Mucosal Immune Responses Associated with NKT Cell Activation and Dendritic Cell Expansion by Nasal administration of α-galactosylceramide in the Nasopharynx
Received: 16-Oct-2015 / Accepted Date: 17-Nov-2015 / Published Date: 24-Nov-2015 DOI: 10.4172/2161-119X.1000216
Abstract
Objective: Intranasal immunization is an effective method to induce mucosal immune responses in the upper respiratory tract.α-galactosylceramide (α-GalCer) is considered as one of the most potent candidates for a mucosal adjuvant. In the present study, mucosal immune responses in the nasopharynx associated with NKT cell activation by nasal administration of α-GalCer were examined for inducing protective immunity in the nasopharynx, with the ultimate goal of developing a mucosal vaccine for preventing upper respiratory infectious diseases. Methods: Mice were administered α-GalCer intranasally as a ligand for NKT cells, without any antigen, weekly total three times. One week after the final administration of α-GalCer, mice were killed, and nasal immune responses were examined. Dendritic cells (DCs) in nasal-associated lymphoid tissue (NALT), a mucosal inductive site, were examined by immunohistochemistry. DCs, NKT cells, and B cells in NALT and nasal passage (NP) were examined by flow cytometry. Cytokine-producing CD4+ T cells were also examined by flow cytometry. Quantification of immunoglobulin (Ig)-producing cells was examined by enzyme-linked immunospot (ELISPOT) assay. In addition, bacterial challenges with live Haemophilus influenzae (Hi) and Streptococcus pneumoniae (Sp) were performed, and bacterial clearance from nasopharynx was examined. Results: After nasal immunization of α -GalCer, DCs increased in NALT. Antibody-producing cells, mainly those that produce IgA, significantly increased in the NP, a mucosal effector site. Interleukin (IL)-17-producing Th 17 cells were also induced in the NP. Bacterial challenges with live Hi and Sp resulted in enhanced clearances of both bacterial species from the nasopharynx. It is interesting that bacterial clearance was impaired by IL-17 neutralization. Conclusion: Nasal vaccination is effective for the induction of protective immunity against upper respiratory infection. The results of the present study demonstrated that nasal administration of α-GalCer could activate NKT cells in the nasopharynx, followed by the maturation of DCs, B cells, and some cytokine-producing CD4+ T cells. These findings suggest that the activation of NKT cells by nasal administration of α-GalCer induced protective immunity in the nasopharynx, possibly involving the interaction with DCs and induction of Th17 cells.
Keywords: α-GalCer; NKT cell, Dendritic cell, Th17, Mucosalimmunity, Nasal vaccine, Haemophilus influenza, Streptococcuspneumoniae
251193Introduction
Mucosal surfaces such as those of the respiratory, gastrointestinal, and genital tracts, are the main entry site of most environmental antigens (Ags) or pathogens, and thus act as the first line of defense. Many studies have focused on developing mucosal vaccines capable of effectively inducing both mucosal and systemic immune responses [1-4]. Intranasal immunization is an effective method to elicit mucosal immune responses in the upper respiratory tract, and has shown efficacy in enhancing bacterial clearance from the nasopharynx, lungs, and other organs [5-7].
NKT cells are a specific subset of immune regulatory cells that express an invariant antigen receptor α-chain encoded by a Vα14- Jα28.1 rearranged gene segment in mice and Vα14-Jα28 in humans [8]. Glycolipids such as α-galactosylceramide (α-GalCer) presented via CD1d can directly activated NKT cells [9]. Upon activation, NKT cells rapidly produce both Th1 and Th2 cytokines, including interferon (IFN)- γ and interleukin (IL)-4, which contribute to the upregulation of both cellular and humoral immune responses [10].
Nontypeable Haemophilus influenzae (NTHi) and Streptococcus pneumoniae (Sp) are major pathogens of upper respiratory tract and cause infectious diseases including otitis media (OM) and rhinosinusitis [11-14]. Since colonization of these pathogens in the nasopharynx is important in the pathogenesis of OM, inhibition of bacterial colonization is effective for preventing upper respiratory infections. Due to recent increases in antibiotic-resistant NTHi or Sp strains, the development of a mucosal vaccine is considered an important public health goal. Previously, we showed that α-GalCer is one of the most potent candidates for a mucosal adjuvant [15,16]. Nasal vaccination with P6 outer membrane protein, which is common to all NTHi strains, and α-GalCer induced P6-specific immunoglobulin (Ig)A and Th1/Th2 immune responses in the nasopharynx, and nasal vaccination with P6 and α-GalCer induced NTHi-specific protective immunity, enhancing bacterial clearance from the nasopharynx [15,16]. Recently, it was shown that the co-administration of α-GalCer with ovalbumin (OVA) can induce full maturation of dendritic cells (DCs), thereby generating functional Ag-specific Th1-type CD4+ and CD8+ T cells that are resistant to OVA-expressing tumors [17]. In addition, α-GalCer administration triggers the in vivo maturation of mesenteric DCs. This contributes to T cell division in vitro and blocks the tolerance induced by both high and low doses of oral OVA [18]. It is also known that α-GalCer can serve as an effective adjuvant for a nasal vaccine that induces substantial protective immune responses against viral infections and tumor growth [19].
