Research Article Open Access
Movement Smoothness Differentiates Voluntary from Parkinsonian Bradykinesia
Nicolas Bayle1,2,6*, Stephen J Fried3, Elisabeth A Kappos4,7, Emilie Hutin1,2, Karen Fung5, Donald J Weisz5 and Jean-Michel Gracies1,2
1Laboratoire Analyse et Restauration du Mouvement, Université Paris Est Créteil, Créteil, France
2Service de Rééducation Neurolocomotrice, Unité de Neurorééducation, Hôpitaux Universitaires Henri Mondor, Créteil, France
3Physiologic Assessment Services, Teaneck, New Jersey, USA
4Department of Neurology, Mount Sinai Medical Centre, New York, USA
5Department of Neurosurgery, Mount Sinai Medical Centre, New York, USA
6Neuroscience Research Australia and University of New South Wales, Sydney, Australia
7Department of Plastic Hand Surgery, University Hospital Basel, Switzerland
- Corresponding Author:
- Nicolas Bayle
Service de Rééducation Neurolocomotrice AP-HP
Hôpitaux Universitaires Henri Mondor
51 Avenue du Maréchal de Lattre de
Tassigny 94000 Créteil, France
Tel: +331 49 81 49 42
Fax: +331 49 81 24 84
E-mail: nicolas.bayle@aphp.fr
Received date: date: Nov 17, 2015; Accepted date: date: Jan 25, 2016; Published date: date: Jan 31, 2016
Citation: Bayle N, Fried SJ, Kappos EA, Hutin E, Fung K, et al. (2016) Movement Smoothness Differentiates Voluntary from Parkinsonian Bradykinesia . J Addict Res Ther 7:264. doi:10.4172/2155-6105.1000264
Copyright: © 2016 Bayle N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Objective: While considered a key symptom, bradykinesia is not specific to Parkinson's disease (PD). Measuring movement smoothness may help distinguish PD-induced from volitional bradykinesia.
Methods: Eight PD patients and 12 healthy subjects performed alternating, maximal speed, small and large elbow flexion-extension movements. Six of the healthy subjects also performed the task while matching the average speed of PD patients. From angular displacement, we derived speed, acceleration, jerk measures and the power spectrum of acceleration frequencies. Acceleration variability was evaluated using the Normalized Average Rectified Jerk (NARJ) and the fast-frequency to movement-frequency (FF/MF) ratio. Ratios of maximal velocities and accelerations in large to those in small movements (L/S velocity and acceleration ratios) were also measured.
Results: NARJ in PD was 189 ± 17% of controls and 151 ± 14% of speed-matched controls (p=0.004; pairwise p=0.003, p=0.051 respectively) in large movements and 146 ± 11% of controls and 139 ± 11% of speed-matched controls (p=0.012; pairwise p=0.011, p=0.067 respectively) in small movements. FF/MF ratio in PD was 277 ± 45% of controls and 200 ± 32% of speed-matched controls (p=0.032; pairwise p=0.028, non-significant, respectively) in large movements and 613 ± 73% of controls and 246 ± 29% of speed-matched controls (p<0.001; pairwise p<0.001, p<0.001 respectively) in small movements. Time since diagnosis, but not age, was correlated with NARJ (p<0.05) and FF/MF ratio (p<0.01) for all movements. L/S ratios did not differentiate PD from speed-matched movements in the study sample.
Conclusion: The two smoothness metrics, NARJ and FF/MF ratio, distinguished PD from volitional slowness and correlated with time since diagnosis. They are candidate physiological markers of PD-induced bradykinesia.
Keywords
Parkinson; Bradykinesia; Smoothness; Jerk metric
Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disorder with motor and non-motor disturbances. Many classical motor features of PD (abnormal posture, bradykinesia, decreased step length) are specifically characterized by movement scaling difficulties. The search for quantitative markers of PD movement disturbances and of progression in PD will enhance our basic understanding of PD and facilitate the investigation of new potential neuro protective therapies.
While James Parkinson did not mention this feature in his original monograph, bradykinesia has become the major diagnostic symptom of idiopathic Parkinson’s disease (PD) in the past decades [1,2]. Indeed, movements are involuntarily slow (bradykinesia) in PD, especially when large amplitudes are required [3,4]. However, slow movements may also occur in somatoform disorders as well as in a number of other neurological, psychiatric or orthopaedic conditions [5]. Yet, in the face of a slow movement, there is currently no universally recognized movement characteristic to identify parkinsonian bradykinesia.
Clinically, when assessing bradykinesia by asking patients to perform rapid alternating movements [6-8], movement slowing is often associated with reduction of movement size, i.e., hypometria [9-12]. However, these subjective, mostly ordinal tests, do not measure hypometria, nor do they allow clinicians to quantify bradykinesia, much less to qualify it [9-12]. In particular, the relative distribution of bradykinesia over large vs. small movements is usually not evaluated. Yet, techniques quantifying bradykinesia have been proposed in laboratory experiments, which have approached the physiological mechanism of bradykinesia, particularly with respect to acceleration profile and smoothness [12-21].
