Motivational Interviewing Impact on Cardiac Rehabilitation Program Outcomes: A Systematic Review of Randomized Controlled Trials
Received: 14-Apr-2022 / Manuscript No. jcpr-22-57843 / Editor assigned: 16-Apr-2022 / PreQC No. jcpr-22-60857 (PQ) / Reviewed: 30-Apr-2022 / QC No. jcpr-22- 60857 / Revised: 05-May-2022 / Manuscript No. jcpr-22-60857 (R) / Published Date: 12-May-2022 DOI: 10.4172/jcpr.1000164
Abstract
Objectives: Cardiovascular disease (CVD) is the leading cause of death globally but can be prevented and treated with lifestyle modifications or health behavior changes. A modified Cochrane method of systematic review was implemented to explore and report evidence and gaps in the literature for studies of motivational interviewing (MI) influence on outpatient cardiac rehabilitation (OPCR) patient outcomes.
Methods: The literature search was conducted using several databases (Medline, Academic Search Premier, Psych Info, PubMed, Sport Discus, Science Direct, CINAHL, Web of Science, APA Psych Articles, Complementary Index, Education Research Complete, Health Source, and Directory Open Access Journals) with a combination of the search terms, ‘cardiac rehab’ and ‘motivational interviewing’. Inclusion criteria included studies that used MI as part of an intervention, conducted in the OPCR setting, randomized control trials (RCT), results for behavioral, psychosocial/humanistic, and/or clinical outcomes were reported, and written in English. Search/review tiers included titles/abstracts and full text.
Results: Of the 398 studies from the initial search, nine RCTs met the criteria and were retained for this review. Of the 9 studies, 7 reported a significant difference in at least one behavioral, psychological/humanistic, and/or clinical outcome such as OPCR attendance, anxiety or depressive symptoms scores, cholesterol values, and/or blood pressure.
Conclusion: MI demonstrated a positive effect on behavioral, psychosocial, humanistic, or clinical outcomes. Heterogeneity in study designs, measures, and outcomes suggest that further research is warranted to elucidate optimal intervention structure in terms of contact frequency and study duration.
Keywords
Motivational interviewing; Cardiac rehabilitation; Cardiovascular disease; Behavior change
Introduction
Cardiovascular disease (CVD) is the term for several conditions, and collectively, these diseases are the leading causes of death globally [1]. An estimated 71.3 million American adults are affected by CVD [2]. Cardiac rehabilitation is an integral part of recovery [3] Outpatient cardiac rehabilitation (OPCR) programs consist of individualized assessments and evaluations of risk factors, such as tobacco use, hypertension, diabetes mellitus, hyperlipidemia, sedentary behaviors, and obesity [4]. Cardiac rehabilitation is associated with strengthening the heart and body after a heart event through physical activity (PA) participation, building healthier habits, improving energy, mood, and preventing future heart problems [5]. Though the benefits of cardiac rehabilitation are clear, utilization and adherence are low [6]. In addition to non-modifiable factors, interventions have aimed to address factors relating to OPCR session attendance and behavior modifications to improve acute and long-term outcomes [7]. Effective interventions that target behavior change are integral to achieve expected outcomes.
Motivational interviewing (MI) is a counseling intervention that has been scientifically tested in various lifestyle behaviors. It was initially applied in the treatment of alcoholism and other addictive behaviors and evolved into counseling strategies in other lifestyle behaviors [8]. MI increases motivation toward target behaviors by eliciting strategies and goals based on the individual’s preferences, values, and routines rather than at the practitioner’s sole discretion. MI is directive, personcentered, and collaboration between the practitioner and the patient. MI is an approach to facilitate a person’s decision-making about engaging in a target health behavior while resisting the urge to “fix” the person. The process involves understanding and exploring motivations while actively listening and encouraging the person to develop ideas to evoke change. MI utilizes evoking person's views and values to enhance their motivation while supporting autonomy and self-efficacy [9]. MI may provide a potential strategy to increase OPCR adherence and core component outcomes of OPCR.
Researchers have studied utilizing MI in various behavior change initiatives related to disease management or prevention [10]. Behavioral modifications, including medication adherence [11], smoking cessation [12], PA [13], and healthy eating, are a part of OPCR [14]. MI may provide an effective strategy in OPCR to address behavioral modifications and adherence to desired behaviors; therefore, a synthesis of the literature to examine evidence-based implementation is necessary that is specific to OPCR settings. To our knowledge, there has not been a literature review exploring and reporting the influences of MI in combination with traditional OPCR in randomized controlled trials (RCTs). This review aims to systematically explore and report evidence and gaps in the literature on the effects of MI on behavioral, psychosocial, and clinical outcomes and report implications for OPCR practice based on the MI intervention protocols implemented in OPCR settings.
Methods
Protocol and literature search
A modified Cochrane method of systematic review was conducted [15]. The modification involves exploring evidence and gaps in the literature while using the rigor of the Cochrane search and review tiers [16]. The lead author completed a comprehensive search of relevant studies using articles originally written in English and published between January 1, 2000, and February 28, 2021. MI was introduced in the 1980s [8]; however, published articles in peer-reviewed journals related to MI research were not found by our team prior to 2000.
