Multicentric Assessment of Efficacy and Safety Outcomes with Pharmacological Thromboprophylaxis in the Setting of Liver Transplant
Received: 31-Jan-2024 / Manuscript No. JGDS-24-128023(PQ) / Editor assigned: 02-Feb-2024 / PreQC No. JGDS-24-128023(PQ) / Reviewed: 16-Feb-2024 / QC No. JGDS-24-128023 / Revised: 21-Feb-2024 / Manuscript No. JGDS-24-128023(R) / Published Date: 28-Feb-2024 DOI: 10.4172/2161-069X.1000784
Abstract
Background: Liver transplant recipients often remain transiently hypercoagulable following transplantation. This is due to the inherent bleeding and clotting risks associated with the surgical procedure and to a disruption in coagulation homeostasis associated with cirrhotic disease. There is no guidance on the application of pharmacological venous thromboembolism (VTE) prophylaxis in this patient population. We sought to investigate the risk of bleeding and VTE in liver transplant recipients (n=38) who received pharmacological prophylaxis (n=11) versus (vs.) those who did not (n=27) during hospitalization. Results: The overall incidence of postoperative bleeding was 55.3%, and was lower in the group that received pharmacological prophylaxis (18.2% vs. 70.4%; p=0.002). The overall rate of major bleeding was 8%, with all 3 cases occurring in the group that did not receive pharmacological prophylaxis. We noted that patients who experienced bleeding in general had lower baseline platelets (p=0.01). Our study found no difference in the incidence of postoperative thromboembolic events, with an overall rate of 2.6% (n=1 case in the group that did not receive pharmacological prophylaxis). Readmission within 30 days due to bleeding or thromboembolic events also did not differ between the groups (0% vs. 4%; p=0.33 and 9% vs. 7%; p=0.97, respectively). Conclusion: Our results suggest that the incidence of post liver-transplant thromboembolic events are low, and that exposure to pharmacological thromboprophylaxis during admission of liver transplantation procedure is not associated with an increased risk of post-operative bleeding.
Keywords
Chronic immune thrombocytopenia; Liver transplant; Pharmacological prophylaxis
Introduction
End-stage liver disease characterized by cirrhosis often leads to a state of hypercoagulability in patients. This is due to a severe homeostatic disruption in balance between clotting factors and anticoagulation proteins which are synthesized in the liver. Recipients of liver transplant are generally at an increased risk for bleeding, with postoperative incidences reported between 8.5%-19.9% [1-5]. There is literature documentation of delayed recovery of anti-coagulant proteins including anti-thrombin III deficiency, and proteins C and S levels [6]. Furthermore, reperfusion changes and reversion of coagulopathy lead to a transient period of post-operative hypercoagulability of unclear duration [6]. In addition, transplant recipients generally receive immunosuppressive medications peri-operatively and early post-transplant, which are associated with thrombogenicity (tacrolimus, cyclosporine, corticosteroids) and myelosuppression (anti-thymocyte globulin, mycophenolate, azathioprine) [7-11]. These physiologic and pharmacological factors have made it difficult to ascertain risk of VTE versus (vs.) bleeding in these patients.
Several studies reported an incidence of VTE from 0.5% to 6.3% in cirrhotic patients; with an overall deep vein thrombosis (DVT) incidence between 2.7% and 8.6% after liver transplantation [12-19]. As VTE is associated with significant morbidity and mortality, it is important to consider prophylaxis in these patients. However current guidelines do not provide recommendations to guide the use of pharmacologic therapy in this setting, and literature in this area is limited. Thus, the use of anticoagulation for VTE prophylaxis remains controversial.
The purpose of this study was to examine the safety and efficacy of pharmacological thromboprophylaxis in the setting of liver transplantation given the inherent risks of bleeding and thrombosis.
