Morpho-Physiological Responses and Nutrient Profile of Rice Cultivars to Salinity
Received: 18-Feb-2019 / Accepted Date: 18-Apr-2019 / Published Date: 29-Apr-2019
Abstract
Salinity is one of the main obstacles in increasing rice production worldwide. Even though rice is considered to be a salt-sensitive or moderately sensitive crop, the development of salt tolerant cultivars is essential to coping with the simultaneous increases in global population and salinization in arid and semi-arid areas. Over the course of the project, 5 rice cultivars from two subspecies (Indica and Japonica) were exposed to salt doses (0, 50 and 100 mM, NaCl) in several independent experiments. The experiments were carried out in high light growth chambers beginning with young plantlets at the three-leaf stage grown hydroponically. The plants were characterized based on morpho-physiological traits and ions profile under salinity treatments. Morpho-physiological evaluation of cultivars after exposure to salinity showed marked variability in response to salinity. The growth retardation response of japonica cultivars to salinity was much greater than that of indica cultivars. Reduction in the root:shoot ratio of plants under salt stress was a common behavior among the cultivars. The chlorophyll fluorescent quantum yield and electrolyte leakage of cultivars decreased and increased respectively after exposure to salinity. The nutrient profile of cultivars under salt stress revealed that the tolerant cultivars exhibit significantly higher K/Na ratios in their leaves than sensitive cultivars. Boron, copper and sulphur didn’t change significantly. The contents of cadmium, cobalt, chromium, nickel and selenium in rice cultivars were below the detection limits. Moreover, salinity in high doses makes molybdenum deficiency the same as iron deficiency in rice plants.
Keywords: Chlorophyll fluorescent; Electrolyte leakage; Indica ; Ion homeostasis; Japonica ; K/Na ratios; Root; Shoot
Introduction
Rice is a dietary staple for more than half of the world's human population (most of Asia and Latin America), making it the most consumed cereal grain (for more details see www.irri.org). Salinity is one of the main obstacles to increasing production by expanding rice growing areas worldwide [1-3]. Rice is defined as salt-sensitive crop by Shannon et al. [3] and as moderately sensitive by Maas and Hoffman [4]. On the contrary, it has been reported that rice gives satisfactory yields even when the electrical conductivity of the saturated soil extract is 20 to 25 dsm-1 in the upper layers [5,6].
The response of rice plants to salinity varies depending on growth stage, but most cultivars are highly sensitive to salinity at the seedling stage [7,8]. There are some reports of more salinity tolerance of rice at germination in contrast to other stages [9,10]. Seed germination is not significantly affected up to 16.3 dsm-1, but was severely inhibited when salinity increased to 22 dsm-1. Osmotic stress is reported to be the major cause of germination suppression at high salt levels [11].
Salinity tolerance and the physiological mechanisms underlying it have presented a big challenge for scientists for many years, yet a clear consensus about how to address this crucial issue remains to be reached [12-14]. Three physiological effects are considered to be the major effects of salinity: (i) An osmotic effect, due to high ion concentrations around roots; (ii) An ionic effect, due to salt build up toxicity; and (iii) An oxidative effect, due to the accumulation of reactive oxygen species [15]. It is hypothesized that plant growth is initially inhibited by a cellular response to the osmotic effects of external salt. In a later response, growth is further inhibited by the toxic effects of excessive salt accumulation within the plant [16]. In saline conditions, high Na+ concentration interrupts K+ absorption and transport, and various other physiological activities. Differences in salt tolerance among rice cultivars can also be caused by differential compartmentalization of the Na+ in the shoot [17,18]. Older leaves have been shown to act as ion sinks that restrict ion entry into meristematic and actively growing, photosynthesising cells [19]. Maintaining the homeostasis of Na+, K+ by increasing the K+:Na+ ratio by selectively transporting K+ from stems and sheathes to green leaves and roots, and intercepting Na+ in stems and sheathes, is reported to be a major salt tolerance mechanism in rice [20]. Ca was found to be crucial in altering the ion selectivity of uptake by plants and in enhancing salt tolerance in many species [21], but results in rice are contradictory. Although amelioration of Na+ toxicity by supplemental Ca2+ has been documented in some studies [9,17,22], others have found little [23] or no significant Ca2+ effect [19] in rice plants under salt stress. Magnesium, by comparison, has received little attention, although it could play a central role in senescence-related processes. Mg2+ is implicated in the regulation of protein synthesis [24]. A decrease in Mg2+ absorption could also be responsible for decreased chlorophyll content [25] and quenching of variable fluorescence due to increased spillover of excitation energy from PS II to PS I [26]. Significant decrease in Mg2+ content of rice cultivars is reported after imposing the plants to salt stress [27].