In the search of markers for parkinsonian bradykinesia the present study has explored four potential metrics, with two hypotheses in mind. The first hypothesis was that the number of acceleration bursts that goes into producing one given movement, indicative of movement smoothness [20,21], would characterize PD bradykinesia versus voluntary slowness, regardless of the movement size. Hence, we evaluated two key parameters: 1) the Normalized Average Rectified Jerk (NARJ) [22]; 2) the ratio of the power in frequencies faster than the movement frequency to that at the movement frequency (FF/MF ratio) in the Fourier power spectrum of the acceleration profile.
The second hypothesis was that under scaled acceleration bursts in PD would cause bradykinesia and hypometria to predominate over large vs. small movements [9]. In quantified terms, there would be an abnormally low ratio of the size of speed or acceleration bursts over large to that over small movements in PD bradykinesia versus voluntary slowness. We thus tested two additional parameters: 3) the ratio of the maximal velocities reached in large to those in small movements (L/S velocity ratio) and; 4) the ratio of the maximal accelerations in large to those in small movements (L/S acceleration ratio). We studied these four parameters in large and small movements performed at maximal speed by PD patients and by healthy subjects, first moving naturally and then deliberately mimicking the movement slowness of PD, both to provide control for movement speed and to search for differences in motor control between PD and voluntary slowness.
Methods
Patients and healthy controls
This study was conducted in compliance with the Mount Sinai School of Medicine Institutional Review Board (IRB) regulations and with the World Medical Association Declaration of Helsinki. All healthy subjects and PD patients provided informed consent. A convenience sample of eight subjects with PD were recruited (1 woman; age 66 ± 7, mean ± SD, range 50-72; Hoehn and Yahr 2.5-4; five subjects had rest tremor). Inclusion criteria for PD subjects were: 1) age 20 to 75; 2) clinical diagnosis of idiopathic PD using the United Kingdom Parkinson’s disease Society Brain Bank (UK-PDSBB) clinical diagnostic criteria [2].
Twelve age-matched healthy subjects (6 women; age 64 ± 5, range 59-75) also participated. The healthy subjects met the following inclusion criteria: 1) age 50 to 75; 2) no history of neurological disorder or exposure to neurotoxic substances and no orthopaedic limitation in the upper limbs; 3) no intake of neurotropic or psychiatric medication, neither shortly before the experiments nor on a chronic basis.
Equipment and procedures
All patients were assessed in the clinically defined levodopa OFFstatus, i.e., after drug withdrawal for at least 12 hours for levodopa and 48 hours for other anti-parkinsonian drugs [23].
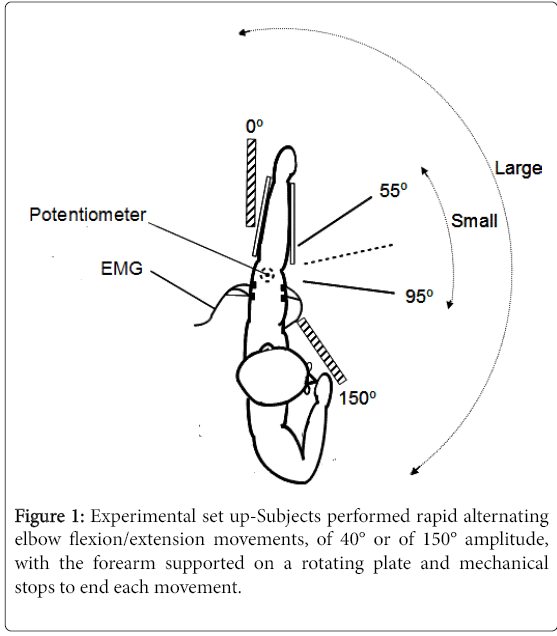
The subjects sat at a table with the forearm supported by a rotating horizontal plate, the shoulder in 60° of abduction (Figure 1). The elbow rested at one end of the plate, with the olecranon positioned over the centre of rotation of the plate. A low-friction potentiometer located beneath the plate was used to measure rotations movements of the plate. The forearm was maintained on the plate in semi-pronated position with foam padding. A low friction bearing ensured the plate could be rotated in the horizontal plane with little force. Two predefined ranges of alternating elbow flexion and extension movements were tested, centred around an intermediate elbow position of 75° flexion: from 0 to 150 degrees of elbow flexion (large movements) and from 55 to 95 degrees of elbow flexion (small movements). Removable blocks on the table were used to mechanically stop each movement at the end of the predefined range. The onset and end of each movement series were cued by audition.
All subjects (PD and controls) were asked to perform continuous 15-second sets of as many alternating large and small movements of elbow flexion-extensions as possible (maximal speed movements) with each arm, for a total of four movement sets. Hands were designated Dominant (D) and Non-Dominant (ND) in controls (based on the handedness Edinburgh inventory-[24]), and More Affected (MA) and Less Affected (LA) in PD subjects, based on clinical assessment (UPDRS) and history. By convention, the more affected hand in PD subjects was compared to the dominant hand in control subjects, and the less affected in PD subjects was compared to the non-dominant hand in controls for all analyses.
Six of the control subjects were then asked to perform the same series of movements at slower speeds approximately matched to the average speed of the PD patients in the series. This was accomplished by giving the subjects rhythmic acoustic cues (metronome) with a frequency matching the mean movement frequency of the PD subjects. These controls had a one-series practice before data was recorded.