Method
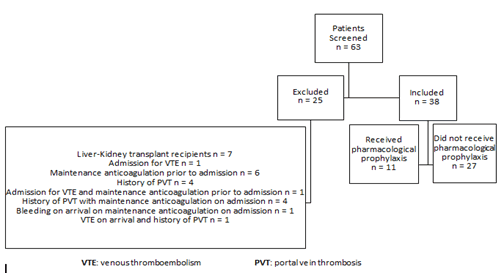
The study was conducted as an observational, retrospective multicentric analysis. We included a sample of adult patients (≥ 18 years old) who were admitted to Mayo Clinic Rochester, Mayo Clinic Arizona, or Mayo Clinic Florida between March 1, 2018-August 1, 2020 and underwent liver transplantation during their admission. Patients were categorized as either having received pharmacologic prophylaxis (subcutaneous (SQ) unfractionated heparin (UFH) or SQ low molecular weight (LMWH)) or no pharmacological prophylaxis during their hospitalization. Initiation of pharmacological prophylaxis (with or without mechanical prophylaxis) was at the discretion of providers. The timing of administration was noted with respect to transplantation. Data was collected for every patient who met inclusion criteria in the treatment group and for a number of patients in the control group, as the majority of patients transplanted across the three centers often did not receive pharmacologic prophylaxis. We excluded patients who received a liver transplant for acute liver failure and patients with confounding factors including maintenance anticoagulation prior to admission, admission for suspected bleeding or venous thromboembolism, a history of portal vein thrombosis, and a diagnosis of congenital or acquired thrombophilia (factor V Leiden, antiphospholipid syndrome, prothrombin G20210A, protein C or S deficiency, prothrombin mutation, or anti-thrombin deficiency) or hemophilia. A 30-day followup period after hospital discharge was used to screen for incidence of bleeding and thromboembolic events.
Baseline characteristics were collected to assess possible risk factors associated with the study's outcome measures. We obtained demographic, clinical, and laboratory data by manual electronic health record extraction. Among the baseline data collected, we included conventional hematologic testing consisting of hemoglobin, platelet count, and international normalized ratio (INR). We also collected thrombo-elastography (TEG) results performed preoperatively prior to transplant at our institution. Studies have shown that the use of conventional coagulation tests is associated with limitations in patients with chronic liver disease due to the imbalance of coagulation state. This is due to their nature as in-vitro tests, which limits their ability to capture the full range of the in-vivo coagulation system dysfunction [20]. The mechanism of TEG relies on viscoelastic properties of whole blood to measure coagulation initiation, clot formation, clot strength, and fibrinolysis. Evidence suggests that there is a strong and consistent correlation between TEG measures of clot formation and clot strength and fibrinogen levels [21,22]. Collecting this information may therefore provide a more accurate view of the state of coagulation in our patient population.
The main outcome of this study was the incidence of postoperative bleeding during hospitalization following transplantation. Secondary outcomes included incidence of postoperative inpatient thromboembolic events, readmission within 30 days due to a bleeding event, and readmission within 30 days due to a thromboembolic event. Bleeding events were classified as major or minor. Major bleeding was defined as bleeding or hematoma requiring return to the operating room, fatal bleeding, or bleeding into a major organ or critical area (intracranial, retroperitoneal, pericardial, intra-spinal, intra-articular, intraocular). Minor bleeding included all other bleeding events including postoperative hematoma, or acute anemia secondary to post-hemorrhagic blood loss. At all three sites the most common liver transplant operation is orthotopic liver transplant with piggyback technique. The operating team usually includes a primary liver transplant surgeon, with first assist who can be either a fellow or an advance practice provider (nurse practitioner or physician assistant), in addition to the usual operating room nursing and ancillary staff. Patients are managed intra-operatively by a liver transplant anesthesiologist with either an anesthesia resident and/or a certified registered nurse anesthetist. Post-operative management is done by the transplant critical care team and liver transplant hepatology. Occurrence of thromboembolic events were confirmed by radiographic data using chart review.
Statistical analysisData were analyzed with the BlueSky Statistics (version 7.40) software. Parametric data were expressed as means with standard deviation. Nonparametric data were presented as the median with the interquartile ranges between quartiles 1 and 3. Baseline characteristics and outcome results were compared between the 2 groups and assessed using the χ2 test of dependence for categorical data, the independent t-test for continuous parametric data, and the Mann Whitney U test for non-parametric data. We also conducted an analysis comparing the characteristics of the cohort that experienced the primary outcome versus those who did not, using the same methods depending on the type of data. All significance tests were two-tailed with a p<0.05 for significance.
Results
Cohort characteristics
We assessed 63 patients with cirrhosis of varying etiology who underwent liver transplantation at the Mayo Clinic Rochester, Mayo Clinic Arizona, or Mayo Clinic Florida within the pre-defined time period. Exclusion criteria were met for 25 patients (Figure 1). The remaining patients (n=38) were allocated into the two comparison groups. Eleven patients received pharmacological prophylaxis at the discretion of their providers (SQ UFH n=3; SQ LMWH n=8) (Table 1). Since the initiation of prophylaxis was not protocolized, there was variability in timing of initiation of therapy as it related to transplantation. Three patients received therapy prior to transplantation and 8 patients received therapy following transplantation (Table 1). It was noted that the patients in this group were all transplanted at the Mayo Clinic Rochester campus (n=11). Patients who were in the group that did not receive pharmacological prophylaxis (n=27) were transplanted across all three Mayo Clinic campuses (Rochester n=7, Arizona n=9, Florida n=11). Most patients also received mechanical thrombo-prophylaxis (82% in the group that received pharmacological prophylaxis, 100% in the control group (p=0.17).