Although rice is not tolerant to excess salinity, it is a crop favored in saline soils and, in fact, is preferred over other tolerant crops during the initial stages of reclamation of many saline soils. This is chiefly due to the system of lowland rice culture that is advantageous to the crop rather than to the tolerance of the crop to soil salinity. The system of lowland rice culture involves the maintenance of standing water almost throughout the growing season, which brings about a significant reduction in root zone salinity by leaching and dilution of the salts. In current project the morpho-physiological characters and the accumulation of ions in several rice cultivars were assessed under different salt doses. The major goals were to evaluate the cultivars and to study the relations between different traits.
Material and Methods
Plant material
Two subspecies of rice cultivars (Orysa sativa L.), Indica and Japonica were used in this project. The seeds of Japonica cultivars (Nipponbare , Tai pai and Zhong hua ) and Indica cultivars (Pokkali and IR75311 ) were provided by the IRRI (International Rice Research Institute). Pokkali and Nipponbare are used as positive control (tolerant) and negative control (sensitive) in salinity studies on rice.
Seed sterilization, germination, and plant growth conditions
Before planting, the seed were surface sterilized. The seeds of cultivars were incubated in 60°C water for 10 min and germinated in plastic boxes on damp filter paper and left in the dark at 28°C for 7 days, and then transferred to light condition at 26°C for 10 days. The germinated seeds were hydroponically grown in polypropylene boxes. For this purpose, the plantlets were fixed to a polypropylene plate, 30 plantlets per plate and floated in 10 L hydroponic solution boxes. The composition of the nutrient solution was 404.23 mgL-1 MgSO4.7H2O, 147.02 mgL-1 CaCl2.2H2O, 114 mgL-1 NH4NO3, 38.39 mgL-1 NaH2PO4, 36.71 mgL-1 FeEDTA, 23 mgL-1 K2SO4, 1.195 mgL-1 MnCl2.2H2O, 0.093 mgL-1 (NH4)6Mo7O24.4H2O, 0.086 mgL-1 H3BO3, 0.037 mgL-1 CuSO4.5H2O and 0.0431 mgL-1 ZnSO4.7H2O. The hydroponic solution was changed weekly in all experiments. After 14 days of growth under normal condition, the plants were subjected to salt stress.
The rice cultivars were subjected to three independent experiments for physiological investigations during salt stress. In these experiments two week old plants cultivated in a hydroponic solution were subjected to one of three salt treatments (0, 50, or 100 mM NaCl) and phenotyped for different morpho-physiological traits on days 0, 7, and 14. The experiments were conducted in high light chambers with 600 μE m-2s-1 light and a photoperiodic setting of 12 hrs/12 hrs day/night. Temperature and relative humidity were set to 26°C/22°C and 75%/70% day/night, respectively. For the first two weeks the seedlings acclimated to this regime while being grown in NaCl-free conditions and then transferred to salt stress treatments. All data were subjected to the analysis of variance and the means were compared using the t test.
Morphological traits and visual salinity scoring
Morphological traits like plant height, tiller number, plant fresh weight and plant dry weight were measured before and after exposure the plants to salt stress. For measuring the dry weight the sampled plants were dried in 65°C for 48 hrs. The plants were visually assessed and scored over the course of their exposure to salt stress based on suggested method of standard evaluation system for salt scoring from IRRI. The inspection was performed at 0, 7 and 14 days after salt exposure and scored visually from 1-9 (Table 1) the higher the value the more sensitivity to stress.
| Score scale | Description |
|---|---|
| 1 | Leaves nearly normal, tips slightly dry |
| 3 | Less than 1/4 of all leaves becoming chlorotic or necrotic |
| 5 | 1/4-1/2 of all leaves becoming chlorotic or necrotic |
| 7 | More than 2/3 of all leaves becoming chlorotic or necrotic |
| 9 | Most of the plants looks dead |
Table 1: The score scales and descriptions of plants under stress.