Data acquisition and analysis - Down-sampling process
Movement frequency was calculated from the total number of movements completed within 15 seconds. Potentiometer signals were recorded using a 1401 analog-digital DAQ (CED, Cambridge, UK) and processed off line using Spike II software (CED, Cambridge, UK). Sampling frequencies in controls were 50 Hz for maximal-speed movements and 2000 Hz for speed-matched movements, and 50 Hz, 2000 Hz or 5000 Hz in PD subjects.
To normalize the number of data points per movement across all experiments, off-line processing involved a down-sampling algorithm, to adjust for varying movement speeds and sampling frequencies depending on the subject or the experimental condition. For the controls moving at maximal speed (50 Hz sampling frequency) no down-sampling was performed, providing a baseline against which other data was down-sampled.
To calculate the down-sampling factor for slow-moving subjects (i.e., PD subjects and controls moving slowly), the ratio of the average movement frequency of the control to the movement frequency of each slow-moving subject was calculated. This number was then multiplied by the sampling frequency of the slow-moving subjects and divided by 50 (50 Hz sampling frequency in controls). This was formulated as:
Down-Sample Factor= (control frequency * sampling frequency of slow subject) / (slow frequency * 50 Hz)
The result was rounded to the nearest integer, yielding a downsampling number. For example, if the average movement frequency for the control subjects (sampled at 50 Hz), was 1 Hz for a given movement series, about 50 data points per movement were recorded. Recordings from a slow-moving subject with a 0.5 Hz movement frequency sampled at 2000 Hz were thus down-sampled by a factor of 80 to ensure the same approximate number of 50 data points per movement.
Determination of mean angular speed and L/S ratios
Potentiometer voltage data were converted to angular rotation, with negative and positive voltages corresponding to flexion and extension directions, respectively. Voltage zero corresponded to the central position of 75° of elbow flexion. First, second, and third order derivatives of the down-sampled angular displacement data were calculated using a central difference, five-point method. These corresponded to speed, acceleration, and jerk profiles, respectively. We calculated the total distance travelled, and determined maximal flexion and extension velocities and accelerations for each movement series. Mean angular speed was calculated by dividing the total distance travelled by 15 seconds.
Ranges of speed and acceleration were defined as the differences between maximal flexion and extension values. For both speed and acceleration the ranges in large movements were compared to those in small movements using large/small ratios (L/S).
Determination of the normalized average rectified jerk (NARJ)
Movement smoothness was quantified using the average rectified jerk (ARJ) [22], given by 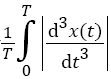 , where T is the total duration of movement and x (t) is the displacement as a function of time. The ARJ is thus highly dependent on movement duration, with a fast movement yielding a higher ARJ than a movement of identical amplitude but longer duration. Thus, movement slowness in PD may lead to a lower ARJ than normal regardless of movement smoothness. Cozens and Bhakta corrected for this by formulating the Normalized Average Rectified Jerk (NARJ), which corrects for the difference in duration between two movements by the cube of the ratio of the movement durations [22]. Thus, after jerk rectification and calculation of the ARJ over a movement series, we multiplied the ARJ by the normalization factor which was, within a given series, the cube of the ratio of the average control movement frequency to that of the subject under analysis, to derive the NARJ.
, where T is the total duration of movement and x (t) is the displacement as a function of time. The ARJ is thus highly dependent on movement duration, with a fast movement yielding a higher ARJ than a movement of identical amplitude but longer duration. Thus, movement slowness in PD may lead to a lower ARJ than normal regardless of movement smoothness. Cozens and Bhakta corrected for this by formulating the Normalized Average Rectified Jerk (NARJ), which corrects for the difference in duration between two movements by the cube of the ratio of the movement durations [22]. Thus, after jerk rectification and calculation of the ARJ over a movement series, we multiplied the ARJ by the normalization factor which was, within a given series, the cube of the ratio of the average control movement frequency to that of the subject under analysis, to derive the NARJ.
Determination of the fast frequency to movement frequency ratio in the acceleration power spectrum (FF/MF ratio) - Resolution of power spectra
A Fourier power spectrum of the acceleration profile was generated over the full movements sets completed in 15 seconds using the Spike 2 software. As the maximum number of bins allowed over a 15-seconds recording using the final sampling frequency (after down-sampling) was never lower than 64, 64 was selected as the common number of bins for all power spectrums. The resolution (Hz) of the power spectrum was half the final sampling frequency divided by 64. For instance, the acceleration power spectrum of controls moving maximally fast (50 Hz sampling frequency, no down-sampling) had 64 bits of data up to 25 Hz, with a resolution of 25/64=0.39 Hz. The recording from a subject moving at half the average speed of controls and recorded with the same 50 Hz sampling frequency was downsampled to 25 Hz (down-sampling number 2). The power spectrum for this subject had 64 bits of data up to 12.5 Hz with a resolution of 12.5/64=0.20 Hz.
For each group (controls, PD subjects, slow-moving controls), the acceleration power spectrum was analysed up to a maximal frequency of 12 times the average movement frequency for large movements, and 6 times the average movement frequency for small movements. For each power spectrum, we calculated the ratio of the power in frequencies faster than the movement frequency (FF, average power in all bins between the movement frequency and the maximal frequency analysed) to that at the movement frequency (MF, weighted average of the power in the two bins at frequencies closest to the movement frequency), deriving an FF/MF ratio.