In this study, we investigated the efficacy of NKT cell activation by nasal administration of α-GalCer for inducing protective immunity in the nasopharynx, with the ultimate goal of developing a mucosal vaccine for preventing upper respiratory infectious diseases.
Materials and Methods
Animals
Specific pathogen-free (SPF) BALB/c mice (6 weeks old) were used in this study, and mice were maintained under SPF conditions throughout all experiments. The study was approved by the Committee on Animal Experiments of Oita University, Oita, Japan, and was performed according to local guidelines for animal experiments.
Administration of α-GalCer
α-GalCer (KRN7000, Kirin Pharma Co., Tokyo), was used for nasal immunization and activating nasal NKT cells. Mice were immunized intranasally on days 0, 7, and 14 with 10 μl phosphate-buffered saline (PBS) containing 2 μg of α-GalCer (α-GalCer group). Control mice were administered PBS without antigen (control group). Mice were killed on day 21, and nasal passages (NPs) and nasal-associated lymphoid tissue (NALT) were collected.
Immunohistochemistry
For histological analysis, mice were killed under deep anesthesia on day 21 and then perfused transcardially with PBS, followed by 10% neutral buffered formalin. Heads were immersed in the same fixative for 6 h and decalcified with 0.12 M EDTA for 2 weeks. After dehydration, tissues were embedded in paraffin. To detect CD11c+ DCs in NALT, a mucosal inductive site in the upper respiratory tract, 12-μm-thick serial and horizontal paraffin sections were prepared for light microscopic examination. Specimens were dehydrated through a graded series of ethanol and treated with 3% H2O2 in absolute methanol for 20 min. Sections were exposed to a 5% normal mouse serum in PBS for 30 min and then incubated for 24 h with biotinylated goat anti-mouse CD11c antibody. After rinsing with PBS, sections were incubated with ABC reagent for 1 h and developed in 0.05% 3,3’-diaminobenzidine-0.01% H2O2 substrate medium in 0.1 M phosphate buffer for 8 min.
Flow cytometry for DCs, NKT cells, and B cells
Mononuclear cells (MNCs: 1 x 106 cells) were isolated from NALT and NP, and the number of CD11c+ DCs, as well as the expression of functional markers on DCs, and NKT cells in NALT were analyzed by flow cytometry as described previously [20,21]. The number of B cells in NALT and NP were also analyzed by flow cytometry. The following monoclonal antibodies (mAbs) were used in this study: fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD11c (HL3), phycoerythrin (PE)-conjugated anti-mouse CD11b (M1/70), Cy-Chrome-conjugated anti-mouse CD8α (53-6.7), Cy-Chrome conjugated anti-mouse CD11c (HL3), FITC-conjugated anti-mouse MHC class I (H-2Kb, AF6-88.5), FITC-conjugated anti-mouse MHC class II (I-A/I-E, 2G9), FITC-conjugated anti-mouse CD80 (B7-1; 16-10A1), FITC-conjugated anti-mouse CD86 (B7-2; GL1), FITCconjugated anti-mouse αβ (TCR β chain, H57-597), FITC-conjugated anti-mouse CD45R/B220 (RA3-6B2), PE-conjugated anti-mouse CD5 (53-7.5), PE-conjugated anti-mouse CD1d tetramer, and CyChromeconjugated anti-mouse anti-mouse CD45R/B220 (RA3-6B2). These mAbs were purchased from BD Pharmingen (San Diego, CA). PEconjugated mouse CD1d tetramer was purchased from BMC (Nagoya, Japan), and CD1d tetramer was loaded with α-GalCer according to the manufacturer’s instructions. MNCs were incubated with various combinations of mAbs, and samples were analyzed by FACS Calibur (Becton Dickinson, Sunnyvale, CA).
Quantification of Ig-producing cells
MNCs were isolated from the NP and NALT, and the numbers of total Ig-producing cells were determined using an enzyme-linked immunospot (ELISPOT) assay, as previously described [15,20]. Briefly, 96-well plates (MultiScreen; Millipore) were coated with goat antimouse Ig (H+L) UNLD (1.0 μg/well) and incubated overnight at 4°C. Plates were washed and then blocked with complete medium for 1 h. Test cells were added at varying concentrations and were cultured at 37°C, 5% CO2 for 4 h. After washing, horseradish peroxidase (HRP)-labeled goat anti-mouse IgM, IgG, or IgA was added (SBA). After overnight incubation at 4°C, the plates were washed and the spots developed at room temperature with 100 μl AEC (Moss) after incubation for 30 min.
Flow cytometry of cytokine-producing CD4+ T cells
MNCs (1 x 106 cells) from the NP were incubated with 100 μg/ mL phorbol myristate acetate and 2 μg/mL ionomycin (Sigma, St. Louis, MO, USA) for 12 h in the presence of Golgi plug (BD Pharmingen). After fixing and permeabilization with Perm/fix solution (BD Pharmingen), cells were incubated with 1 μg/mL Fc Block (BD Pharmingen) for 15 min, and then stained with following mAbs: CyChrome-conjugated anti-mouse CD4 (H129.19), FITC-conjugated anti-mouse IFN-γ (XMG1.2), PE-conjugated anti-mouse IL-17 (TC11- 18H10; BD Pharmingen) or PE-conjugated anti-mouse IL-22. After intracellular staining, samples were analyzed by FACS Calibur [21].