Chlorophyll fluorescence measurements
The quantum yield of PSII of cultivars grown under control and salt stress conditions were determined using pulse-amplitude modulated system of portable fluorometer PAM-2000 (WALZ, Effeltrich, Germany) after 7 days exposure to salinity. This fluorometer allows data to be collected under the ambient light, temperature and moisture conditions without pre-dark adaptation of samples [28]. All measurements were performed on the second or third fully expanded leaves. The effective quantum yield in PSII was calculated using the equation ΔF/(F'm)=(F'm-Fs)/(F'm) where F'm and Fs represent the maximum and steady state fluorescence in the light condition respectively (for nomenclature see [29]).
Electrolyte leakage
The electrolyte leakage was measures base on electrical conductivity of aqueous bathing solution containing plant tissues before and after boiling it as depicted in [30].
Nutrition measurements
Na+ and K+ were measured on shoot after 7 days exposure to salinity. Plant material was weighed (80-100 mg fresh weight), extracted with hydrochloric acid (0.1 M) and heated at 80°C for 20-30 min. Ions were measured using flame photometer (model 410, Corning). The recorded data transformed to mM/g fresh weight after estimating the calibration curve. Element concentrations were determined with inductively coupled plasma-atomic emission spectrometry (ICP-AES; Applied Research Laboratories, Accuris, Ecublens, Switzerland) using an IRIS Advantage Duo ER/S (Thermo Fisher). Elemental quantification was validated using IC-CTA-VTL2 Virginia tobacco leaves as a certified reference material.
Results
Morpho-physiological evaluation of cultivars
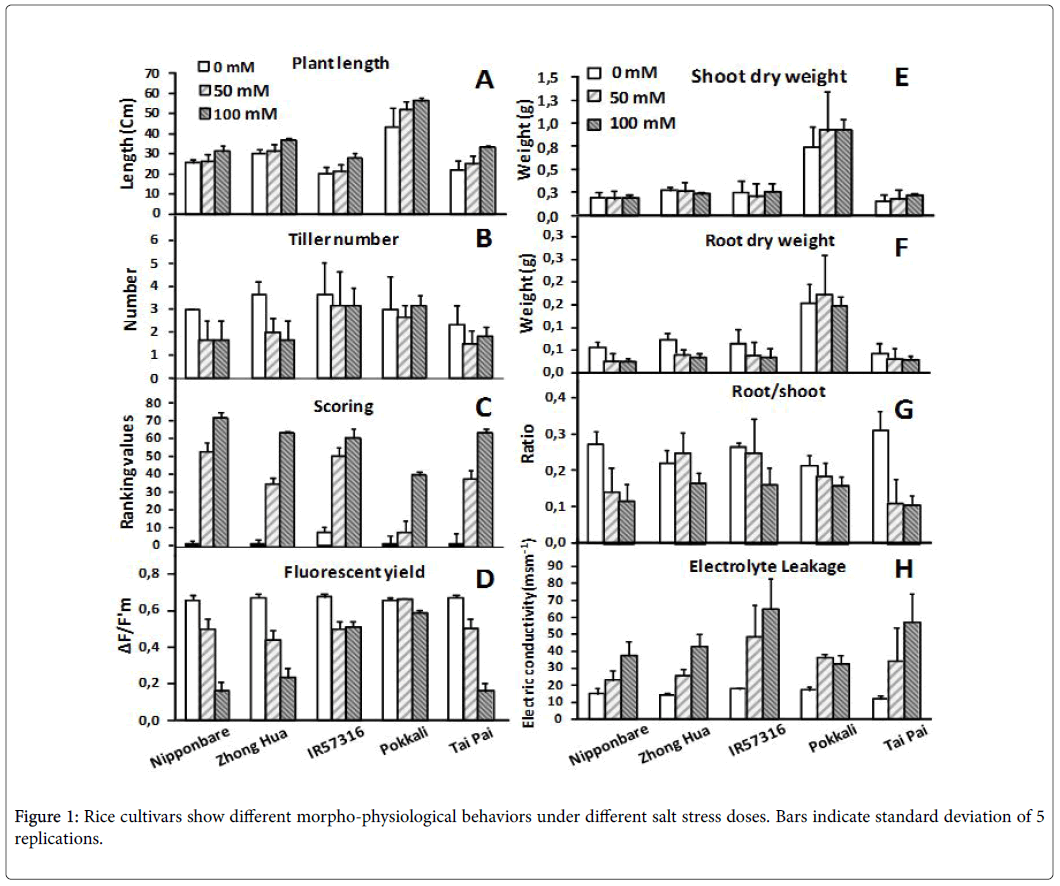
The cultivars were evaluated based on morpho-physiological traits after 7 days exposure to different doses of salinity. Tiller number and plant length didn’t change significantly over the time course among cultivars (Figures 1A and B). In a visual inspection we scored the plants, ranked the scores, and then calculated the mean of ranks for each cultivar. Based on the results, all lines changed phenotypically following salt stress treatments. As a rule, the lower the score, the more tolerant the cultivar; in this experiment, the Pokkali cultivar demonstrated the lowest score of all the cultivars at the highest salt dose (100 mM) (Figure 1C). As already explained, salinity stress promotes changes in chlorophyll fluorescence parameters and quantum yields, and both of these parameters were considerably