Table 1: Pharmacological prophylaxis agents received during admission
| Thromboprophylaxis Agents | Pre-transplant | Post-transplant |
|---|---|---|
| Unfractionated Heparin (UFH) | n=2 | n=1 |
| Low Molecular Weight Heparin (LMWH) | n=1 | n=7 |
Patient median age was 59.5 (IQR: 43-64.8) years old without gender predominance. Individuals tended to be overweight or obese, with an overall median body mass index of 27 (IQR: 25-30) kg/m2. The most common indication for liver transplant was cirrhosis secondary to hepatocellular carcinoma or cholangiocarcinoma (39.5%). All patients whose indication for transplant was cirrhotic Nonalcoholic Steatohepatitis (NASH) did not receive pharmacological prophylaxis (p=0.26). None of the predisposing medical factors assessed were different between the groups including history of major bleeding, history of thromboembolic events, history of cancer, or aspirin or steroid therapy during admission. Admission liver function did not differ between the groups as quantified by the Model for End Stage Liver Disease-sodium score (MELD-Na median 31 (IQR: 13-21) vs. 22 (IQR: 15-31); p=0.31). However, the group that received pharmacological prophylaxis had a lower Child-pugh score that was not statistically significant (median 7 (IQR: 6.0-9.5) vs. 10 (IQR: 8.0-11.5); p=0.06). Baseline Aspartate Aminotransferase (AST), Alanine Transaminase (ALT), and total bilirubin did not differ between the groups. There was no difference in baseline laboratory markers for clotting and bleeding including hemoglobin, platelets, INR, or perioperative TEG.
We also assessed baseline risk for VTE with the Padua Prediction Score, which was calculated from patient risk factors [22]. A score ≥ 4 points indicates high VTE risk with a recommendation for initiation of pharmacologic prophylaxis (i.e. UFH, LMWH) in patients without contraindications (platelets<100 × 109 L−1, major bleeding, creatinine clearance of <30 mL/min), or initiation of mechanical prophylaxis if a patient is at higher bleeding risk. A score <4 points indicates low VTE risk with consideration of thrombo-prophylaxis on a case-by-case basis [23]. Patients who received pharmacological prophylaxis all had scores <4 (100%) as compared to patients in the control group that had more variation (81.5% of patients had a score <4 vs. 18.5% ≥ 4). There was a statistically significant difference when comparing the 2 groups for both score ranges (scores ≥ 4: p=0.02; scores <4: p=0.02). Complete statistical data from baseline characteristics are summarized in Table 2.
Table 2: Baseline characteristics
| Characteristic | Overall Cohort (n=38) |
Did not receive pharmacological prophylaxis (n=27) | Received pharmacological prophylaxis (n=11) | P-value |
|---|---|---|---|---|
| Gender-male, % (n) | 53.6% (20) | 55.6% (15) | 45.5% (5) | 0.59 |
| Age at transplant in years (Median (IQR 1-3)) | 59.5 (43-64.8) | 61 (43.5-65) | 51 (40-61) | 0.23 |
| Body Mass Ratio (BMI) in kg/m2 (Median (IQR 1-3)) | 27 (25-30) | 27 (24.5-29) | 30 (26-33) | 0.32 |
| Transplantation Center, % (n) | ||||
| Mayo Clinic Rochester | 47% (18) | 25.9% (7) | 100% (11) | <0.05 |
| Mayo Clinic Arizona | 23.7% (9) | 33.3% (9) | 0% (0) | 0.013 |
| Mayo Clinic Florida | 29% (11) | 40.7% (11) | 0% (0) | 0.003 |
| Type of organ donor, % (n) | ||||
| Donation after Cardiac Death (DCD) | 21% (8) | 29.6% (8) | 0% (0) | 0.003 |
| Donation after Brain Death (DBD) | 71% (27) | 70.3% (19) | 72.7% (8) | 0.89 |