NTHi and Sp clearance from the nasopharynx
On day 21, bacterial challenge was performed and bacterial clearance was examined, as previously described [15,20]. Briefly, NTHi (strain 76) and Sp were cultured on chocolate agar plates and blood agar plates alternately overnight at 37°C in 5% CO2, collected by scraping, and then resuspended in PBS (1010 CFU/ml) for nasal challenge. A 10- μl aliquot (108 CFU) of the live NTHi (NTHi group) or Sp (Sp group) suspension was injected intranasally. Then, 24 h after nasal challenge, mice were killed and nasal wash samples were collected. Aliquots from each sample were serially diluted 10-fold with sterile PBS, and 10-μl aliquots of the diluted samples were spread on chocolate agar plates (NTHi group) or blood agar plates (Sp group) for quantification of live bacteria. After overnight incubation at 37°C in 5% CO2, the bacterial colonies were counted.
Neutralization of nasal IL-17 and IL-22 was performed to clarify the role of Th17 cells in protective immunity. To neutralize IL-17 and IL-22 in the nasopharynx, mice were twice intranasally administered 10 μg anti-mouse IL-17 mAb (50104; R&D Systems) and/or IL-22 mAb (142928; R&D Systems) in 10 μl PBS on days 16 and 20 [16]. Bacterial clearance was then examined, as described above (groups: control, α-GalCer, α-GalCer+anti-IL-17, α-GalCer+anti-IL-22, and α-GalCer+anti-IL-17+anti-IL-22).
Statistical analysis
Mann-Whitney U-test was used to compare data. P-values less than 0.05 were considered significant.
Results
DCs and NKT cells in NALT
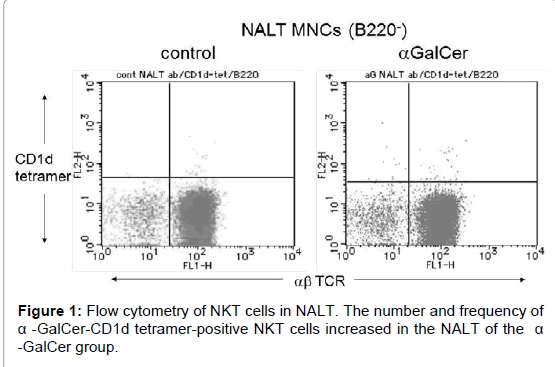
The number and frequency of NKT cells were investigated by flow cytometry. The control group contained 1.49 × 102 cells of NKT cells in the NALT per mouse. The number and frequency of α-GalCer-CD1d tetramer-positive NKT cells increased in the NALT of the α-GalCer group (Table 1 and Figure 1). The α-GalCer group contained 3.88 × 102 cells of NKT cells in the NALT per mouse.
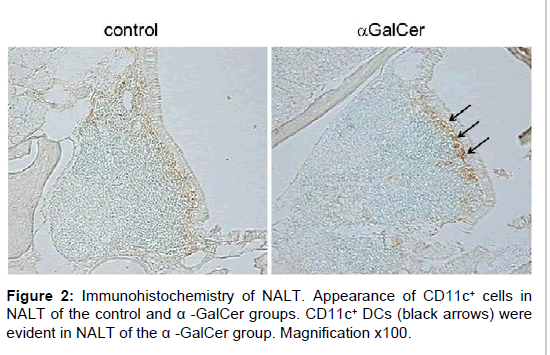
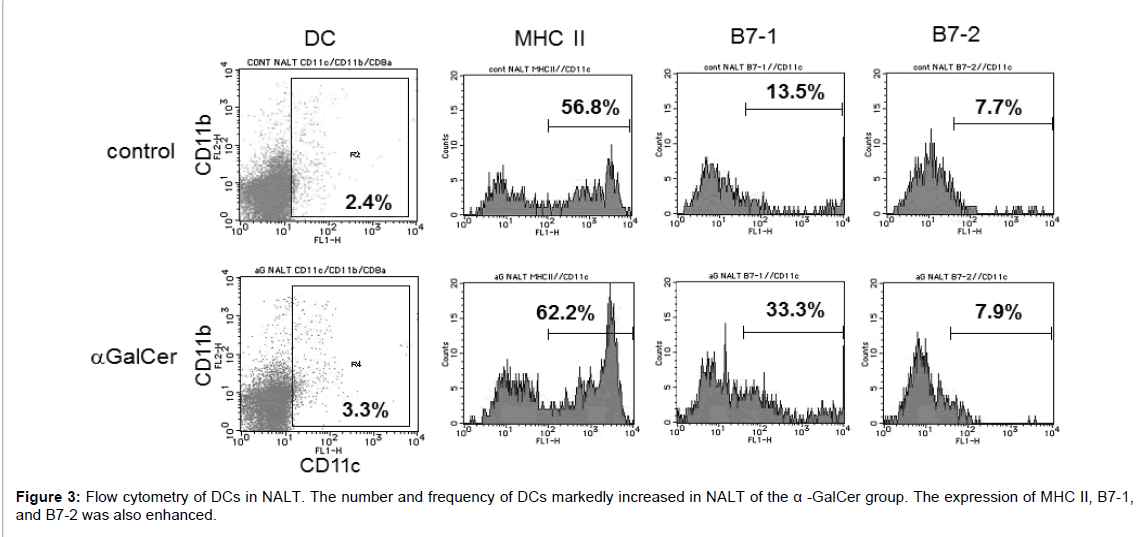
Immunohistochemical analysis showed that CD11c+ DCs were present in NALT of the α-GalCer group, with fewer CD11c+ DCs in NALT of the control group. Following nasal immunization with α-GalCer, CD11c+ DCs were evident in NALT of the α-GalCer group (Figure 2). The increase in DCs was confirmed by flow cytometry. The control group contained 1.07 × 103 cells of DCs in the NALT per mouse. The number of CD11c+ DCs increased in NALT of the α-GalCer group. The α-GalCer group contained 1.99 × 104 cells of DCs in the NALT per mouse. Statistical significant difference was detected between the control group and immunized group (Table 1). Among the increased DCs in NALT of the α-GalCer group, the expression of MHC class II, and B7-1 was markedly enhanced, when compared with those of the control group (Figure 3).