affected as salt concentrations increased (Figure 1D). We detected drastic changes in sensitive cultivars (Nipponbare , Zhong Hua and Tai pai ) regarding fluorescent yields under stress. In contrast Pokkali obviously cope with intermediate and high salinity treatments and showed less change in chlorophyll fluorescence parameters and quantum yields. This may simply be due to the rapid vegetative growth rate of Pokkali –more vegetative mass could equalize ion accumulation and influence the reduction of electron acceptor of PSII. Salt treatments didn’t dramatically influence the shoot and root dry weight of rice cultivars (Figures 1E and F). It appears that 7 days is not enough time to significantly influence biomass performance. However, reduction in the root:shoot ratio of plants under salt stress was a common behavior among the cultivars (Figure 1G). This reduction was high in sensitive cultivars (e.g. Tai Pai and Nipponbare ) and less in tolerant ones (e.g. Pokkali ).
Electrolyte leakage is a good indicator of the degree of cellular damage, as it allows quick assessment of the intactness of cell membranes [31]. In the absence of stress, electrolyte leakage did not vary among cultivars, but the presence of NaCl in the nutritive solution induced leakage. This induction was dose dependent in all the cultivars except Pokkali, which maintained its membrane integrity even at a salt dose of 100 mM NaCl (Figure 1H).
Nutrient profiling of rice cultivars under salt stress
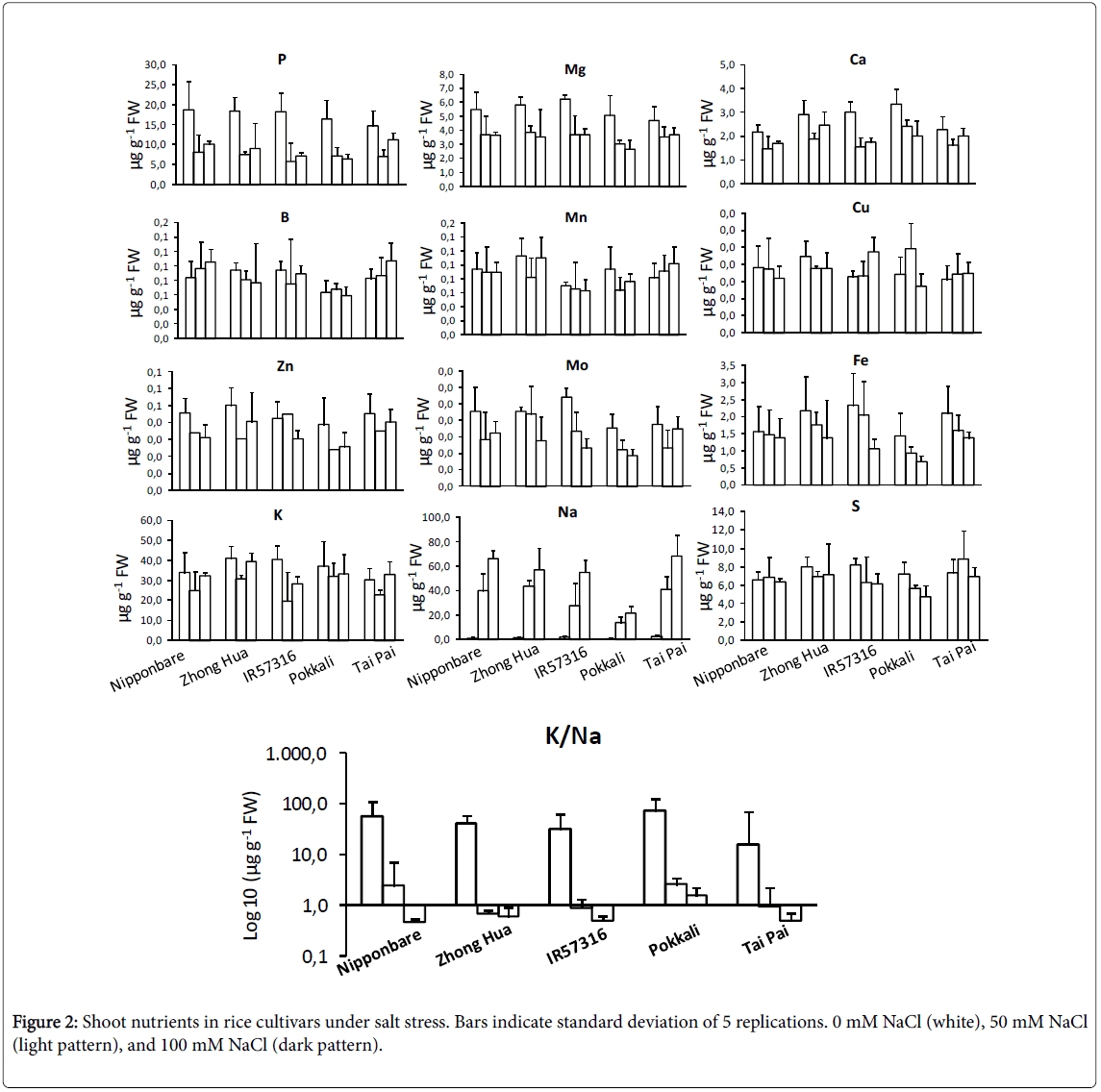
Inductively Coupled Plasma/Atomic Emission Spectrometry (ICPAES) was used to study profile changes in shoot micro and macronutrient contents. Phosphorous, magnesium, calcium and zinc were significantly decreased under salt condition (Figure 2). This decrease was independent of the NaCl dose. On the other hand, molybdenum and iron contents did decrease in a dose-dependent manner. Boron, copper and sulphur didn’t change significantly. The contents of cadmium, cobalt, chromium, nickel and selenium in rice cultivars were below the detection limits. As expected, K+ levels were negatively correlated with increasing salinity. Na+ levels increased to a significantly higher degree in sensitive rather than tolerant cultivars. None of the cultivars showed a significant trend toward potassium depletion. We couldn’t detect an obvious difference among cultivars for most of elements at the NaCl concentrations tested. A screening system for salinity resistance using K/Na ratio has been reported for rice cultivars [32,33]. In this study, the most tolerant cultivar, Pokkali , showed significantly higher K/Na values under severe salt treatment (100 mM NaCl) in comparison to other cultivars (Figure 2).