Relevant electronic databases were searched using the combinations of keywords for cardiac rehabilitation (cardiac rehab, cardiovascular rehab, cardiac rehabilitation, or cardiovascular rehabilitation) and motivational interviewing (motivational interview or motivational interviewing). The relevant databases searched included Medline, Academic Search Premier, Psych Info, PubMed, Sport Discus, Science Direct, the Cumulative Index to Nursing and Allied Health Literature [CINAHL], Web of Science, APA Psych Articles, Complementary Index, Education Research Complete, Health Source, and Directory Open Access Journal. The search was limited to full-text articles in English that were published in peer-reviewed academic journals.
Selection process and data extraction
The target population consisted of persons actively participating in OPCR.
Inclusion criteria were the following: 1) utilized MI as a named part of the intervention, 2) conducted in an OPCR setting, 3) peer-reviewed publication, 4) used robust methodological design (randomized controlled trial - RCT), and 5) studied the impact on behavioral, psychosocial/humanistic, and clinical outcomes. Exclusion criteria included narrative reviews, case studies, dissertations, protocol articles, abstracts, qualitative studies, and commentaries.
All studies were reviewed to ensure they met the selection criteria. The lead author completed an initial scan of the titles and abstracts to exclude articles that did not fit the inclusion criteria. Two researchers conducted full-text reviews of each article and discussed consensus articles where the retain/reject decision differed. The final retained articles were organized into a standardized data extraction tool by the lead author.
Relevant electronic databases were searched using the combinations of keywords for cardiac rehabilitation (cardiac rehab, cardiovascular rehab, cardiac rehabilitation, or cardiovascular rehabilitation) and motivational interviewing (motivational interview or motivational interviewing). The relevant databases searched included Medline, Academic Search Premier, Psych Info, PubMed, Sport Discus, Science Direct, the Cumulative Index to Nursing and Allied Health Literature [CINAHL], Web of Science, APA Psych Articles, Complementary Index, Education Research Complete, Health Source, and Directory Open Access Journal. The search was limited to full-text articles in English that were published in peer-reviewed academic journals.
Selection process and data extraction
The target population consisted of persons actively participating in OPCR.
Inclusion criteria were the following: 1) utilized MI as a named part of the intervention, 2) conducted in an OPCR setting, 3) peer-reviewed publication, 4) used robust methodological design (randomized controlled trial - RCT), and 5) studied the impact on behavioral, psychosocial/humanistic, and clinical outcomes. Exclusion criteria included narrative reviews, case studies, dissertations, protocol articles, abstracts, qualitative studies, and commentaries.
All studies were reviewed to ensure they met the selection criteria. The lead author completed an initial scan of the titles and abstracts to exclude articles that did not fit the inclusion criteria. Two researchers conducted full-text reviews of each article and discussed consensus articles where the retain/reject decision differed. The final retained articles were organized into a standardized data extraction tool by the lead author.
Data collection and quality assessment
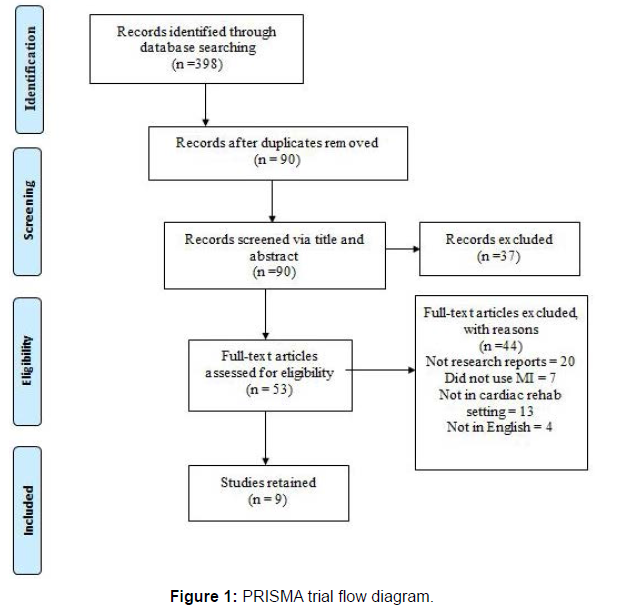
The Cochrane Risk of Bias Tool [15] was used to review the retained articles to evaluate the methodological quality. There are five domains of bias in the Cochrane Tool: selection bias, performance bias, attrition bias, reporting bias, and detection bias, which were evaluated for all retained studies. Studies were categorized as “low risk,” “high risk,” or “unclear risk” when the information was limited or not provided (Figure 1).
Results
The initial search revealed 398 studies through the database search.
The PRISMA flow diagram (see figure 1) illustrates the results per search and review tier and the rationale for rejections. Articles were removed if they were not 1) available in full-text (n=73), 2) peer-reviewed (n=86), or 3) written in English (n=10). After removing duplicates (n=139), 90 articles remained. Articles were examined based on title and abstracts resulting in 37 articles rejected due to 1) not utilizing MI (n=11) 2) not completed in OPCR (n=23) 3) was a case study (n=2), and 4) protocol (n=1). Of the articles retained, 53 underwent full-text review, resulting in a rejection of 44 articles.