| Living Related Donor (LRD) | 2.6% (1) | 0% (0) | 9.1% (1) | 0.34 |
| Living Unrelated Donor (LUD) | 5.3% (2) | 0% (0) | 18.2% (2) | 0.17 |
| Indication for transplant, % (n) | ||||
| Alcoholic Cirrhosis | 13.2% (5) | 11.1% (3) | 18.2% (2) | 0.61 |
| Autoimmune Cirrhosis | 5.3% (2) | 3.7% (1) | 9.1% (1) | 0.59 |
| Cirrhosis secondary to hepatocellular carcinoma/or cholangiocarcinoma | 39.5% (15) | 33.3% (9) | 54.5% (6) | 0.26 |
| NASH Cirrhosis | 15.8% (6) | 22.2% (6) | 0% (0) | 0.01 |
| Ischemic Cholangiopathy | 2.6% (1) | 3.7% (1) | 0% (0) | 0.33 |
| Cryptogenic Cirrhosis | 7.9% (3) | 11.1% (3) | 0% (0) | 0.08 |
| Primary Biliary Cirrhosis | 2.6% (1) | 0% (0) | 9% (1) | 0.34 |
| Primary Sclerosing Cholangitis | 13.2% (5) | 14.8% (4) | 9% (1) | 0.62 |
| Transplant listing on admission | ||||
| Not Listed | 7.9% (3) | 7.4% (2) | 9.1% (1) | 0.87 |
| Listed | 89.5% (34) | 92.6% (25) | 81.2% (9) | 0.43 |
| Status 7 (Temporarily Inactive) | 2.6% (1) | 0% (0) | 9.1% (1) | 0.34 |
| Mechanical thromboprophylaxis during admission | 94.7% (36) | 100% (27) | 81.2% (9) | 0.17 |
| ICU stay prior to transplant, % (n) | 23.7% (9) | 22.2% (6) | 27.3% (3) | 0.76 |
| Length of stay in days in the ICU prior to transplant (Median (IQR 1-3)) |
1 (0.5-1) | 1 (0.6-1) | 1 (0.75-3.5) | 0.91 |
| ICU stay post-transplant, % (n) | 94.7% (36) | 92.6% (25) | 100% (11) | 0.16 |
| Overall Length of stay in days (Median (IQR 1-3)) | 8 (6-10) | 7 (6-9.5) | 10 (8-23) | 0.07 |
| MELD-Na Score on admission (Median (IQR 1-3)) | 21.5 (15-31.5) | 22 (15-31) | 31 (13-21) | 0.31 |
| Child-Pugh Score on admission (Median (IQR 1-3)) | 9 (7-10.8) | 10 (8-11.5) | 7 (6-9.5) | 0.06 |
| Presence of ascites prior to transplantation, % (n) | 76.3% (29) | 81.5% (22) | 63.6% (7) | 0.25 |
| Liver Function Panel on admission (Median (IQR 1-3)) | ||||
| Aspartate aminotransferase (AST) IU/L | 98 (48.3-222.8) | 82 (42.5-123.5) | 246 (128.5-442.5) | 0.23 |
| Alanine transaminase (ALT) IU/L | 82 (41.8-191.5) | 62 (36.5-123.5) | 194 (82.5-438.5) | 0.18 |
| Total bilirubin (T-Bili) µmol/L | 3.3 (2.1-13.8) | 3.5 (2.25-7.15) | 3 (1.45-24.2) | 0.48 |
| Padua Score prior to transplant, % (n) | ||||
| High (= 4 points) | 13.2% (5) | 18.5% (5) | 0% (0) | 0.02 |
| Low (< 4 points) | 86.8% (33) | 81.5% (22) | 100% (11) | 0.02 |
| MELD-Na: Model for end-stage liver disease-sodium, IQR: Interquartile range | ||||
| Predisposing past medical history, % (n) | ||||
| History of bleeding | 34.2% (13) | 37% (10) | 27% (3) | 0.57 |
| History of malignancy | 44.7% (17) | 41% (11) | 54.5% (6) | 0.46 |
| History of thrombosis | 5.3% (2) | 4% (1) | 9% (1) | 0.59 |
| Predisposing hospital risk Factors, % (n) | ||||
| Aspirin use during admission | 47.4% (16) | 44% (12) | 36% (4) | 0.66 |
| Steroid use during admission | 10.5% (4) | 7% (2) | 18% (2) | 0.43 |
| Duration of mechanical ventilation, % (n) | ||||
| < 1 day | 78.9% (30) | 74% (20) | 91% (10) | 0.19 |
| 1 day | 18.4% (7) | 22% (6) | 9% (1) | 0.29 |
| > 1 day | 2.6% (1) | 4% (1) | 0% (0) | 0.34 |
| Hematology/Coagulation Labs on Admission (Median (IQR 1-3)) | ||||
| Hemoglobin (Hgb) g/dL | 1.7 (1.3-2.4) | 9.8 (8.7-11.95) | 10.4 (9.1-11.75) | 0.99 |
| Platelet (PLT) × 109 L-1 | 9.9 (9.0-12) | 84 (50-139) | 85 (71.5-189.5) | 0.21 |
| International Normalized Ratio (INR) | 1.7 (1.3-2.4) | 1.7 (1.4-2.4) | 1.7 (1.3-2.1) | 0.68 |
| Perioperative Thromboelastography (TEG) (Median (IQR 1-3)) | ||||
