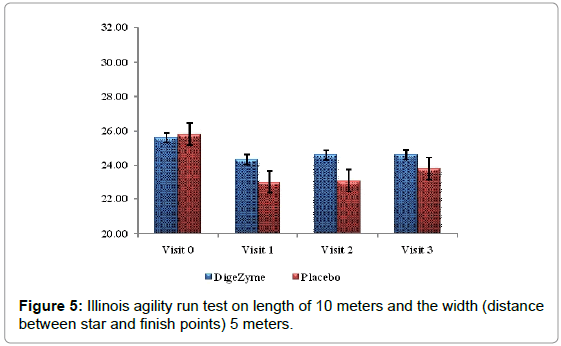
Illinois agility run test
Agility was measured using Illinois agility run test. The tests were conducted on a course 10 meters in length and 5 meters in width. Agility time was recorded using a stopwatch. The start, finish and the two turning points were marked with four cones and another four cones were placed down the center equal distance spaced 3.3 meters apart. Subjects were made to lie on their front (head to the start line) and hands by their shoulders. The run started from a standing start on the command ‘Go’ the stopwatch was started, and the subjects were instructed to get up as quickly as possible and run around the course in the direction indicated, without knocking the cones over, to the finish line, at which the timer was stopped [26].
Outcome measures
McGill pain questionnaire comprising of 10 subscales from 0 (no pain) to 10 (worst possible pain) was used to assess pain [27,28]. An algometer was used to experimentally induce pain on a predefined point on the patellar tendon five centimetres above the center of the patella, tenderness was assessed.
Subjects ranked their pain perception on a scale from 0 to 10. On day 3, assessments were taken at baseline (pre-exercise), post-exercise and further at 24, 48 and 72 hours post-exercise for each arm of the study. Secondary outcomes included assessments of inflammation, muscle damage, flexibility, and the amount of energy expended prior to exercise.
Biomarkers creatine kinase and lactate dehydrogenase were monitored pre exercise and 72 hours post exercise.
Safety profile of the supplement was assessed by routine haematology, kidney and liver function tests. Adverse events were monitored throughout the study.
Statistical analysis
All testing was done using Statistical Analysis Software (SAS) having version 9.2. All analyses was conducted using the intent-to-treat population. Patients with no data recorded for a particular parameter were automatically excluded from the analyses of that parameter. For all the data set variables analysis of covariance (ANCOVA) and Wilcox on signed rank sum test were used and the level of significance was set as P<0.05.
Results
Subject disposition
A total of 20 subjects were enrolled into the study. There were no subject withdrawals or dropouts in this study.
Physical characteristics
The population was essentially healthy without significant concomitant disease or medication intake. Screening characteristics of participants are presented in (Table 2). There were no statistically significant differences between subjects in the placebo (n=10) and the DigeZyme® (n=10) group. On the day of screening, the mean weight of all the enrolled subjects was 59.3 ± 4.64 kgs; mean height was 163.8 ± 4.99 cm and the mean BMI was 22.2 ± 1.50 kg/m2.
| Demographics | Values |
|---|---|
| Height (cm) | |
| N | 20 |
| Mean(SD) | 163.8 (4.99) |
| Median | 164.5 |
| Min, Max | 154, 172 |
| Weight (kg) | |
| N | 20 |
| Mean(SD) | 59.3 (4.64) |
| Median | 59.2 |
| Min, Max | 51, 67 |
| Body Mass Index (kg/m2) | |
| N | 20 |
| Mean(SD) | 22.2(1.50) |
| Median | 22.3 |
| Min, Max | 20, 25 |
| Race | |
| Central American | 0 |
| East Asian | 0 |
| South Asian | 20 |
| South American | 0 |
| South East Asian | 0 |
| Western European | 0 |
| White | 0 |
Table 2: Subject demographics characteristics.