| Group | CD11c+ DC † | NKT cell† | ||
|---|---|---|---|---|
| frequency (%)‡ | number (cells)§ | frequency (%)‡ | number (cells)§ | |
| a-GalCer | 3.33 ± 0.15 * | 1.99 x 104 * | 0.11 ± 0.032 | 3.88 x 102 |
| Control | 2.20 ± 0.12 | 1.07 x 103 | 0.06 ± 0.017 | 1.49 x 102 |
| † Nasal-associated lymphoid tissue (NALT) cells were analyzed by three-color flow cytometry. NKT cells were determined as a-GalCer-CD1d tetramer-positive cells among B220-, CD4+ cells. ‡ Frequency of CD11c+ dendritic cells (DCs) and B220- NKT cells in NALT mononuclear cells. Values are expressed as mean SE. § Absolute number of CD11c+ DCs and NKT cells in NALT per mouse. Values were calculated according to the absolute number of isolated lymphocytes and the mean frequency of CD11c+ DCs or NKT cells. * p<0.05 compared with control group. |
||||
Table 1: Dendritic cells and NKT cells in NALT.
B cells in NALT and NP
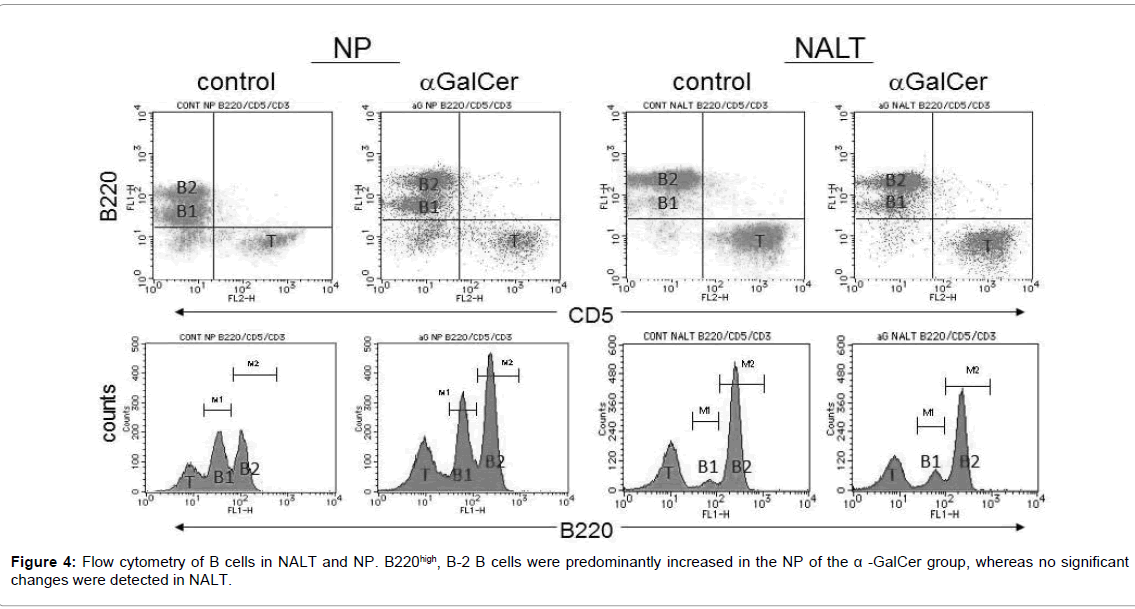
The number of B cells was also confirmed by flow cytometry. The control group contained 2.49 × 105 cells of B220high, B-2 B cells in the NP per mouse. Following nasal immunization with α-GalCer, B220high, B-2 B cells were predominantly increased in the NP, a mucosal effector site. The α-GalCer group contained 3.27 × 105 cells of B220high, B-2 B cells in the NP per mouse. Statistical significant difference was detected between the control group and immunized group (Table 2 and Figure 4).Whereas no significant changes were detected in NALT, a mucosal inductive site.