Discussion
We characterized all rice cultivars morpho-physiologically. The results confirmed the superiority of Pokkali (as a positive control) and posteriority of Nipponbare (as a negative control) in comparison to other cultivars or studying responses to salt stress. The fluorescent yield in sensitive rice cultivars was seriously affected by high salt dose but didn’t show significant change in tolerant cultivars. It has been reported that chlorophyll fluorescence is a suitable screening technique for assessing the effect of environmental stresses on plants [34-36]. Salinity stress promoted drastic changes in chlorophyll fluorescence parameters due to its adverse effect on PSII activity and destruction of chlorophyll pigments via accumulation of ions that may influence the reduction of PSII’s electron acceptor (Quinone A). Fv/Fm ratio, which is an excellent indicator of PSII quantum yield, is considerably affected by salt stress [35,36]. Among the rice cultivars evaluated, there is a great deal of diversity as regards responses to salt stress. Pokkali and Nipponbare are extremely tolerant and sensitive rice cultivars, respectively, and the other cultivars lie between, except Tai Pai , which showed higher sensitivity than Nipponbare in our study. Pokkali and Nipponbare have, in fact, been frequently used in previous studies due to their high salt tolerance and sensitivity [17,37-39].
As already explained, the cultivars’ chlorophyll fluorescence parameters varied during salt stress. It seems that the tolerant cultivars have mechanisms to offset the adverse effect on activity of PSII and maintain the chlorophyll pigments in high salt doses rather than sensitive cultivars. These results are in agreement with the results of Moradi and Ismail [35] but are contrary with the results of Dionisio- Sese and Tobita [40] who reported no significant change in fluorescent yields during salt stress in rice plants.
Electrolyte leakage is a physiological feature that can be exploited to assess membrane deterioration due free radical-induced lipid peroxidation during salt stress [41-43]. The induction of change in membrane permeability for all cultivars was dose dependent, the only exception being the tolerant cultivar, Pokkali , which maintained selective membrane permeability even at a salt dose of 100 mM (Figure 1H).