| R Time | 6.5 (5.7-7.7) | 6.6 (5.4-8) | 6 (5.8-7.5) | 0.81 |
| K Time | 2.1 (1.7-3) | 2.2 (1.7-3.1) | 2 (1.7-2.2) | 0.27 |
| Alpha angle | 62.1 (53.8-66.4) | 62.4 (52.1-66.8) | 62.1 (60.3-65.7) | 0.88 |
| Max amplitude | 56.6 (48.6-61.3) | 55.4 (48-61.2) | 59.3 (51.8-61.7) | 0.28 |
| LYS30 | 0.15 (0-0.8) | 0 (0-0.78) | 0.45 (0.33-0.75) | 0.29 |
| NASH: Non-alcoholic Steatohepatitis; MELD: Model for End-Stage Liver Disease; ICU: Intensive Care Unit; R Time: Reaction Time, time of start from test to clot formation; K Time: Kinetics of Clot, time taken to achieve certain level of clot strength; Alpha angle: Speed at which fibrin build-up and cross-linking occurs; Max amplitude: Measure of the ultimate strength of the fibrin clot; LYS30: Percentage decrease in amplitude at 30 minutes after maximum amplitude | ||||
Clinical outcomes
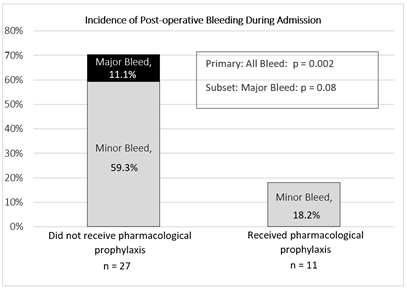
The incidence of postoperative bleeding was lower in the group that received pharmacological prophylaxis (18.2% vs. 70.4%; p=0.002). All cases of major bleeding (n=3) occurred in the control group (0% vs. 11.1%; p=0.08) (Figure 2). It details the types of bleeding events noted in each group. Both cases of postoperative bleeding noted in the group that received pharmacological prophylaxis occurred in patients who received postoperative LMWH from the day of transplant until discharge. Both were not on concomitant aspirin therapy. Data collected on blood products administered on postoperative day 0 showed that patients who received pharmacological prophylaxis required a lower volume of blood products, at a median of 7 units (IQR: 4-14) vs. 18 units (IQR: 3-39); p=0.01). Median time to postoperative bleeding event was 1 day (IQR: 0.5-2.5). A subset analysis was conducted and revealed that patients who experienced an event had lower baseline platelets (median 79 × 109 L−1 (IQR: 45- 96) vs. 152 × 109 L−1 (IQR: 78-182); p=0.01); and a trend towards lower maximum amplitude values on perioperative TEG analysis (median 52.4 (IQR: 47.4-59.8) vs. 60.3 (IQR: 51.5-61.2)), but this did not meet statistical significance (p=0.08). There was one postoperative thromboembolic event during post-transplant hospitalization (overall rate 2.6%). One patient in the control group with a high baseline Padua Score had an intraoperative thrombosis. Of note, this patient was also noted to have significant posthemorrhagic acute blood loss. No other thromboembolic events were noted in our study population, correlating to no difference between the groups (3.7% vs. 0%; p=0.34).
There was no difference in readmission rate within 30 days due to bleeding events (9.1% vs. 3.7%; p=0.59), with 1 readmission due to perihepatic hematoma in the group that received pharmacological prophylaxis (UFH during admission prior to transplant) and 1 readmission due to recurrent incision site bleeding in the group that did not receive prophylaxis. The latter case involved a patient who also had significant postoperative hemorrhagic bleeding during admission. Rate of readmission within 30 days due to thromboembolic events was the same between the groups (9.1% vs. 7.4%; p=0.97), with 1 readmission due to hepatic artery thrombosis (HAT) in the group that received pharmacological prophylaxis (UFH, followed by LMWH during admission prior to transplant), and two readmissions in the group that did not receive pharmacological prophylaxis due to pulmonary embolism (PE) and HAT (Table 3).