Articles were primarily excluded due to being 1) in abstract form (n=1), 2) not in English (n=4), 3) methods/protocol articles (n=10), 4) reviews/commentaries (n=9), 5) conducted in non-OPCR setting (n=13), 6) not using MI (n=7). After the final full-text review tier, researchers retained a total of nine RCTs (Table 1).
| Source | Participants (% Female) | Mean Age (yr + SD) | Interventionist Type | Setting | Duration and Dose | Primary Outcomes | Secondary Outcomes | Clinical Outcomes |
|---|---|---|---|---|---|---|---|---|
| Beckie, 2011[24] | 133 (100) | 63.0 ± 12.0 | Clinical Psychologist or Registered Nurse | CR Facility | 60-minutes Wk 1, 6 and 12. |
Sessions attended (p = .007) | SF-36 MCS (p = .026) SF-36 PCS (p > .05) |
|
| Beckie, 2011 [25] | 133 (100) | 63.0 ± 11.0 | Clinical Psychologist or Registered Nurse | CR Facility | 60 min Week 1, 6 and 12 |
CES-D (p = .013) | Sessions attended (p = .007) | |
| Beckie, 2010 [20] | 133 (100) | 63.0 ± 11.0 | Clinical Psychologist or Registered Nurse | CR Facility | 60 min Wk 1 and 6 |
Sessions attended (p <.001) | CES-D (p = .572) SAI (p = .734) SF-36– PCS (p = .934) SF-36- MCS (p = .340) |
|
| Chair, 2012 [21] | 73 (35.6) | 66.8 ± 10.3 | Registered Nurse | CR Facility | 30-40 min 4 sessions |
MA (p = .154) BMI (p = .151) |
HADS-A (p = .004) SF-36 -MCS (p = .010) SF-36 -PCS (p = .002) HADS-D (p = .116) |
LDL (p = .001) SBP (p = .735) |
| Chair, 2013 [22] | 73 (35.6) | 66.8 ± 10.3 | Registered Nurse | CR Facility | 30-40 min Wk 1,3,5 &7 Then 1x/mo @ , 9 mo and 12 mo |
MA (p = .005) BMI (p = > .05) |
HADS-A (p = .001) HADS-D (p .001) SF-36-MCS (p =.001) SF-36 -PCS (p = .001) |
SBP (p = .001) DBP (p = .001) LDL (p = .001) |
| McGrady, 2014 [27] | 64 (26.5) | 60.3± 11.7 | Provider not stated | Setting not stated Group sessions |
30-min 4 sessions |
Sessions attended (p = .001) | BAI (p = .882) BDI-II (p = .001) SF-36 -MCS (p .006) SF- 36-PCS (p = .001) |
|
| Pietrabissa, 2017 [23] | 21 (23.8) | 60.5 ± 8.2 | Psychotherapist | Setting not stated | 30-45 min 3 Sessions |
Weight (p = .005) BMI (p=.604) |
SF-36-PCS (p .287) SF-36 - MCS (p = 0.555) HADS-A (p = .452) HADS-D (p = .985) |
LDL (p = .398) SBP (p = .878) |
| Rouleau, 2018 [28] | 50 (18.0) | 59.8 ± 11.2 | Clinical Psychology PhD Student | CR facility, University office, or at their home | 30-60 min 1 Session |
Sessions attended (p = .008) | Intention (p = .001) | |
| Ter Hoeve, 2018 [26] | 161 (19.8) | 58.8 ± 9.0 | Physical Therapist | CR facility Group Setting |
60-75 min 6 Sessions |
# of Steps (p = .021) % wear time (p = .002) 6,500/day (p = .004) MVPA/Week (p = .069) |
||
| BAI: Beck Anxiety Inventory; BDI-II: Beck Depression Inventory II; CESD-10: The Center for Epidemiological Studies Depression Scale; CR: Cardiac Rehab; DBP: Diastolic Blood Pressure; HADS: Hospital Anxiety and Depression Scale; LDL: Low-Density Lipoproteins; SBP: Systolic Blood Pressure; SF-36 MCS: Short Form Survey-Mental Health; SF-36 PCS: Short Form Survey- Physical Health; STAI-S: State Anxiety Inventory. | ||||||||
Table 1: Characteristics of retained studies (n = 9).
Table 1 displays a summary of the retained RCT study characteristics and outcomes. Sample size, gender, CVD diagnosis, interventionist type, duration, and dose varied between the retained studies. Nine studies combined included a total of 1,757 patients with sample sizes ranging from 42-489 patients. Participant ages ranged from 51-87 years, and 770 (43.8%) were male. Diagnoses in the retained articles consisted of myocardial infarction (n= 144), CABG (n= 183), angina (n= 130), coronary angioplasty (n= 269), heart failure (n= 2), and other (n= 48). Four studies did not include diagnoses [17-20]. Five studies reported angina, two reported: “stable angina” [21, 22]. Three of the studies included diagnoses as "other," "not specified," and "stroke/TIA. "The retained studies were published between 2004 to 2017 and were conducted in Hong Kong [18, 19], the Netherlands [23], Canada [25], Italy [20], and the United States [17, 21, 22, 24] (Table 2).
| Source | Selection Bias | Performance Bias | Attrition Bias | Reporting Bias | Detection Bias | |
|---|---|---|---|---|---|---|
| Sequence Generation | Allocation Concealment | Blinding | Incomplete Data | Free of selective reporting | Other threats to validity | |
| Beckie, 2010 [20] | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk |
| Beckie, 2011 [24] | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk |
| Beckie, 2011 [25] | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk |
| Chair, 2012 [21] | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk |
| Chair, 2013 [22] | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk |
| McGrady, 2014 [27] | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk | Unclear Risk |
| Pietrabissa, 2017 [23] | Low Risk | Low Risk | Low Risk | Low Risk | Unclear Risk | Low Risk |
| Rouleau, 2018 [28] | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Unclear Risk |
| Ter Hoeve, 2018 [26] | Low Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Unclear Risk |
Table 2: Assessment of methodological quality using Cochrane Risk of Bias Tool.