Efficacy evaluation
Delayed Onset Muscle Soreness-quality of life was analyzed throughout the study period as primary efficacy measure. The ‘p’ value suggests that there was a statistically significant change in these symptoms from baseline to final visits, between the placebo and active arms. Statistical analysis using Analysis of Co-Variance (ANCOVA) showed the primary efficacy parameters were statistically significant (p<0.05) between the multienzyme complex and placebo groups (Table 3). Furthermore, comparative mean values of efficacy assessments between Multi enzyme complex and placebo groups across various visits (baseline, day 1, day 2 and day 3) are presented for efficacy parameters (Figures 1-8). Patients who were on Multi enzyme complex had statistically significant difference for the efficacy parameters on day 3 when compared with placebo.
| Subjective Parameters | |||
|---|---|---|---|
| Measure | Investigational Products | Significance | |
| Hand held Dynamometer readings result [grip strength] kg |
DigeZyme® Baseline 72 hours Post Exercise |
Placebo Baseline 72 hours Post Exercise |
0.4956 |
| 36.2 (7.86) 37.6 (8.32) | 35.2 (6.84) 36.2 (5.26) | ||
| Thigh muscle (point of pain) Algometer Reading’s kg/cm2 |
9.5 (0.58) 5.3 (1.18) | 9.3 (1.03) 3.9 (1.45) | 0.0436* |
| Calf muscle (point of pain) Algometer Reading’s kg/cm2 |
9.1 (1.09) 3.9 (1.20) | 9.4 (1.11) 3.3 (1.03) | 0.1397 |
| Total Time Taken Illinois Agility Run test (Seconds) |
25.6 (3.43) 24.6 (4.11) | 25.2 (1.69) 23.8 (2.91) | 0.7246 |
| The McGill pain questionnaire (total pain score) |
29.5 (2.55) 48.7 (8.86) | 28.1 (1.45) 61.3 (7.07) | 0.0061* |
| Objective Parameters (Serum markers (U/L) | |||
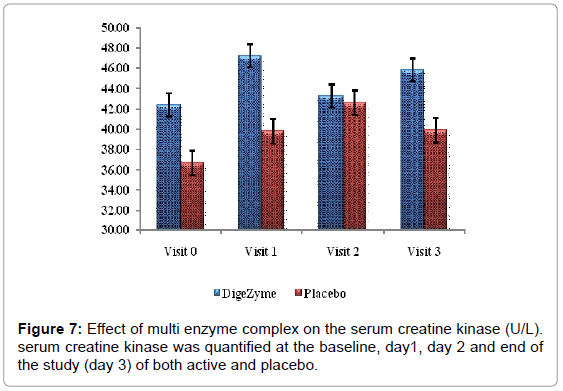
| Serum Creatine Kinase | 42.4 (11.69) 45.8 (10.49) | 36.7 (4.30) 40.5 (5.42) | 0.5735 |
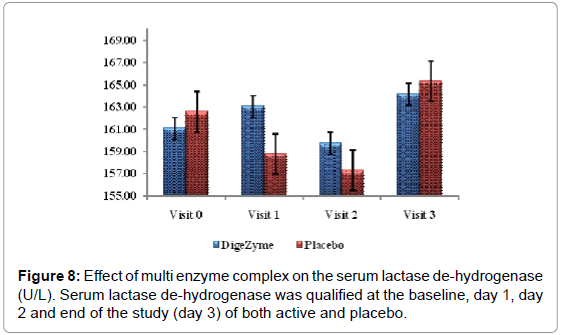
| Serum Lactate De-hydrogenase | 161.1 (35.56) 164.2 (31.17) | 162.6 (28.40) 161.4 (25.44) | 0.2556 |
Table 3: Efficacy analysis.
Pain assessment
McGill pain questionnaire is a multidimensional pain instrument; the final score is a sum of all the ten individual pain questions. The first nine McGill pain questionnaires were based on assessment of pain (current pain, least pain, and worst pain) while the tenth on the degree of numbness and its interference with function.
When differences between multi enzyme complex and placebo were compared, the pain score showed high statistical significance (p=0.0061).
Tenderness assessment
On the tenderness quotient, subjects taking multi enzyme complex demonstrated significantly less tenderness, 72 hours after exercise (p=0.042).
Muscle damage assessments
Liberation of biochemical substances such as creatine kinase, lactate dehydrogenase, protein metabolites and myoglobin occurs from muscle cells approximately 24 hours post exercise and have been found in plasma up to 48 hours [29]. Creatine kinase being a surrogate index of muscle damages more indicative of damage or gaps in the sarcolemma [30]. The CK response was less in the multi enzyme complex group suggesting the membrane integrity was maintained to greater extent than the placebo group.
24 hours post exercise, subjects on the placebo group showed trend towards a higher level of CK than the multi enzyme complex group. This trend continued through the 72 hour assessment. Difference between the multi enzyme complex group and placebo in CK values was not statistically significant. Lactate dehydrogenase also showed a similar phenomenon, which trended higher at 24 and 72 hours postexercise in the placebo group (Figures 7 and 8).