Table 3: Results from secondary outcomes
| Outcome Measures | Overall Cohort (n=38) |
Did not receive pharmacological prophylaxis (n=27) | Received pharmacological prophylaxis (n=11) | P-Value |
|---|---|---|---|---|
| Incidence of postoperative thrombosis during admission, % (n) | 2.6% (1) | 3.7% (1) | 0% (0) | 0.34 |
| Readmission within 30 days due to bleeding event, % (n) | 5.3% (2) | 3.7% (1) | 9.1% (1) | 0.59 |
| Readmission within 30 days due to hrombotic event, % (n) | 7.9% (3) | 7.4% (2) | 9.1% (1) | 0.97 |
Discussion
To our knowledge there are no large prospective, randomized controlled trials that assess safety of VTE pharmacologic prophylaxis in cirrhotic patients undergoing liver transplantation. Retrospective studies have been performed to evaluate the risks and benefits of implementing VTE prophylaxis in cirrhotic patients. Smith et al. investigated the use of prophylactic agents on the incidence of VTE and bleeding risks in patients with chronic liver disease (CLD). The study included 410 patients with 225 (55%) receiving thromboprophylaxis during a hospital admission for or with a diagnosis of CLD. Of these patients, 154 (38%) received mechanical prophylaxis, 49 (12%) received pharmacological prophylaxis, and 22 (5%) were on a combination of both strategies. Pharmacological interventions included UFH, LMWH, and fondaparinux. Overall incidence of VTE in this study was 0.7% and bleeding rate of 3.7%. Of the 15 patients who experienced a bleeding event, 9 (60%) were on mechanical prophylaxis, 1 (7%) on pharmacologic, 3 (20%) on combination, and 2 (13%) were on no prophylaxis therapy. The investigators found an association between bleeding and CLD secondary to alcohol and INR>2.0 [24]. Similarly, Yerke, et al. assessed 1106 patients with liver disease who either received or did not receive pharmacological VTE prophylaxis (UFH, LMWH, fondaparinux). The control group was more likely to experience the composite endpoint of VTE or major bleeding as compared to the group that received prophylaxis (8.7% vs. 5.1%; p=0.002). This was however driven by higher rates of major bleeding (6.9% vs. 2.9%, p<0.001) rather than similar rates of VTE (1.9% vs. 2.2%, p=0.62).
The results of our observational retrospective analysis reflect similar findings in the liver transplant population. Our data show that the use of pharmacological thromboprophylaxis was not associated with an increase in the risk of bleeding in liver transplant recipients in the setting of hospital admission during which transplantation occurred. In our study we observed a higher incidence of overall bleeding associated with the group that did not receive pharmacological prophylaxis (p=0.002), and a trend towards higher incidence of major bleeding in these patients (p=0.06). Although baseline liver function and coagulation markers did not differ between the 2 groups, subset analysis demonstrated that patients who had a bleeding event while admitted had lower platelets (p=0.01). These patients also had a trend towards lower maximum amplitudes of the preoperative TEG (p=0.08). Although it did not meet statistical significance, it does suggest reduced platelet function. Since the initiation of pharmacological prophylaxis was not protocolized, it could be inferred that patients were generally not started on prophylaxis therapy if there was a perceived increased risk for bleeding at baseline. Although the 2 patients who experienced bleeding on pharmacologic prophylaxis were on similar regimen (LMWH following transplant until discharge), it would be prudent not to draw specific conclusions with regards to the use of LMWH due to the small sample size and the variety of pharmacologic prophylaxis regimens noted in the cohort. The safety of LMWH use in this setting clearly warrants further investigation.
We observed an overall 2.6% incidence of postoperative thromboembolic events during admission (n=1), with no statistical difference between the 2 groups. Our data reflect findings from previous studies in liver disease patients. We also assessed for risk of thrombosis at baseline using the Padua Prediction Score. Our results show that patients who received pharmacological prophylaxis had low scores and vice versa. This observation may indicate that in practice, the different factors of the score alone may not hold sufficient weight to influence the clinical decision to initiate pharmacological prophylaxis. Given that the primary etiology of concern in these patients is coagulopathy, physicians are likely also using baseline coagulation labs to increase their decision-making power. This hypothesis is also supported by our observation of lower baseline platelets in patients who ultimately experienced bleeding in our study population. The single case of thrombosis in our study occurred in a patient who had a high baseline Padua score and did not receive pharmacological thromboprophylaxis. However, this patient also experienced postoperative bleeding, which illustrates the coagulability imbalance following liver transplant reported in literature. This suggests that better tools are needed to help predict the risk of thrombosis as well as bleeding in this patient population.