Table 2 presents the ratings from the methodological quality assessment of the nine retained studies. Three reviewers analyzed the quality until a consensus was met. The retained studies overall displayed a low risk of bias. As all studies were RCTs, there was a reduction in population bias and variance due to randomization. MItrained professionals could not be blinded due to the nature of MI, limiting confirmation bias. Allocation concealment was deemed low risk across the studies.
The reported inclusion criteria implemented in the retained studies required the following: an acute coronary syndrome diagnosis [17-25], referred by a primary physician, were < 1-month post-cardiac event and received medical therapy and angioplasty [24,25], spoke the native language [20,23,25], no hearing/cognitive impairments [25], attended an initial OPCR orientation [25], lived within a specific radius [25], and obtained certain scores on assessments [20] All studies reported clear descriptions of the intervention protocols. Mental health nurses [18, 19], physical therapists [23], nurses and clinical psychologists [17, 21, 22], and clinical psychology Ph.D. students [21, 23] delivered the interventions. McGrady et al. did not mention which type of practitioner delivered the MI sessions [24]. All interventions used MI as the primary intervention in studies using a face-to-face mode for encounters [17-25]. Ter Hoeve et al. offered more than one face-to-face option, such as at home, in the clinic, or at a university health setting [23]. Each face-to-face session was between 15-60 minutes 2-4 times throughout the study. Two studies noted MI sessions were conducted in groups [23, 24], five were conducted individually [17, 18, 20-22, 25], and two did not specify [18, 19].
Reported target outcomes in the studies included behavioral improvement in medication adherence [18, 23], PA [23], attendance at OPCR [17, 21, 24, 25], psychosocial depressive symptoms [22], anxiety [18-20, 24] and humanistic quality of life [17-21, 24]. Various instruments were used in the studies to measure the targeted outcomes. Overall, seven of the nine studies reported an effect after MI over the traditional treatment process in one or more target outcomes relevant to cardiac rehabilitation; these studies reported a statistically significant effect when looking at PA [23], medication adherence [19], adherence to OPCR [21, 22, 24, 25], depressive symptoms [19, 22, 24], anxiety [18, 19], and quality of life [18, 21, 24].
MI training and fidelity
All but one study mentioned the interventionist had been trained in MI [24]. Rouleau et al. indicated the interventionist had 200 hours of supervised MI training and attended two days of advanced workshops [25] whereas, nurses in the Hong Kong studies completed required MI workshops [18, 19]. Seven of nine studies mentioned the interventionist was MI trained, they did not describe the training in detail [17, 20-25]. Seven studies indicated the interventionist providing the MI was previously trained [17-19, 21-23, 25]. One study indicated the interventionist had experience with MI [25], and two studies did not mention the training or experience of their interventionist [20, 24] Six studies noted the MI sessions were audio-recorded [17-22] to determine fidelity. One study indicated they tested for fidelity [20] but did not report their fidelity scores or the findings.
Behavioral outcomes results
Medication adherence
Two studies examined medication adherence. These studies were conducted by the same author and demonstrated the difference in target outcomes over two different periods. Chair et al. [18] found an increase in medication adherence in both the control and MI intervention groups from baseline to post-intervention, but the difference was not significant. Chair et al. [19] found after 12 months of MI the intervention group had significantly higher rates of medication adherence than the control group. The intervention group had lower adherence rates at 12 months compared to baseline self-reported medication adherence.
Physical activity
One study examined physical activity [23]. Ter Hoeve et al. measured physical activity by step count and time in moderate-tovigorous physical activity (MVPA) using a pedometer [23]. This study compared face-to-face MI, traditional OPCR and telehealth MI OPCR and traditional OPCR. The face-to-face MI patients received about 405 minutes of MI. Significant differences were found in the OPCR with face-to-face MI group compared to the OPCR only group for step count (p=0.035) and pedometer wear time (p=0.002). No variables were significantly different between the telehealth MI OPCR group and the control group. There was no difference between OPCR with face-to-face MI and OPCR with telehealth MI regarding step count and pedometer wear time [23].
OPCR Sessions
Four studies measured the number of sessions completed as a primary factor. McGrady et al. did not find a significant difference in sessions completed between groups [24]. The remaining three studies had a significantly greater number of OPCR sessions attended in the intervention group than the control group [21, 22, 25]. MI interviews ranged from 30-180 minutes between interventions. A single 30 minute session of MI resulted in the intervention group attending significantly more sessions than the control group [25]. The other three studies had a significantly higher number of sessions attended and consisted of three 60 minute sessions, with the potential for 180 minutes of MI [17, 21, 22]. Collectively these studies showed that MI utilization increased session adherence by an average of 5 sessions over the course of OPCR.