Statistical analysis
Descriptive statistics yielded average values and standard deviations for each quantitative continuous variable (mean speed, NARJ, FF/MF ratios, velocity and acceleration L/S ratios). Mann-Whitney and twosample unequal variance two-tailed t-tests compared PD subjects with both sets of controls (maximal speed and slow-moving) for NARJ, velocity and acceleration L/S ratios and FF/MF ratios (dependent variables). Univariate linear regression analyses evaluated the importance of time since diagnosis of PD and age as potential predictor of NARJ, and L/S and FF/MF ratios. The significance of linear relationships was evaluated with an F-test (ANOVA). Bonferroni corrections were used to account for multiple comparisons and significance was set at the 0.05 level. Data were analysed using SPSS 18.0 (SPSS Inc., Chicago, ILL).
Results
Mean angular speeds
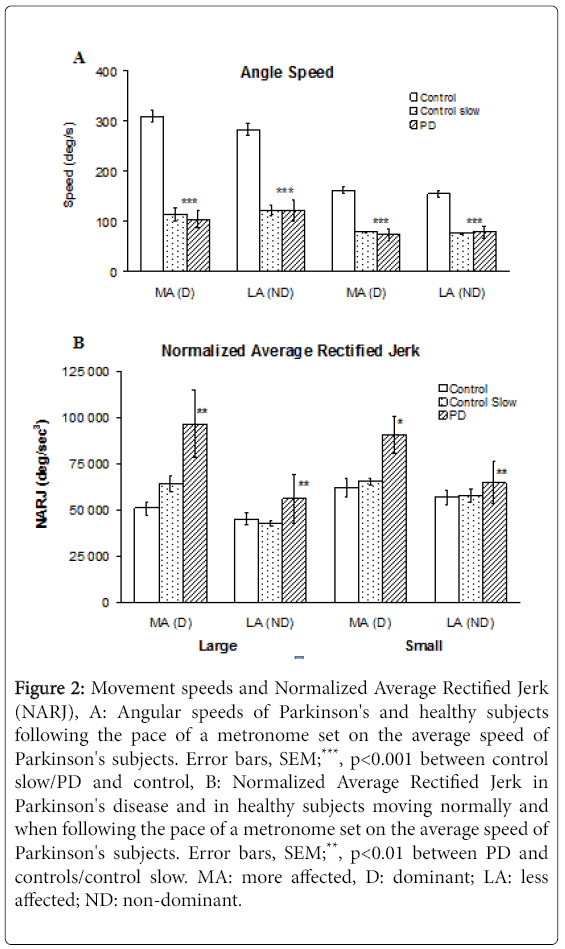
PD subjects moved at 103 ± 48° sec-1 (mean ± SD) /122 ± 50° sec-1 with the more/less affected hand in large movements and at 73 ± 31° sec-1/79 ± 31° sec-1 with the more and less affected hand in small movements. On average, these values represent 33% (p<0.001) and 45% (p<0.001) of control speeds over large and small movements respectively when considering the dominant hand in controls vs. the more affected hand in PD, with differences of lesser magnitude when comparing the less affected hand of PD subjects to the non-dominant hand of controls (Figure 2A). Mean angular speeds of control subjects mimicking PD slowness differed from PD speeds by a maximum 9% (NS).
Figure 2: Movement speeds and Normalized Average Rectified Jerk (NARJ), A: Angular speeds of Parkinson's and healthy subjects following the pace of a metronome set on the average speed of Parkinson's subjects. Error bars, SEM;***, p<0.001 between control slow/PD and control, B: Normalized Average Rectified Jerk in Parkinson's disease and in healthy subjects moving normally and when following the pace of a metronome set on the average speed of Parkinson's subjects. Error bars, SEM;**, p<0.01 between PD and controls/control slow. MA: more affected, D: dominant; LA: less affected; ND: non-dominant.
Normalized average rectified jerk
Normalized Average Rectified Jerk values for PD in the more affected hand were 189% (p=0.003) and 146% (p=0.0011) of controls for fast large and small movements respectively, and 151% (p=0.007) and 139% (p=0.012) of controls for speed-matched large and small movements respectively (Figure 2B).
Fast frequency to movement frequency ratio in the acceleration power spectrum
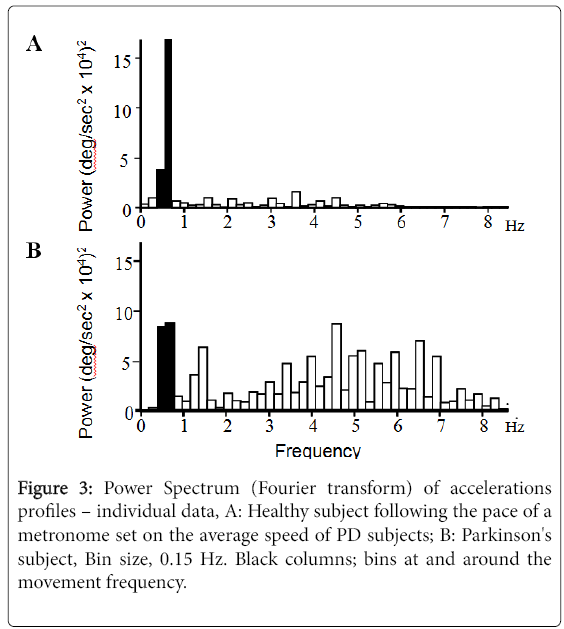
Figure 3 displays individual Fourier power spectra of the acceleration profile for a control subject (Figure 3A).