Flexion and extension measurements
On analysing the pre exercise leg flexion measurements, they were found to be equal between the groups for the left leg. Whereas the flexion measurement was found to be significantly greater (p=0.049) for the right leg in the placebo group.
When the post exercise flexion measurements were analysed, only the 24-hour right leg flexion measurement was found to be significant (p=0.004) in the multi enzyme complex group.
Safety evaluations
Vital signs such as Blood Pressure, Respiratory Rate, Pulse Rate and any abnormal laboratory parameters were considered for safety evaluations. No clinically significant changes were recorded for descriptive physical examination in both the groups (Multi enzyme complex and placebo). The safety of multi enzyme complex was assessed using adverse event data (occurrence, intensity, and relationship to study drug). No adverse events were noticed in the study.
Discussion
Multi enzyme complex capsules contain alpha-amylase, neutral protease, lipase, lactase and cellulase. The capsule containsfree broad acting enzymes obtained from the fermentation process with Aspergillusoryzae, including amylase, protease and lipase. This manufacturing technique ensures that the gastric enzymes from Aspergillusoryzae are delivered at distinct sites, the stomach and the small intestine, respectively. Various studies have shown protease supplementation may attenuate muscle soreness after downhill running [31].
A subsequent series of four studies have evaluated papain, in combination with other proteases, in small samples of male athletes, especially with regard to its effectiveness in attenuating DOMS post eccentric exercise. Two of the studies were able to show better flexion in the tested limb post eccentric load which was hypothesised to be mediated by regulation of leukocyte activity and inflammation. Two further studies showed an improvement in contractile function and subjective pain and tenderness ratings but not in biochemical measures of DOMS. Further interpretation of these studies is difficult as all four used a combination of papain with other proteolytic enzymes (e.g. bromelain, amylase, lysozyme, and trypsin) [8].
In the present study we sought to investigate the effects of multi enzyme complex on delayed onset muscle soreness induced by eccentric exercise.
Delayed onset muscle soreness (DOMS) due to eccentric muscle activity is associated with inflammatory responses and production of reactive oxygen species (ROS) that sustain both inflammation and oxidative stress. After eccentric exercise, damage to the contractile element of the muscle leads to the occurrence of DOMS. The results of this study suggest that supplementation with Multi enzyme complex supplementation helps in the recovery of this contractile fraction of the muscles.
In the present study multi-enzyme complex capsules demonstrated significant improvement in subjective pain and tenderness, with no significant improvement in levels of markers of inflammation, muscle damage or muscle flexion. Multi-enzyme complex contains a multiple enzymes that are indicated for relieving the symptoms of DOMS.
The findings of this study suggest that multi enzyme complex can have several potential clinical applications. Protease supplementation when coupled with a well-managed training programme can result in more rapid recovery of the damage caused to contractile mechanism by DOMS.
Registration
The trial was registered on the Clinical Trial Registry of India with the registration number CTRI/2015/09/006148.
References
- Dierking JKATC, Bemben MG (1998) Delayed onset muscle soreness. Strength & Conditioning 20: 44-48.
- Armstrong RB (1990) Initial events in exercise-induced muscular injury. Med Sci Sports Exerc 22: 429-435.
- Francis KT, Hobbler T (1987) Effects of aspirin on delayed muscle soreness. J sports Med Physical Fitness 27: 333-337.
- Proske U, Morgan DL (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537: 333-345.
- Aoi W, Naito Y, Takanami Y, Kawai Y, Sakuma K, et al. (2004) Oxidative stress and delayed-onset muscle damage after exercise. Free Radic Biol Med 37: 480-487.
- Joanna Vaile, Shona Halson, Nicholas Gil, Brian Dawson (2008) Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. Eur J Appl Physiol 102: 447-455.
- Sellwood KL, Brukner P, Williams D, Nicol A, Hinman R (2007) Ice-water immersion and delayed-onset muscle soreness: a randomised controlled trial. Br J Sports Med 41: 392-397.
- Craig JA, Bradley J, Walsh DM, Baxter GD, Allen JM (1999) Delayed onset muscle soreness: lack of effect of therapeutic ultrasound in humans. Arch Phys Med Rehabil 80: 318-323.
- Kraemer WJ, Bush JA, Wickham RB, Denegar CR, Gómez AL, et al. (2001) Influence of compression therapy on symptoms following soft tissue injury from maximal eccentric exercise. J Orthop Sports PhysTher 31: 282-290.