Psychosocial and humanistic outcomes results
Depression and anxiety symptoms
Six studies examined depressive symptoms. The Center for Epidemiological Studies Depression Scale (CESD-10) [17, 22], Beck Depression Inventory-II (BDI-II) [24], and Hospital Anxiety and Depression Scale (HADS) [18-20] were used to measure levels of depressive symptoms. Three found significant decreases in depressive symptoms scores [22, 24, 25], and three did not [17, 18, 20]. MI sessions ranged from 30-450 minutes between these interventions. Two studies found the MI groups had significantly lower depressive symptoms scores after 12 weeks of MI [25] and 12 months of MI than the control group [19]. Beckie et al. found a group-by-time interaction resulting in significantly lower depressive symptoms scores after 12 weeks compared to the control group [22].
Five studies examined anxiety as an outcome. The Beck Anxiety Inventory (BAI) [24] and the State Anxiety Inventory (STAI-S) [17], Hospital Anxiety and Depression Scale (HADS) [18, 19] were used to measure anxiety. Two studies found a significant effect [18,19] and three did not find a significant difference [17, 20, 24], and all used a different tool to measure anxiety; HADS-A [20], BAI [24], STAI-S [17]. Chair et al. found a significant decrease in HADS-A scores after the 12- month intervention. However, a group-by-time interaction was not found [19]. Chair et al. reported a group-by-time interaction, but the control group decreased in anxiety significantly compared to the intervention group following the short-term intervention [18].
Quality of life
Quality of life was measured using the Medical Outcomes Study (MOS) SF-36 (mental and physical health component summary scores) [17-21, 24]. Of the six studies that measured quality of life with the SF- 36, four studies found a statistically significant difference within the SF- 36 mental component summary score between the MI and control [18- 21, 24], and two did not [20, 26] of the six studies that examined anxiety with the SF-36 regarding the physical component summary score, three studies reported a statistically significant difference between the MI group and the control [18, 19, 24], and three did not [17, 20, 21].
Clinical Outcomes Results
Cardiovascular biomarkers
Three of the retained studies assessed clinical aspects such as lowdensity lipoproteins (LDL) and blood pressure [18-20]. In terms of LDL, two studies found a significant difference between groups, with the MI intervention group having greater decreases [18, 19], while one study did not [20]. One study found a statistically significant overall decrease in blood pressure [19] and two did not find a significant difference between the MI intervention and control groups [18, 20].
Anthropometrics
Four of the nine studies identified Body Mass Index (BMI) as a primary outcome. None of the studies found a significant difference between the control and intervention groups for BMI [18-20, 24].
Discussion
The purpose of this systematic review was to explore, synthesize, and report the literature examining the use of MI and its influence on outcomes in cardiac rehabilitation programs. These results suggest MI interventions have demonstrated an impact on both primary and secondary outcomes.
Two studies examined medication adherence varied in time, but both found positive effects in the MI group compared to the control. Chair et al. was longer in duration and consisted of 10 MI sessions than four conducted in Chair et al. Neither study reported barriers to medication adherence. Describing barriers could help identify specific characteristics leading to MI’s success [11]. A previous systematic review examining medication adherence in addition to MI reported similar increases in adherence following a MI intervention [11].
Face-to-face MI resulted in significantly higher step count and prolonged bouts of physical activity. MI's addition to standard care moderately increases PA in individuals with chronic disease [27]. Telehealth interventions did not have the same results as face-to-face MI. During that time, they discussed goal setting and self-monitoring. The face-to-face MI group may have had adequate duration and frequency of interviews, and these patients were able to work towards goals over many sessions as opposed to five phone calls. The amount of time the telehealth group received MI was not reported. Therefore, dose information is unknown. The telehealth MI group did not have the experience of sitting face-to-face with the interventionist and could have missed out on physical cues that have been reported to be effective, such as eye contact or acknowledging they are listening [28]. Additionally, the face-to-face MI group was in a group setting compared to the telehealth MI group conducted in one-on-one sessions with an interventionalist. It has been reported when face-to-face MI is incorporated with telehealth, outcomes improve, but telehealth alone does not produce the same benefits [29].
Four of the five studies that had the number of sessions attended as an outcome [17, 21, 22, 25] reported significantly higher session attendance for the MI group than the control group. MI may provide relationship building that encourages session attendance [30]. Session adherence is important for cardiac outcomes because each session provides additional education, exercise time, different techniques to living with a chronic disease and prevent disease progression.
Studies indicate higher levels of depressive symptoms and anxiety at baseline decrease adherence [31, 32]. In previous studies that evaluated depressive symptoms [17-20, 22, 24] no significant differences were found, despite a decrease in reported symptoms for the intervention group. MI alone has not been reported to improve depressive symptoms, but promotes seeking treatment when combined with other therapies [33, 34]. These studies utilized similar interventionist and available exposure time to MI [17-20, 24]. MI in addition to psychological treatments for anxiety, successfully reduces anxiety symptoms in populations that suffer from generalized anxiety and post-traumatic stress disorder in cardiac patients [35-38]. MI may not have been successful in these interventions because MI focuses on increasing the motivation fovr change and in regard to anxiety and depression motivation might not be the issue [39], but can be used to promote adherence to other treatment options [40].