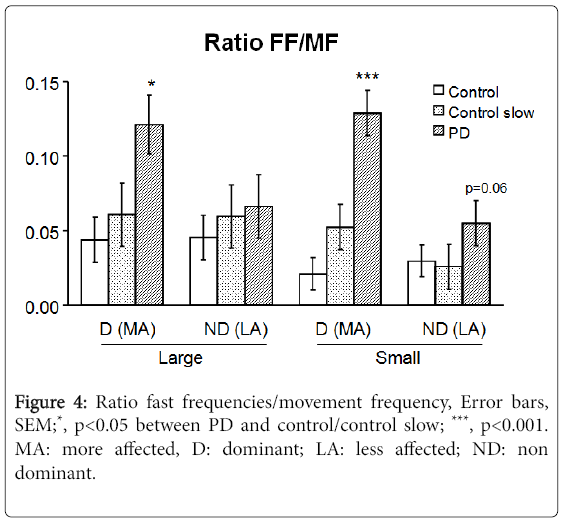
Moving at approximately the same speed as a PD patient (Figure 3B), showing increased power in frequencies greater than the movement frequency in PD. The fast frequency to movement frequency average power ratio (FF/MF ratio) in PD subjects was 277% (p=0.028) and 613% (p<0.001) of controls for fast large and small movements respectively and 200% (p=0.032) and 246% (p<0.001) of controls for speed-matched large and small movements respectively (Figure 4).
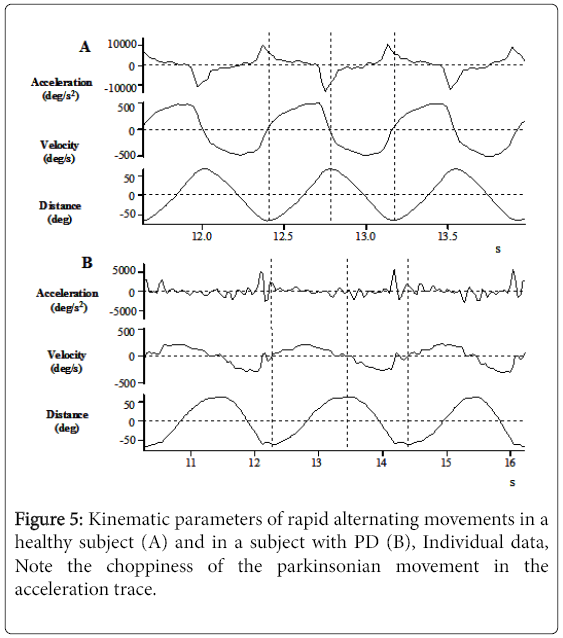
To illustrate speed and smoothness differences, Figure 5 displays two individual, adjusted down-sampled data of angular displacement, speed, and acceleration profiles for a healthy (Figure 5A) and a PD subject (Figure 5B), both moving at maximum speed. The speed differences are notable when considering the x-axes and the smoothness differences are visible when comparing the acceleration profiles.
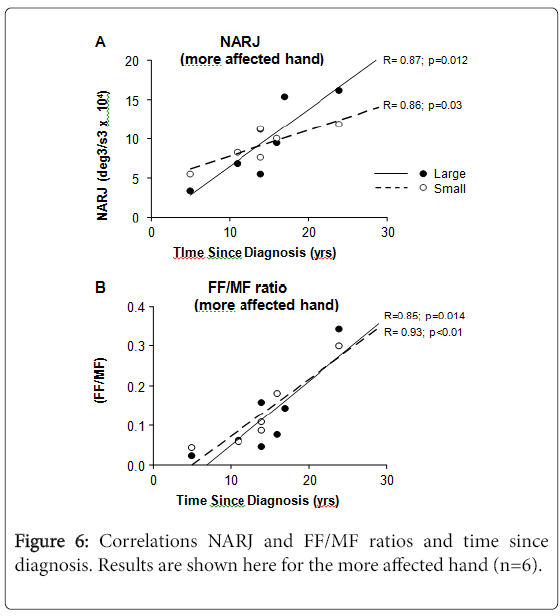
NARJ and FF/MF ratios vs. time since diagnosis
The NARJ and FF/MF ratios for PD patients correlated with time since diagnosis (Figure 6), both correlations being stronger for the more than for the less affected hand, and for large than for small movements. Such correlations were absent with age (data not shown).