Literature reports the most common factors associated with VTE in the general population as hospitalization (52%), cancer (48%), and surgery (42%) [25,26]. Additional reported risk factors for patients who have undergone liver transplantation include previous history of VTE, endstage renal disease, discharge to rehabilitation centers, receiving factor VII during surgery, hepatitis C due to increased level of antiphospholipid antibodies, postoperative pneumonia, and diabetes [27,28]. Yip, et al. sought to determine risk factors that influence the VTE incidence and the effectiveness of prophylaxis in patients who underwent initial liver transplant during 12-year span (n=999). A retrospective historical comparison analysis was conducted with patients who did not receive pharmacological prophylaxis and patients who received a standardized prophylactic regimen using SQ UFH following initiation of an institutional protocol. Baseline white blood cell count, platelet count, INR, age, or MELD were the same between both groups. Patients who received UFH (n=288) had lower rates of VTE as compared to those who did not (1.0% vs. 3.5%; p=0.03). A multivariate analysis confirmed that peripherally inserted central catheter (PICC) placement was associated with 6.3 times higher likelihood of developing post-transplant VTE within the 30-day and SQ UFH was associated with 5 times lower odds for developing VTE [29]. We were unable to statistically assess the influence of independent patient risk factors on the incidence of postoperative bleeding and of thromboembolic events due to the nature of our data set. We were however able to identify an association between lower baseline platelets and increased risk of bleeding in liver transplant recipients.
Limitations
The results of this study should be interpreted within the context of the presence of limitations. First, this study was an observational retrospective review, with unbalanced comparison arms and a small sample size. The study did not account for additional procedural manipulations during transplantation such as Roux-en-Y. It is also important to consider potential bias of treatment practice differences between the 2 groups since patients who received pharmacological prophylaxis were all transplanted at one center, Mayo Clinic Rochester. The Padua is commonly used in medical patients but has not been specifically validated in critically ill patients. Lastly, the variability of the pharmacological prophylaxis regimen received in the study group, in combination with the small sample size, limits interpretation and stratification of the observed outcomes as it pertains to specific agents.
Conclusion
Pharmacological thromboprophylaxis in cirrhotic patients pending liver transplantation did not decrease the risk of postoperative thrombosis, nor did it increase the risk of postoperative bleeding in this patient population. Given the low rate of thrombosis, future studies should focus on assessing which patient factors are associated with incidence of thrombosis in order to better stratify the decision of which patients would benefit most from receiving pharmacological prophylaxis. Furthermore, data is needed to help determine which agent is associated with lowest incidence of postoperative bleeding while providing effective VTE protection.
Disclosure
The authors of this manuscript have no industry relations or financial conflicts of interest to disclose.
Funding
This research received no specific grant from any funding agency.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgement
None.
Conflict of Interest
None.
References
- Anderson FA, Wheeler HB (1995) Venous thromboembolism. Risk factors and prophylaxis. Clin Chest Med 16(2): 2350-251.
- Tripodi A, Primignani M, Chantarangkul V, Clerici M, Giuseppe M, et al. (2009) An imbalance of pro vs anti-coagulation factors in plasma from patients with cirrhosis. J Gastroenterol 137(6): 2105-2111.
- Khoury T, Ayman AR, Cohen J, Daher S, Shmuel C, et al. (2016) The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion 93(2): 149-159.
- Moghadamyeghaneh Z, Alameddine M, Jue J, Guerra G, Selvaggi G, et al. (2018) A nationwide analysis of re-exploration after liver transplant. HPB 20(3): 2160-221.
- Schrem H, Klumann A, Focken M, Emmanouilidis N, Oldhafer F, et al. (2016) Post-operative hemorrhage after liver transplantation: Risk factors and long-term outcome. Ann Transplant 21(1): 46-55.
- Stahl RL, Duncan A, Hooks MA, Henderson JM, Millikan WJ, et al. (1990) A hypercoagulable state follows orthotopic liver transplantation. J. Hepatol 12(3): 553-558.
- Bedreli S, Straub K, Achterfeld A, Willuweit K, Katsounas A, et al. (2019) The effect of immunosuppression on coagulation after liver transplantation. Liver Transpl 25(7): 1054-1065.
- Ormrod D, Jarvis B (1998) A review of the use of thymoglobulin® in the prevention and treatment of acute renal allograft rejection. Bio Drugs 14(2): 255-273.
- Mukherjee S, Mukherjee U (2009) A comprehensive review of immunosuppression used for liver transplantation. J Transplant 2009: 701464.