LDL and blood pressure were the most common clinical outcomes examined. Two studies found a significance in LDL levels between the intervention and control group [18, 19]. Chair et al. saw a significant decrease in blood pressure [19], with a 12-month protocol compared to Chair et al. and Pietrabissa et al. who used a 3-month protocol. Chair et al. included 300-450 total minutes of MI, whereas Chair et al. and Pietrabissa et al. consisted of 120-180 minutes and 45 minutes, respectively. Therefore, the intervention group individuals had a more extended time to be educated and worked toward changing target behaviors relevant to reduce blood pressure [19]. The use of MI is more distal to improve clinical outcomes as a behavioral strategy. Behavior strategies such as MI can promote higher levels of self-care to improve clinical outcomes [41].
Pietrabissa et al. showed participants who engaged in a MI intervention with 90-135 minutes of MI resulted in weight loss [20]. No retained studies that identified BMI as a primary outcome found a significant difference in BMI reduction between intervention and control groups. These findings, although in a unique context, are at odds with meta-analyses that investigate MI for weight management among adults [42, 43] which demonstrate significant weight and BMI loss when compared to control groups. The studies examining BMI ranged from 3-12 months. While these studies reported weight and BMI, there is no indication on whether the participants had body composition changes. Measuring lean mass and fat mass, for example, would help understand whether weight changes were caused by gaining muscle mass and losing fat mass.
The nine studies utilized a variety of interventionists. In the two studies conducted by Chair et al., the interventionist was a nurse [18, 19]. In contrast a psychotherapist conducted the MI intervention in Pietrabissa et al. [20] One potential explanation for non-significant findings for blood pressure, lipid profiles, and depressive and anxiety symptoms could be variability in the way patients may have viewed their interviewer practitioner type. Two studies using nurse interventionists resulted in significant differences in medication adherence [18, 19]. The influence of medication adherence could be attributed to the participants trusting the nurses more than physical therapists or students. The mental health aspect measured by the SF-36 identified a primary difference in the intervention as being the interventionist. [18-21, 24, 26] Those who saw a significant change in scores received MI from a medical professional such as a nurse interventionalist or exercise physiologist. Additionally, an individual’s willingness to set weight loss goals with a psychotherapist or nurse may differ from other practitioners like sports specialists or dieticians. Research indicates that the interventionist may be uncomfortable discussing weight-related health if they lack adequate knowledge of the topic [44].
These results suggest a trained MI interventionist interviewing patients for at least 30 minutes and as little as one encounter, there is likely to be an increase in session attendance. Program completion is the primary outcome for patients attending OPCR. The inclusion of MI-trained practitioners to help discuss person-centered goal setting for behavior changes can impact other outcomes and can be a valuable contribution to achieving overall improved outcomes. There did not appear to be a difference in impact on outcomes between sessions delivered by group or individually, so this may be a factor of individual patient preference. An individual is more likely to relate to the strategies used and information/goal setting provided with individualized programming [45]. Tailored programs can elicit behavior changes stemming from the perceived autonomy received during the MI person-centered care. MI increases the opportunity for change by exploring what the patient can do or want to improve their health [46]. If patients continue to progress, they are more likely to grow in confidence and adhere to their program [47]. Incorporating evidence-based MI training for practitioners in an OPCR practice setting may be warranted to help retain participants and achieve better outcomes.
None of the studies retained for this review included fidelity scores, limiting our ability to determine the level of MI implementation. This is a common finding in other reviews of MI for health behaviors [48, 49]. Few studies fully described the actual MI training the interventionists underwent, which also is a common finding among other reviews [42, 48]. MI is complex and multifaceted, person-centered by nature, including describing the MI training and intervention fidelity assessment is critical to determining MI consistent practices and future interventions would improve the literature by reporting this information due to the consistent absence of this information. Anxiety can be a barrier to behavior change. It would be beneficial to know during decision-making processes about appropriate strategies to address anxiety in cardiac patients. The amount of time the patients received MI varied throughout the nine retained interventions with a minimum of 30 minutes and a maximum of 450 minutes. Regarding the role MI dose contributes to changes in behaviors, it would be pertinent to determine what frequency or duration are the main factors patients are responsive to provide the best treatment [16].
The methods used in the review strive for validity and reliability; however, there are limitations within this review. First, the literature selected had a criterion written initially in English, which could introduce some bias in retained studies that may not have included MI-based OPCR interventions conducted in other countries. Only nine studies were retained, a modest body of work and three of the nine retained studies focused on females, limiting generalizability. Another limitation regarding MI training reporting is how consistent the interviews with MI were, especially for the articles that did not report intervention fidelity. It is difficult to draw direct conclusions regarding clinical outcomes such as cholesterol and blood pressure affected by MI. It is necessary to measures variables related to these factors such as PA, nutrition diary, and medication adherence. None of the retained articles included smoking cessation as a variable though it is abundant within MI literature and being a focal point in OPCR. Future researchers may consider adding smoking cessation in upcoming interventions.