Antibody-producing cells
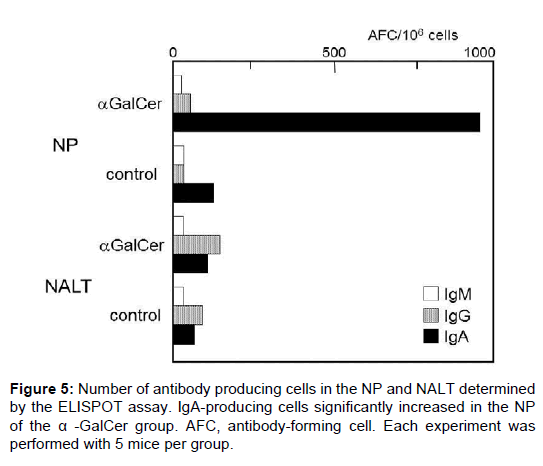
The number of antibody-producing cell was determined by ELISPOT assay. A small number of antibody-producing cells was detected in the NP and NALT of the control group. Following nasal immunization with α-GalCer, the number of IgA-producing cells significantly increased in the NP of the α-GalCer group (Figure 5). Fewer IgA-producing cells were evident in NALT than in the NP. We also found no significant difference in IgG- and IgM-producing cells between the groups. This result shows that nasal immunization with α-GalCer induced B-cell activation and mucosal IgA production in the mucosal effector site (the NP).
Cytokine-producing CD4+ T cells in the NP
The number of cytokine-producing CD4+ T cells in the NP was also confirmed by flow cytometry. The control group contained 9.47 × 102 cells of IL-17-producing T cells in the NP per mouse. Following nasal immunization with α-GalCer, IL-17-producing T cells were increased in NP. The α-GalCer group contained 2.52 × 103 cells of IL-17-producing T cells in the NP per mouse. Statistical significant difference was detected between the control group and immunized group (Table 3). There was no significant difference in the number of IL-22-producing T cells between the control group and immunized group. As Th17 is the typical T cell that produces IL-17 and IL-22, this result seems to indicate that Th17 is involved in host defense in the upper respiratory tract induced by nasal immunization with α-GalCer.
NTHi and Sp clearance from the nasopharynx
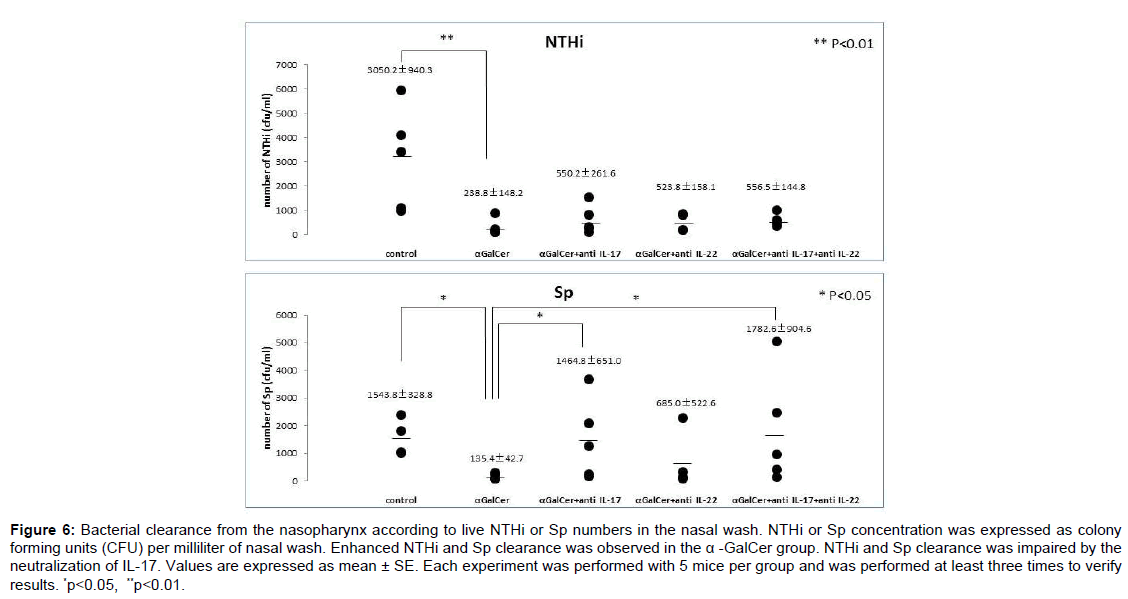
Bacterial clearance from the nasopharynx was investigated according to live NTHi or Sp numbers in the nasal wash. After the bacterial challenges, a large number of NTHi or Sp was detected in the nasal wash of the control group. After nasal immunization, clearance of both NTHi and Sp was significantly enhanced in mice immunized with α-GalCer, as indicated by the reduced live NTHi and Sp numbers in nasal washes of the α-GalCer group, respectively. Statistical significant differences in the clearance of both NTHi and Sp were detected between the control group and immunized group (Figure 6).