- Huang LQ, Whitworth JA, Chesterman CN (1995) Effects of cyclosporin A and dexamethasone on haemostatic and vasoactive functions of vascular endothelial cells. Blood Coagul Fibrinolysis 6(5): 438-445.
- Bombeli T, Muller M, Straub PW, Haeberli A (1996) Cyclosporine-induced detachment of vascular endothelial cells initiates the intrinsic coagulation system in plasma and whole blood. J Lab Clin Med 127 (6): 621-634.
- Sogaard KK, Horvath PE, Gronbaek H, Jepsen P, Vilstrup H, et al. (2009) Risk of venous thromboembolism in patients with liver disease: A nationwide population-based case-control study. Am J Gastroenterol 104(1): 96-101.
- Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM (2010) Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest 137(5): 1145-1149.
- Aldawood A, Arabi Y, Aljumah A, Alsaeedi A, Rishu A, et al. (2011) The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J 9(1): 1.
- Northup PG, McMahon MM, Ruhl AP, Altschuler SA, Caldwell SH, et al. (2006) Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol 101(7): 1524-1528.
- Gully D, Teal E, Suvannasankha A, Chalasani N, Liangpunsakul S (2008) Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci 53(11): 3012-3017.
- Garcia-Fuster MJ, Abdilla N, Fabia MJ, Fernandez C, Oliver V, et al. (2008) Venous thromboembolism and liver cirrhosis. Rev Esp Enferm Dig 100(5): 259-262.
- Annamalai A, Kim I, Sundaram V (2014) Incidence and risk factors of deep vein thrombosis after liver transplantation. Transplant Proc 46(10): 3564-3569.
- Ishitani M, Angle J, Bickston S, Caldwell S, Isaacs R, et al. (1997) Liver transplantation: Incidence and management of deep venous thrombosis and pulmonary emboli. Transplant Proc 29(7): 2861-2863.
- Lloyd-Donald P, Vasudevan A, Angus P, Gow P, Glassford N, et al. (2020) Comparison of thromboelastography and conventional coagulation tests in patients with severe liver disease. Clin Appl Thromb Hemost 26(9): 1-10.
- Lloyd-Donald P, Vasudevan A, Angus P, Gow P, Martensson J, et al. (2020) Comparison of thromboelastography and conventional coagulation tests in patients with severe liver disease. Clin Appl Thromb Hemost 26:1076029620925915.
- Peng HT, Nascimento B, Beckett A (2018) Thromboelastography and thromboelastometry in assessment of fibrinogen deficiency and prediction for transfusion requirement: A descriptive review. Biomed Res Int 2018(11): 1-24.
- Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B (2010) A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua Prediction Score. J Thromb Haemost 8(11): 2450-7.
- Smith CB, Hurdle AC, Kemp LO, Sands C, Twilla JD (2013) Evaluation of venous thromboembolism prophylaxis in patients with chronic liver disease. J Hosp Med 8(10): 569-573.
- Yerke J, Bauer SR, Bass S, Torbic H, Militello M, et al. (2019) Effectiveness of venous thromboembolism prophylaxis in patients with liver disease. World J Hepatol 11(4): 379-390.
- Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, et al. (2004) Deep vein thrombosis and pulmonary embolism in two cohorts: The longitudinal investigation of thromboembolism etiology. Am J Med 117:19-25.
- Salami A, Qureshi W, Kuriakose P, Moonka D, Yoshida A, et al. (2013) Frequency and predictors of venous thromboembolism in orthotopic liver transplant recipients: A single-center retrospective review. Transplant Proc 45(1): 315-319.
- Annamalai A, Kim I, Sundaram V (2014) Incidence and risk factors of deep vein thrombosis after liver transplantation. Transplant Proc 46(10): 3564-3569.
- Yip J, Bruno DA, Burmeister C, Kazimi M, Yoshida A, et al. (2016) Deep vein thrombosis and pulmonary embolism in liver transplant patients: Risks and prevention. Transplant Direct 2(4): 68
Citation: Pierre N, Liu V, Soto-Arenall M, Niven A, Ceba RC, et al. (2024) Multicentric Assessment of Efficacy and Safety Outcomes with Pharmacological Thromboprophylaxis in the Setting of Liver Transplant. J Gastrointest Dig Syst.14:784. DOI: 10.4172/2161-069X.1000784
Copyright: © 2024 Pierre N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1908
- [From(publication date): 0-2024 - Nov 07, 2025]
- Breakdown by view type
- HTML page views: 1603
- PDF downloads: 305