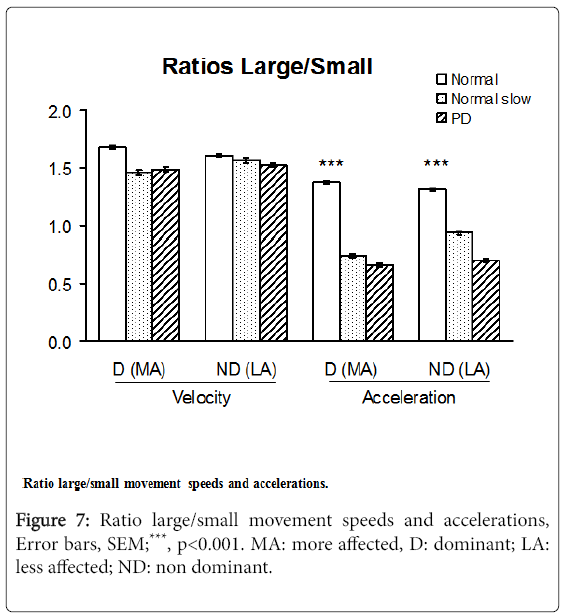
Velocity and acceleration large/small movement ratios
The acceleration Large/Small movements ratios in PD patients ranged from 48 to 53% of those in healthy subjects moving at maximum speed (p<0.001), differences that were less pronounced for the velocity L/S ratios. The acceleration L/S ratios, however, did not distinguish PD subjects from slow-moving controls (Figure 7).
Discussion
In this pilot study of large and small amplitude rapid alternating movements in subjects with PD and in healthy controls matching PD slowness, the only methods that discriminated PD slowness from mimicked slowness were the two methods that quantified movement smoothness by measuring the irregularity of the acceleration profile, in two different ways: the normalized average rectified jerk (NARJ) and the fast to movement frequency ratio (FF/MF) in the power spectrum of acceleration frequencies. These two metrics also confirmed the more affected side in each patient and correlated with time since diagnosis (but not with age). The NARJ and FF/MF ratio in the power spectrum of acceleration frequencies thus distinguish PD bradykinesia vs. voluntary slowness, as associated with multiple acceleration bursts [25,26]. These two metrics should now be evaluated as candidate physiological markers for PD as against other bradykinesia-inducing disorders.
The present findings also showed that speed of movement was lower with the more affected (as determined clinically and by history) than the less affected hand in PD and similar in speed-matched controls and in PD. The latter observation confirmed that the cueing of movement frequency in the speed-matched healthy controls based on averaged PD frequency was effective.
Limitations of the study
In this pilot study the sample sizes of both PD patients and healthy subjects were small and subjects were taken as a sample of convenience, which may limit generalizability of the data. Yet, the findings about NARJ and FF/MF ratios were significant in the small sample. Similarly, of the 12 healthy subjects participating in the study, a convenience sample of six subjects was asked to serve as the second control, “speed-matched” group. As this was not a random sample, it might also affect internal validity. In addition, assessors were not masked in our paradigm, even though the results obtained here were objective parameters derived without human intervention (potentiometer data).
There may also exist disadvantages in the experimental paradigm we used, but these might have led to underestimate bradykinesia in PD patients: (i) The onset of each movement series was cued by beeps, as opposed to letting subjects initiate each movement series based on internal preparedness; for PD subjects this feature may have led to underestimate bradykinesia [25,26]; (ii) Blocks mechanically stopped movements at the end of each predefined range, precluding evaluation of voluntary acceleration reversals. Instead, the present set-up was appropriate to study the acceleration profile during repeated single uni-directional movements only, not during direction reversals; (iii) acceleration profiles were determined using the third order differential from movement using a potentiometer, instead directly from an accelerometer; however, the potential reduction of signal/nose ratio due to computing third order differentials did not prevent from seeing clear differences between voluntary mimicked slowness and parkinsonian slowness.
From a clinical point of view, presence or absence of rest tremor in the subjects (5/8 had rest tremor in this group) is unlikely to have impacted the recorded NARJ or FF/MF ratio, as the fast frequencies were widely distributed throughout all frequencies between the movement frequency and 8 Hz, unlike any specific rest tremor frequency. Finally, it might have been valuable to explore correlations of the two smoothness metrics with clinical parameters, such as UPDRS sub-scores or other quantitative clinical tests. As UPDRS was not performed at the same time as participation to the experiments for all PD subjects, these correlations could not be performed here and will have to be explored in another, specifically designed study.
Looking for smoothness reduction as a way to improve characterization of bradykinesia in Parkinson’s disease, using an increased Normalized Average Rectified Jerk.
Movement slowness of PD has been explained by the inability to generate initial agonist bursts of sufficient duration [27] or size [28-33] and therefore by insufficient movement acceleration [11]. In Alexander’s model, this is due to inappropriate preparedness (low “internal” excitability) of the premotor and motor cortex by the basal ganglia [34]. However, movement slowness is insufficient to characterize PD, as a number of syndromes other than PD slow voluntary movements, including somatoform disorders [35]. Because of this lack of specificity, the positive predictive value of bradykinesia for the diagnosis of idiopathic PD has been shown as suboptimal [36,37]. Additionally, there is currently no universally accepted biomarker for PD, i.e., no quantitative technique that would help the clinician with early detection, follow-up, and assessment of treatment effects [38]. Most tools used in standard clinical examination are subjective and qualitative [5-8].