| Group | NP† | NALT† | ||
|---|---|---|---|---|
| frequency (%) ‡ | number (cells) § | frequency (%) ‡ | number (cells) § | |
| a-GalCer | 40.1 ± 0.50* | 3.27 x 105* | 53.3 ± 0.63 | 4.60 x 105 |
| Control | 30.6 ± 1.03 | 2.49 x 105 | 58.4 ± 0.19 | 3.63 x 105 |
| † Nasal passage tissue cells and nasal-associated lymphoid tissue cells were stained to detect B220 expression to distinguish B2 B cells (B220high) from B1 B cells (B220low) and analyzed by flow cytometry. ‡ Frequency of B2 B cells. Values are expressed as mean ± SE. § Absolute number of B2 B cells in NP per mouse. Each value was calculated in according to the absolute number of isolated lymphocytes and the mean frequency of B2 B cells. *p<0.05 compared with control group. |
||||
Table 2: B cells in the NP and NALT.
| Group | cytokine† | frequency (%) ‡ | number (cells) § |
|---|---|---|---|
| a-GalCer | IL-17 | 2.02 ± 0.02* | 2.52 x 103 * |
| IL-22 | 1.02 ± 0.03 | 1.25 x 103 | |
| Control | IL-17 | 0.85 ± 0.07 | 9.47 x 102 |
| IL-22 | 1.03 ± 0.02 | 1.02 x 103 | |
| † NP cells were subjected to intracellular cytokine staining, and cytokine-producing cells were analyzed by flow cytometry. ‡ Frequency of IL17+, or IL-22+ CD4+ T cells in whole CD4+ T cells. Each value is expressed as the mean ± SE. § Absolute number of cytokine-producing CD4+ T cells in NP per mouse. Each value was calculated in according to the absolute number of isolated lymphocytes and the mean frequency of IL17+, or IL-22+ CD4+ T cells. * p<0.05 compared with control group. |
|||
Table 3: Cytokine-producing CD4+ T cells in the NP.
To investigate the role of IL-17 and IL-22 in protective immunity, bacterial clearance was also examined after neutralization of IL-17 and/or IL-22. Of interest, impaired Sp clearance was observed in the mice administered α-GalCer after additional intranasal administration of anti-IL-17 mAb and after administration of both anti-IL-17 and anti-IL-22. Statistical significant differences were detected between the α-GalCer group and IL-17-neutralization groups (α-GalCer+ anti-IL-17, and α-GalCer+ anti-IL-17+anti-IL-22; Figure 6). Whereas neutralization of IL-17 and/or IL-22 did not affect NTHi clearance enhanced by nasal immunization with α-GalCer. In protective nasopharyngeal immunity, a difference in key cytokines was observed in Sp and NTHi infections. This difference might depend on whether the pathogen is an extracellular or intracellular parasite, because the Th17 response facilitates the clearance of extracellular pathogens [22]. This result indicates that Th17 cells contribute partially to non-specific protective immunity, especially against extracellular parasites, induced by nasal administration of α-GalCer.
Discussion
Nasal vaccination is considered to be effective for the induction of protective immunity against upper respiratory infectious diseases, including OM and rhinosinusitis. [6,20,23-26]. The results of the present study demonstrated that nasal administration of α-GalCer alone could activate NKT cells in the nasopharynx, followed by the maturation of DCs, B cells, and some cytokine-producing CD4+ T cells. In our previous study we demonstrated that nasal immunization with P6 protein of NTHi and α-GalCer is effective in eliciting Agspecific secretory IgA in the nasopharynx and specific IgG responses in the systemic compartment. Nasal vaccination with 10 μg P6 and 2 μg α-GalCer successfully induced NTHi-specific protective immunity, enhancing bacterial clearance from the nasopharynx [15,16]. In this study, the clearance of Sp as well as NTHi was partially enhanced by nasal administration of 2 μg α-GalCer alone, without bacterial Ags, since nasal immunization with bacterial Ags and α-GalCer could induce more effectively Ag-specific immune responses. Although the level of expansion in NKT cells and DCs in the present study was lower than in our previous study [15,16], certain immune responses against pathogenic bacteria were induced by nasal administration of α-GalCer. Thus, in the present study we demonstrated that the activation of NKT cells by nasal administration of α-GalCer induces broadly protective immunity in the upper respiratory tract. These responses were characterized by increased local IgA-producing cells and protection against NTHi or Sp challenge. To our knowledge, this is the first study to show the protective effect of NKT cells against both NTHi and Sp by nasal administration of α-GalCer (without any antigen).
Figure 6: Bacterial clearance from the nasopharynx according to live NTHi or Sp numbers in the nasal wash. NTHi or Sp concentration was expressed as colony forming units (CFU) per milliliter of nasal wash. Enhanced NTHi and Sp clearance was observed in the a -GalCer group. NTHi and Sp clearance was impaired by the neutralization of IL-17. Values are expressed as mean ± SE. Each experiment was performed with 5 mice per group and was performed at least three times to verify results. *p<0.05, **p<0.01.