Conclusion
More research is warranted to elucidate specific details of effective MI interventions in the OPCR setting. However, there is growing evidence that it may be beneficial for OPCR to consider gaining MI training and skills to use when interacting with patients. The results suggest incorporating MI into OPCR, the number of sessions patients attended increased, PA increased, and clinical measures such as LDL and blood pressure also improved. Future research should examine evidence gaps, including the difference interventionist may have on MI delivery, specific intervention structure factors including frequency, duration, looking at potential gender differences in response to an impact from MI in OPCR, report training and fidelity information, and conduct further research in more diverse segments of the population.
References
- Mc Namara K, Alzubaidi H, Jackson JK (2019) Cardiovascular disease as a leading cause of death: how are pharmacists getting involved? Integr Pharm Res Pract 8: 1-11.
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, et al. (2021) Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation.
- Centers for Disease Control and Prevention (2020) How Cardiac Rehabilitation Can Help Heal Your Heart.
- Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, et al. (2007) Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 115: 2675-2682.
- Griffo R, Ambrosetti M, Tramarin R, Fattirolli F, Temporelli PL, et al. (2013) Effective secondary prevention through cardiac rehabilitation after coronary revascularization and predictors of poor adherence to lifestyle modification and medication. Results of the ICAROS Survey. Int J Cardiol 167: 1390-1395.
- O'Connor CM, Whellan DJ, Lee KL, et al. (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1439-1450.
- Ades PA, Balady GJ, Berra K, Franklin BA, Froelicher V, et al. (2020) The Journal of Cardiopulmonary Rehabilitation and Prevention at 40 Years and Its Role in the Evolution of Cardiac Rehabilitation. J Cardiopulm Rehabil Prev 40: 2-8.
- Miller WR, Stephen R (2004) Talking Oneself Into Change: Motivational Interviewing, Stages of Change, and Therapeutic Process. J Cogn Psychother 18: 299-308.
- Rollnick S, Miller W, Butler C (2013) Motivational Interviewing in Health Care: Helping Patients Change Behavior. 3rd edn. New York, NY: The Guilford Press.
- Rubak S, Sandbaek A, Lauritzen T, Christensen B (2005) Motivational Interviewing: a systematic review and meta-analysis. Br J Gen Pract 55: 305-312.
- Palacio A, Garay D, Langer B, Taylor J, Wood BA, et al. (2016) Motivational Interviewing Improves Medication Adherence: a Systematic Review and Meta-analysis. J Gen Inten Med 31: 929-940.
- Heckman CJ, Egleston BL, Hofmann MT. (2010) Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control 19: 410-416.
- Brodie DA, Inoue A (2005) Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs 50: 518-527.
- Vanwormer JJ, Boucher JL (2004) Motivational Interviewing and Diet Modification: A Review of the Evidence. Diabetes Educ 30: 404-419.
- Higgins JP, Thomas J, Chandler J, et al. (2020) Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane, The UK Equator Centre, University of Oxford, UK.
- Poudel N, Kavookjian J, Scalese MJ (2020) Motivational Interviewing as a Strategy to Impact Outcomes in Heart Failure Patients: A Systematic Review. Patient 13: 43-55.
- Beckie TM, Beckstead JW (2010) Predicting cardiac rehabilitation attendance in a gender-tailored randomized clinical trial. J Cardiopulm Rehabil Prev 30: 147-156.
- Chair SY, Chan SW, Thompson DR, Leung KP, Ng SK, et al. (2012) Short-term effect of motivational interviewing on clinical and psychological outcomes and health-related quality of life in cardiac rehabilitation patients with poor motivation in Hong Kong: a randomized controlled trial. Eur J Prev Cardiol 19: 1383-1392.
- Chair SY, Chan SW, Thompson DR, Leung KP, Ng SK, et al. (2013) Long-term effect of motivational interviewing on clinical and psychological outcomes and health-related quality of life in cardiac rehabilitation patients with poor motivation in Hong Kong: a randomized controlled trial. Clin Rehabil 27: 1107-1117.
- Pietrabissa G, Manzoni GM, Rossi A, Castelnuovo G (2017) The MOTIV-HEART Study: A Prospective, Randomized, Single-Blind Pilot Study of Brief Strategic Therapy and Motivational Interviewing among Cardiac Rehabilitation Patients. Front Psychol 8: 83.
- Beckie MB, J. (2011) The Effects of a Cardiac Rehabilitation Program Tailored for Women on Their Perception of Health. J Cardiopulm Rehabil Prev 31: 25-34.
- Beckie TM, Beckstead JW, Schocken DD, Evans ME, Fletcher GF (2011) The effects of a tailored cardiac rehabilitation program on depressive symptoms in women: A randomized clinical trial. Int J Nurs Stud 48: 3-12.
- Ter Hoeve N, Sunamura M, Stam HJ, Boersma E, Geleijnse ML, et al. (2018) Effects of two behavioral cardiac rehabilitation interventions on physical activity: A randomized controlled trial. Int J Cardiol 255: 221-228.
- McGrady A, Burkes R, Badenhop D, McGinnis R (2014) Effects of a brief intervention on retention of patients in a cardiac rehabilitation program. Appl Psychophysiol Biofeedback 39: 163-170.
- Rouleau CR, King-Shier KM, Tomfohr-Madsen LM, Bacon SL, et al. (2018) The evaluation of a brief motivational intervention to promote intention to participate in cardiac rehabilitation: A randomized controlled trial. Patient Educ Couns 101: 1914-1923.