In that context, we attempted to improve the characterization of PD bradykinesia, using two hypotheses. In the first hypothesis, lack of smoothness best characterizes PD movement as against normal movement matched for slowness. According to this hypothesis, smoothness measures in PD should be specifically low compared to normal movements matched for speed. In the second hypothesis, the best characteristic of parkinsonian bradykinesia would be the selective difficulty to scale acceleration bursts for large movements compared to small movements: a ratio of the acceleration bursts over large to those over small movements might be specifically low, compared to normal movements and normal movements matched for speed.
The first of these two hypotheses has been verified in this study. First, the NARJ was similar between control maximal speed movements and speed-matched movements, indicating that healthy subjects do not lose smoothness when slowing their movements. Second, the NARJ, known to be minimized in normal human movement, [39-41] was increased in PD movements, compared with speed-marched normal movements, which extends previous findings [42,43]. In addition, the NARJ proved more abnormal in large movements and seemed to better detect the milder abnormalities in the less affected hand (Figure 2B and 4), compared with the FF/MF ratio. Both markers of reduced smoothness correlated with time since diagnosis (Figure 6).
Comparison of large vs. small movements - L/S ratios
Large movements are affected earlier and more severely than small movements in PD, which may point to hypometria as a primary clinical characteristic of this disease [7,11,44,45]. Figure 2 shows 33% reduction of the speed of large movements in PD vs. 45% of normal in the speed of small movements but the difference was not significant in this small sample. L/S ratios were thus more sensitive in distinguishing PD patients from healthy subjects when looking at acceleration ratios than at speed ratios. This is consistent with the concept of PD bradykinesia as primarily due to an acceleration abnormality [46-48].
The present study suggests that quantifying smoothness using the Normalized Average Rectified Jerk, and to a lesser degree the Fast Frequency to Movement Frequency ratio in the acceleration power spectrum, may distinguish PD bradykinesia from volitional slowness. Perspectives include the diagnostic study of these parameters in larger populations with movement slowing from other neurologic, orthopaedic and psychiatric causes. Future studies should also explore early or mild PD cases, and test the sensitivity of these two metrics in differentiating very early parkinsonian movement (with still preserved movement speed) from normal movement.
Acknowledgement
Nicolas BAYLE, Emilie HUTIN and Elisabeth A KAPPOS participated in manuscript writing and data analysis. Karen FUNG and Donald J WEISZ developed the set up to collect data. Stephen J FRIED collected data and processed the data. Jean-Michel GRACIES conceived and supervised the study and assisted with manuscript writing and reviewing. None of the authors was granted specific funds to carry out this study.
References
- Parkinson J (1817) An essay on the shaking palsy. Whittingham and Rowland, London 1817.
- Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease.J Neurol Neurosurg Psychiatry 51: 745-752.
- Flowers KA (1976) Visual "closed-loop" and "open-loop" characteristics of voluntary movement in patients with Parkinsonism and intention tremor.Brain 99: 269-310.
- Sheridan MR, Flowers KA (1990) Movement variability and bradykinesia in Parkinson's disease.Brain 113 : 1149-1161.
- Lang AE, Koller WC, Fahn S (1995) Psychogenic parkinsonism.Arch Neurol 52: 802-810.
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, et al. (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results.Mov Disord 23: 2129-2170.
- Bloem BR, Brundin P (2014) How I examine my patient: the art of neurological examination for Parkinson's disease.J Parkinsons Dis 4: 563-565.
- Abdo WF, van de Warrenburg BP, Burn DJ, Quinn NP, Bloem BR (2010) The clinical approach to movement disorders.Nat Rev Neurol 6: 29-37.
- Berardelli A, Dick JP, Rothwell JC, Day BL, Marsden CD (1986) Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson's disease.J Neurol Neurosurg Psychiatry 49: 1273-1279.
- Stamatakis J, Ambroise J, Crémers J, Sharei H, Delvaux V, et al. (2013) Finger tapping clinimetric score prediction in Parkinson's disease using low-cost accelerometers.Comput Intell Neurosci 2013: 717853.
- Teo W, Rodrigues JP, Mastaglia FL, Thickbroom GW (2013) Comparing kinematic changes between a finger-tapping task and unconstrained finger flexion-extension task in patients with Parkinson's disease.Exp Brain Res 227: 323-331.
- Broderick MP, Van Gemmert AW, Shill HA, Stelmach GE (2009) Hypometria and bradykinesia during drawing movements in individuals with Parkinson's disease.Exp Brain Res 197: 223-233.
- Okada M, Okada M (1983) A method for quantification of alternate pronation and supination of forearms.Comput Biomed Res 16: 59-78.
- Beuter A, Mergler D, de Geoffroy A, Carrière L, Bélanger S, et al. (1994) Diadochokinesimetry: A study of patients with Parkinson's disease and manganese exposed workers.Neurotoxicology 15: 655-664.
- Hermsdörfer J, Marquardt C, Wack S, Mai N (1999) Comparative analysis of diadochokinetic movements.J Electromyogr Kinesiol 9: 283-295.
- Joebges M, Mrowka M, Schimke N, Shing M, Dengler R, et al. (2003) Three-dimensional computerized analysis of diadochokinetic movements of Parkinsonian patients.Acta Neurol Scand 108: 415-423.