α-GalCer is a ligand of NKT cells and thus can directly activate them when presented by CD1d. NKT cells both directly and indirectly modulate the functions of many other cell types, including NK cells and T cells. These interactions are bidirectional, as NKT cells receive signals from antigen-presenting cells (APCs), such as DCs, and vice versa [27]. Activation of NKT cells through recognition of α-GalCer-CD1d complexes results in the rapid production of Th1 and Th2 cytokines, such as IFN-γ and IL-4 [9,28], and the increased expression of the CD40 ligand [29], which induces DC maturation [30,31]. Interaction of NKT cells and DCs is also known to be important for the induction of protective immunity [10,15,18]. In the present study, although these mucosal immune responses were activated without Ag exposure under SPF but not germ-free conditions, the clearance of NTHi and Sp might have been enhanced because of cross-reactive antibodies, antimicrobial peptides, a proliferation-inducing ligand (APRIL) and/or induction of B-cell activating factor (BAFF), by activation of DCs and NKT cells in the nasal mucosa. The mucosal microenvironment and interaction with commensal bacteria are also essential for the development and maintenance of mucosal IgA and homeostasis [32]. Protective mucosal IgA against pathogenic bacteria could be induced by the activation of mucosal DCs carrying commensal bacteria [33]. Thus, the activation of nasal NKT cells by α-GalCer administration resulted in DC expansion and maturation in the nasal mucosa, and might contribute to the induction of nasopharyngeal protective immunity under SPF but not germ-free condition. In addition, activation of innate immune cells such as natural killer cells, or another immune pathway via Th17 cells might also be involved, and further investigation might be necessary to clarify this mechanism and to develop a broadly effective vaccine in the clinical setting.
For the pathway involving Th17 cells, the interaction between NKT and Th17 cells was examined by measuring Th17 cytokine-producing CD4+ T cells. Following nasal immunization with α-GalCer, IL-17- producing cells were increased in NP, so this result seems to indicate that Th17 cells are involved in host defense against Sp and NTHi in the upper respiratory tract. Although Th17 cells were originally identified as pro-inflammatory mediators of autoimmune disease [34], emerging data suggest that they play crucial roles in immune defense against extracellular and intracellular pathogens, including bacterial and fungal species, especially at mucosal surfaces [35,36]. Th17 cells contribute to protection by functioning as antimicrobial peptides, enhancing neutrophil recruitment, and modulating DC function [37,38]. Recently, nasal immunization has been shown to preferentially promote Th17 immune responses [39]. Furthermore, vaccine-induced Th17 cells have been shown to improve immune protection against Sp in the upper and lower respiratory tract [40,41]. Regarding the response against NTHi, we also previously demonstrated the involvement of Th17 cells in the response induced by nasal vaccination in the nasopharynx [16]. In conclusion, the results of the present study indicate that the activation of NKT cells by nasal administration of α-GalCer might positively modulate the mucosal immune system, possibly involving interaction with DCs and Th17 cells, in the nasopharynx.
References
- Czerkinsky C,Anjuere F, McGhee JR, George-Chandy A, Holmgren J, et al. (1999) Mucosal immunity and tolerance: relevance to vaccine development.Immunol Rev 170: 197-222.
- Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, et al. (1998) Mucosal immunization with HIV-peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. ProcNatlAcadSci 95: 1709-1714.
- Berzofsky JA,Ahlers JD, Belyakov IM (2001) Strategies for designing and optimizing new generation vaccines.Nat Rev Immunol 1: 209-219.
- Kozlowski PA,Neutra MR (2003) The role of mucosal immunity in prevention of HIV transmission.CurrMol Med 3: 217-228.
- Wu HY, Nahm MH, Guo Y, Russel MW, Briles DE (1997) Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcupneumoniae. J Infect Dis 175: 839-846.
- Sabirov A, Kodama S, Hirano T, Suzuki M, Mogi G (2001) Intranasal immunization enhances clearance of nontypeableHaemophilusinfluenzae and reduces stimulation of tumor necrosis factor alfa production in the murine model of otitis media. Infect Immun 69: 2964-2971.
- Kurono Y, Yamamoto M, Fujihashi K, Kodama S, Suzuki M, et al. (1999) Nasal immunization induces Haemophilusinfluenzae-specific Thand Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity.J Infect Dis 180: 122-132.
- Kronenberg M(2005) Toward an understanding of NKT cell biology: progress and paradoxes.Annu Rev Immunol 23: 877-900.
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, et al. (1997) CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides.Science 278: 1626-1629.
- Taniguchi M, Seino K, Nakayama T (2003) The NKT cell system: bridging innate and acquired immunity.Nat Immunol 4: 1164-1165.
- Murphy TF(2003) Respiratory infections caused by non-typeableHaemophilusinfluenzae.CurrOpin Infect Dis 16: 129-134.
- Lynch JP 3rd,Zhanel GG (2010) Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines.CurrOpinPulm Med 16: 217-225.
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, et al. (2009) Hib and pneumococcal global burden of disease study team. burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374: 893–902.
- Levine OS, Knoll MD, Jones A, Walker DG, Risko N, et al. (2010) Global status of Haemophilusinfluenzae type b and pneumococcal conjugate vaccines: evidence, policies, and introductions.CurrOpin Infect Dis 23: 236-241.