- Frey LLA, Evans S, Knudtson J, Nus S, Scholl K (2008) Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain 9: 714-721.
- Herbert RD, de NM (2007) Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database Syst Rev.
- Connolly DA, Sayers SP, McHugh MP (2003) Treatment and prevention of delayed onset muscle soreness. J Strength Cond Res 17: 197-298.
- Cockburn E, Hayes PR, French DN, Stevenson E, St Clair GA (2008) Acute milk-based protein-CHO supplementation attenuates exercise-induced muscle damage. Appl Physiol Nutr Metab 33: 775-783.
- Connolly DA, Lauzon C, Agnew J, Dunn M, Reed B (2006) The effects of vitamin C supplementation on symptoms of delayed onset muscle soreness. J Sports Med Phys Fitness 46: 462-467.
- Beck TW, Housh TJ, Johnson GO, Schmidt RJ, Housh DJ, et al. (2007) Effects of a protease supplement on eccentric exercise-induced markers of delayed-onset muscle soreness and muscle damage. J Strength Cond Res 21: 661-667.
- Kingsley MI, Kilduff LP, McEneny J, Dietzig RE, Benton D (2006) Phosphatidylserine supplementation and recovery following downhill running. Med Sci Sports Exerc 38: 1617-1625.
- Braun WA, Flynn MG, Armstrong WJ, Jacks DD (2005) The effects of chondroitin sulfate supplementation on indices of muscle damage induced by eccentric arm exercise. J Sports Med Phys Fitness 45: 553-560.
- Lenn J, Uhl T, Mattacola C, Boissonneault G, Yates J, et al. (2002) The effects of fish oil and isoflavones on delayed onset muscle soreness. Med Sci Sports Exerc 34: 1605-1613.
- Goodwin JS (1984) Mechanism of action of nonsteroidal anti-inflammatory agents. Am J Med 104:2S-8S.
- Evans WJ, Cannon JG (1991) The metabolic effects of exercise-induced muscle damage. Exerc Sport Sci Rev 19: 99-125.
- Inner field I (1957) The anti-inflammatory effect of parenterally administered proteases. Ann N Y Acad Sci 68: 167-77.
- Woolf RM, Snow JW, Walker JH, Broadbent T (1965) Resolution of an artificially induced hematoma and the influence of a proteolytic enzyme. J Trauma 5: 491-493.
- Cirelli MG (1964) Clinical experience with bromelains in proteolytic enzyme therapy of inflammation and edema. Medical Times 92: 919-922.
- Miller PC, Derr JS, Bailey SP, Hall EE, Barnes ME (2004) The effects of protease supplementation on skeletal muscle function and DOMS following downhill running. Journal of Sports Sciences 22: 365-372.
- Kelln BM, McKeon PO, Gontkof LM, Hertel J (2008) Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil 17: 160-170.
- Getchell B (1979) Physical fitness: a way of life. Wiley J Sons, New York.
- Melzack R (1975) The McGill pain questionnaire: major properties and scoring methods. Pain 1: 277-299.
- Sullivan MJL, Rodgers W, Wilson MP, Fraser SN (2002) An experimental investigation of the relation between catastrophizing and activity intolerance. Pain 100: 47-53.
- Clarkson PM, Nosaka K, Braun B (1992) Muscle functions after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc 24: 512-520.
- Jackman SR, Witard OC, Jeukendrup AE, Tipton KD (2010) Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med Sci Sports Exerc 42: 962-970.
- Au RY, Al-Talib TK, Au AY, Phan PV, Frondoza CG (2007) Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthr Cartil 15: 1249-1255.
Relevant Topics
- Aminoacid Suppliments
- Bodybuilding Nutrition
- Clinical Sports Nutrition
- Creatine Sports Nutrition
- Diet
- Fitness Nutrition
- Food and Nutrition
- Gym Suppliments
- Herbal Suppliments
- Micronutrients
- Natural Suppliments
- Nutrition Sport Fitness
- Nutritional Health
- Protein Diet
- Protein Suppliments
- Sports Nutrition Suppliments
- Vitamin Supplement
Recommended Journals
Article Tools
Article Usage
- Total views: 17660
- [From(publication date):
December-2016 - Jan 31, 2025] - Breakdown by view type
- HTML page views : 16554
- PDF downloads : 1106