- Fimbel EJ, Domingo PP, Lamoureux D, Beuter A (2013) Automatic detection of movement disorders using recordings of rapid alternating movements. J Neurosci Methods 146: 183-90.
- Pope PA, Praamstra P, Wing AM (2006) Force and time control in the production of rhythmic movement sequences in Parkinson's disease.Eur J Neurosci 23: 1643-1650.
- Müller T, Harati A (2010) Diadochokinetic movements differ between patients with Parkinson's disease and controls.J Neural Transm (Vienna) 117: 189-195.
- Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, et al. (2012) Postural sway as a marker of progression in Parkinson's disease: a pilot longitudinal study.Gait Posture 36: 471-476.
- Maetzler W, Mancini M, Liepelt-Scarfone I, Müller K, Becker C, et al. (2012) Impaired trunk stability in individuals at high risk for Parkinson's disease.PLoS One 7: e32240.
- Cozens JA, Bhakta BB (2003) Measuring movement irregularity in the upper motor neurone syndrome using normalised average rectified jerk.J Electromyogr Kinesiol 13: 73-81.
- Lang AE, Houeto JL, Krack P, Kubu C, Lyons KE, et al. (2006) Deep brain stimulation: Preoperative issues.Mov Disord 21 Suppl 14: S171-196.
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburghinventory. Neuropsychologia 9:97-11324.
- Ma HI, Trombly CA, Tickle-Degnen L, Wagenaar RC (2004) Effect of one single auditory cue on movement kinematics in patients with Parkinson's disease.Am J Phys Med Rehabil 83: 530-536.
- Ringenbach SD, van Gemmert AW, Shill HA, Stelmach GE (2011) Auditory instructional cues benefit unimanual and bimanual drawing in Parkinson's disease patients.Hum Mov Sci 30: 770-782.
- Teasdale N, Phillips J, Stelmach GE (1990) Temporal movement control in patients with Parkinson's disease.J Neurol Neurosurg Psychiatry 53: 862-868.
- Rand MK, Stelmach GE, Bloedel JR (2000) Movement accuracy constraints in Parkinson's disease patients.Neuropsychologia 38: 203-212.
- Hallett M, Khoshbin S (1980) A physiological mechanism of bradykinesia.Brain 103: 301-314.
- Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson's disease.Brain 124: 2131-2146.
- Stelmach GE, Worringham CJ (1988) The preparation and production of isometric force in Parkinson's disease.Neuropsychologia 26: 93-103.
- Stelmach GE, Teasdale N, Phillips J, Worringham CJ (1989) Force production characteristics in Parkinson's disease.Exp Brain Res 76: 165-172.
- Phillips JG, Martin KE, Bradshaw JL, Iansek R (1994) Could bradykinesia in Parkinson's disease simply be compensation?J Neurol 241: 439-447.
- Alexander GE, Crutcher MD, DeLong MR (1990) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, "prefrontal" and "limbic" functions.Prog Brain Res 85: 119-146.
- Sage JI, Mark MH (2015) Psychogenic parkinsonism: clinical spectrum and diagnosis.Ann Clin Psychiatry 27: 33-38.
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico-pathological study of 100 cases.J Neurol Neurosurg Psychiatry 55: 181-184.
- Rajput AH, Rozdilsky B, Rajput A (1991) Accuracy of clinical diagnosis in parkinsonism-A prospective study.Can J Neurol Sci 18: 275-278.
- Parkinson Progression Marker Initiative (2011) The Parkinson Progression Marker Initiative (PPMI).Prog Neurobiol 95: 629-635.
- Hogan N (1984) An organizing principle for a class of voluntary movements.J Neurosci 4: 2745-2754.
- Hogan N, Flash T (1987) Moving gracefully: Quantitative theories of motor coordination. Trends Neurosci 10: 170-174.
- Nagasaki H (1989) Asymmetric velocity and acceleration profiles of human arm movements.Exp Brain Res 74: 319-326.
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH (1997) Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control.Exp Neurol 146: 159-170.
- Mizuno M, Fukuzawa K, Soejima K, Tsunoda S, Iwata M (2010) [Kinematic aspects of the step movement in Parkinson's disease].Rinsho Shinkeigaku 50: 74-80.
- Gracies JM, Danisi F, Bucobo JC, Brin MF, Olanow CW (1999) Assessing alternating movement in Parkinsonism: Two types of testing. Park Dis Rel Dis 5:S50.
- Van Gemmert AW, Adler CH, Stelmach GE (2003) Parkinson's disease patients undershoot target size in handwriting and similar tasks.J Neurol Neurosurg Psychiatry 74: 1502-1508.
- Contreras-Vidal JL, Stelmach GE (1995) A neural model of basal ganglia-thalamocortical relations in normal and parkinsonian movement.Biol Cybern 73: 467-476.
- Contreras-Vidal JL, Teulings HL, Stelmach GE (1995) Micrographia in Parkinson's disease.Neuroreport 6: 2089-2092.
- Torres EB (2013) The rates of change of the stochastic trajectories of acceleration variability are a good predictor of normal aging and of the stage of Parkinson's disease.Front Integr Neurosci 7: 50.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 11733
- [From(publication date):
February-2016 - Dec 03, 2024] - Breakdown by view type
- HTML page views : 10942
- PDF downloads : 791