- Beckie TM, Beckstead JW (2010) Predicting Cardiac Rehabilitation Attendance in a Gender-Tailored Randomized Clinical Trial. J Cardiopulm Rehabil Prev 30: 147-156.
- O'Halloran PD, Blackstock F, Shields N, Holland A, Iles R, et al. (2014) Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil 28: 1159-1171.
- Cole B, Pickard K, Stredler-Brown A. (2019) Report on the Use of Telehealth in Early Intervention in Colorado: Strengths and Challenges with Telehealth as a Service Delivery Method. Int J Telerehabil 11: 33-40.
- Patel ML, Wakayama LN, Bass MB, Breland JY (2019) Motivational interviewing in eHealth and telehealth interventions for weight loss: A systematic review. Prev Med 126: 105738.
- Britt E, Hudson SM, Blampied NM (2004) Motivational interviewing in health settings: a review. Patient Edu Couns 53: 147-155.
- Glanzer KM, Emery CF, Frid DJ, Banyasz RE. (2002) Psychological Predictors of Adherence and Outcomes Among Patients in Cardiac Rehabilitation. J Cardiopulm Rehabil 22: 40-46.
- Jackson JL, Emery CF (2013) Emotional distress, alexithymia, and coping as predictors of cardiac rehabilitation outcomes and attendance. J Cardiopulm Rehabil Prev 33: 26-32.
- Holt C, Milgrom J, Gemmill AW (2017) Improving help-seeking for postnatal depression and anxiety: a cluster randomised controlled trial of motivational interviewing. Arch Womens Ment Health 20: 791-801.
- Keeley RD, Brody DS, Engel M, Burke BL, Nordstrom K, et al. (2016) Motivational interviewing improves depression outcome in primary care: A cluster randomized trial. J Consult Clin Psychol 84: 993-1007.
- Hsieh MY, Ponsford J, Wong D, Schonberger M, Taffe J, et al. (2012) Motivational interviewing and cognitive behaviour therapy for anxiety following traumatic brain injury: a pilot randomised controlled trial. Neuropsychol Rehabil 22: 585-608.
- Barrera TL, Smith AH, Norton PJ (2016) Motivational Interviewing as an Adjunct to Cognitive Behavioral Therapy for Anxiety. J Clin Psychol 72: 5-14.
- McHugh F, Lindsay G, Hanlon P, Hutton I, Brown M, et al. (2001) Nurse led shared care for patients on the waiting list for coronary artery bypass surgery: a randomised controlled trial. Heart 86: 317-323.
- Guo P. (2015) Preoperative education interventions to reduce anxiety and improve recovery among cardiac surgery patients: a review of randomised controlled trials. J Clin Nurs 24: 34-46.
- Davies SJC, Jackson PR, Potokar J, Nutt DJ (2004) Treatment of anxiety and depressive disorders in patients with cardiovascular disease. BMJ 328: 939-943.
- Randall CL, McNeil DW (2017) Motivational Interviewing as an Adjunct to Cognitive Behavior Therapy for Anxiety Disorders: A Critical Review of the Literature. Cogn Behav Pract 24: 296-311.
- Laxy M, Mielck A, Hunger M, Schunk M, Meisinger C, et al. (2014) The Association Between Patient-Reported Self-management Behavior, Intermediate Clinical Outcomes, and Mortality in Patients With Type 2 Diabetes: Results From the KORA-A Study. Diabetes Care 37: 1604-1612.
- Suire KB, Kavookjian J, Feiss R, Wadsworth DD. (2021) Motivational Interviewing for Weight Management Among Women: a Meta-Analysis and Systematic Review of RCTs. Int J Behav Med 28: 403-416.
- Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, et al. (2011) Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 12: 709-723.
- Bradbury D, Chisholm A, Watson PM, Bundy C, Bradbury N, et al. (2018) Barriers and facilitators to health care professionals discussing child weight with parents: A meta‐synthesis of qualitative studies. Br J Health Psychol 23: 701-722.
- Broekhuizen K, Kroeze W, Poppel MNV, Oenema A, Brug J (2012) A systematic review of randomized controlled trials on the effectiveness of computer-tailored physical activity and dietary behavior promotion programs: an update. Ann Behav Med 44: 259-286.
- Resnicow K, McMaster F (2012) Motivational Interviewing: moving from why to how with autonomy support. Int J Behav Nutr Phys Act 9: 19.
- Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, et al. (1998) Intensive Lifestyle Changes for Reversal of Coronary Heart Disease. JAMA 280: 2001-2007.
- Suire KB, Kavookjian J, Wadsworth DD (2020) Motivational Interviewing for Overweight Children: A Systematic Review. Pediatrics 146: e20200193.
- Barnes RD, Ivezaj V (2015) A systematic review of motivational interviewing for weight loss among adults in primary care. Obes Rev 16: 304-318.
Indexed at, Google Scholar,, Crossref
Indexed at,, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at,, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Winkler DJ, Suire KB, Kavookjian J, Wadsworth DD (2022) Motivational Interviewing Impact on Cardiac Rehabilitation Program Outcomes: A Systematic Review of Randomized Controlled Trials. J Card Pulm Rehabi 6: 164. DOI: 10.4172/jcpr.1000164
Copyright: © 2022 Winkler DJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3722
- [From(publication date): 0-2022 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 3228
- PDF downloads: 494