- Noda K, Kodama S, Umemoto S, Abe N, Hirano T, et al. (2010) Nasal vaccination with P6 outer membrane protein and a-galactosylceramide induces nontypeableHaemophilusinfluenzae-specific protective immunity associated with NKT cell activation and dendritic cell expansion in nasopharynx. Vaccine 28: 5068-5074.
- Noda K, Kodama S, Umemoto S, Nomi N, Hirano T, et al. (2011) Th17 cells contribute to nontypeableHaemophilusinfluenzae-specific protective immunity induced by nasal vaccination with P6 outer membrane protein and a-galactosylceramide. MicrobiolImmunol 55: 574-581.
- Fuji S, Shimizu K, Smith C, Boifaz L, Steinman RM (2003) Activation of natural killer T cells by a-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med 198: 267-279.
- Chung Y, Chang WS, Kim S, Kang CY (2004) NKT cell ligand alpha-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation.Eur J Immunol 34: 2471-2479.
- Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, et al. (2005) a-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol 175: 3309-3317.
- Kodama S, Hirano T, Noda K, Abe N, Suzuki M (2010) A single nasal dose of fms-like tyrosine kinase receptor-3 ligand, but not peritoneal application, enhances nontypeableHaemophilusinfluenzae–specific long term mucosal immune responses in the nasopharynx. Vaccine 28: 2510-2516.
- Hiroi T, Iwatani K, Iijima H, Kodama S, Yanagita M, et al. (1998) Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur I Immunol 28: 3346-3353.
- Siciliano NA, Skinner JA, Yuk MH (2006) Bordetellabronchiseptica modulates macrophage phenotype leading to the inhibition of CD4+ T cell proliferation and the initiation of a TH17 immune responce. J Immunol 177: 7131-7138.
- Kodama S, Suenaga S, Hirano T, Suzuki M, Mogi G (2000) Induction of specific immunoglobulin A and Th2 immune responses to P6 outer membrane protein of nontypeableHaemophilusinfluenzae in middle ear mucosa by intranasal immunization. Infect Immun 68: 2294-2300.
- Kiyono H, Fukuyama S (2004) NALT- versus Peyer's-patch-mediated mucosal immunity.Nat Rev Immunol 4: 699-710.
- Abe N, Kodama S, Hirano T, Eto M, Suzuki M (2006) Nasal vaccination with CpGoligodeoxynucleotide induces protective immunity against nontypeableHaemophilusinfluenzae in the nasopharynx. Laryngoscope 116: 407-412.
- Bertot GM, Becker PD, Guzman CA, Grinstein S (2004) Intranasal vaccination with recombinant P6 protein and adamantylamide dipeptide as mucosal adjuvant confers efficient protection against otitis media and lung infection by nontypeable Haemophilus influenzae. J Infect Dis 189: 1304-1312.
- Cerundolo V, Silk JD, Masri SH, Salio M (2009) Harnessing invariant NKT cells in vaccination strategies.Nat Rev Immunol 9: 28-38.
- Spada FM,Koezuka Y, Porcelli SA (1998) CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells.J Exp Med 188: 1529-1534.
- Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, et al. (2002) CD1-dependent dendritic cell instruction.Nat Immunol 3: 1163-1168.
- Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM (2004) The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation.J Exp Med 199: 1607-1618.
- Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, et al. (2003) NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells.J Immunol 171: 5140-5147.
- Macpherson AJ,Uhr T (2004) Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria.Science 303: 1662-1665.
- Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P (2008) The immune geography of IgA induction and function.Mucosal Immunol 1: 11-22.
- Bettelli E,Korn T, Oukka M, Kuchroo VK (2008) Induction and effector functions of T(H)17 cells.Nature 453: 1051-1057.
- O'Connor W Jr,Zenewicz LA, Flavell RA (2010) The dual nature of T(H)17 cells: shifting the focus to function.Nat Immunol 11: 471-476.
- Mucida D,Salek-Ardakani S (2009) Regulation of TH17 cells in the mucosal surfaces.J Allergy ClinImmunol 123: 997-1003.
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, et al. (2007) IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after immunization and during Mycobacterium tuberculosis challenge. Nat Immunol 8: 369-377.
- Bai H, Cheng J, Gao X, Joyee AG, Fan Y, et al. (2009) IL-17/Th17 promote typeT cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol 183: 5886-5895.
- Zygmunt BM,Rharbaoui F, Groebe L, Guzman CA (2009) Intranasal immunization promotes th17 immune responses.J Immunol 183: 6933-6938.
- Zhang Z, Clarke TB, Weiser JN (2009) Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice.J Clin Invest 119: 1899-1909.
- Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, et al. (2008) Interleukin-17A mediates acquired immunity to pneumococcal colonization.PLoS Pathog 4: e1000159.
Citation: Umemoto S, Kodama S, Hirano T, Noda K, Suzuki M (2015) Mucosal Immune Responses Associated with NKT Cell Activation and Dendritic Cell Expansion by Nasal administration of a-galactosylceramide in the Nasopharynx. Otolaryngology 5:216. DOI: 10.4172/2161-119X.1000216
Copyright: © 2015 Umemoto S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11506
- [From(publication date): 11-2015 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 10624
- PDF downloads: 882