In an ion assay, we detected a marked change in the levels of some macronutrients (including Na+ and K+) and in micronutrients (including molybdenum and iron) after exposing the various cultivars to salinity. However, the trends of change were different in different cultivars; an increase in sodium and a decrease in potassium occurred in sensitive cultivars, but not in tolerant cultivars. The results of this experiment are consistent with previous studies [32,44]. Ion exclusion and osmatic tolerance are two major mechanisms of tolerance against salinity in rice. Ion exclusion causes more exclusion of Na+ and Clthrough efflux of ions from the root cells and in consequence fewer ions accumulate in leaves of tolerant cultivars [45-47]. The changes in molybdenum and iron contents were dose dependent, decreasing as salt dose increased. Molybdenum in complex by a specific organic protein is essential for plants as it is required by a number of enzymes that catalyze key reactions in nitrogen assimilation, purine degradation, phytohormone synthesis, and sulfite detoxification [48,49]. Moreover, a crosstalk between molybdenum and iron metabolisms is presumed because (i) Uptake mechanisms for molybdate and iron affect each other, (ii) Most molybdo-enzymes do also require iron-containing redox groups such as iron-sulfur clusters or heme, (iii) Molybdenum metabolism has recruited mechanisms typical for iron-sulfur cluster synthesis, and (iv) Both molybdenum cofactor synthesis and extra-mitochondrial iron-sulfur proteins involve the function of a specific mitochondrial ABC-type transporter [50]. As concluded in current project, increasing in salinity doses resulting in molybdenum deficiency associated with reduced molybdo-enzyme activities and reductions in plant growth.
Conclusion
Salinity stress could influences grain production of rice in two critical growth stages, young seedlings after transplanting to soil and during seed setting of the mature plants. The current project concentrated mainly on the morpho-physiological aspect of salinity effects on young plantlets-three leaf stage plants. The cultivars were markedly variable in respond to salinity; in general, Japonica cultivars were much more negatively affected by high salinity than were Indica cultivars. We found chlorophyll fluorescence yield as a rapid and nondestructive screening technique to assess the effect of salinity stress on plants. The nutrient profile confirmed the significant change in some macro and micro nutrients under different salinity doses. The K/Na ratio was detected as a convenient trait for screening the sensitive and tolerance rice cultivars under a hydroponic system. The salinity tolerant genotypes possess the ability of ion homeostasis particularly for K+ and Na+ in compare to sensitive genotypes. Moreover, salinity in high doses makes molybdenum deficiency the same as iron deficiency in plants. The regulating mechanisms of ion homeostasis in rice when exposed to salinity, depends on the transport proteins in plasma membrane of the cells. Molecular function of ion channels and transporters under salt stress in rice is the perspective of future researches.
Acknowledgement
We thank Dr. Joachim Kopka and Alexander Erban of the Max- Planck Institute of Molecular Plant Physiology for the kind help and precious advices on the manuscript.
References
- Ponnamperuma FN (1984) Straw as a source of nutrients for wetland rice. In Organic Matter and Rice. IRRI, Los Banos, Philippines, pp 117-136.
- Zeng L, Shannon MC (2000) Salinity effects on seedling growth and yield components of rice. Crop Sci 40: 996-1003.
- Shannon MC, Rhoades JD, Draper JH, Scardaci SC, Spyres MD (1998) Assessment of salt tolerance in rice cultivars in response to salinity problems in California. Crop Sci 38: 394-398.
- Maas EV, Hoffman GJ (1977) Crop salt tolerance-current assessment. J Irrigation and Drainage Division, ASCE 103 (IRI): 115-134.
- Van Alphen JG (1975) Salt affected soils in Peru. International Institute for Land Reclamation and Improvement, Wageningen. Annual Report pp 7-13.
- Yadav JSP, Girdhar IK (1981) The effects of magnesium: calcium ratios and sodium adsorption ratio values of leaching water on the properties of calcareous versus non-calcareous soils. Soil Sci 131: 194-198.
- Lutts S, Kinet JM, Bouharmont J (1995) Changes in plant response to NaCl during development of rice (Oryza sativus L.) varieties differing in salinity resistance. J Exp Bot 46: 1843-1852.
- Zeng L, Poss JA, Wilson C, Draz A-SE, Gregorio GB, et al. (2003) Evaluation of salt tolerance in rice genotypes by physiological characters. Euphytica 129: 281-292.
- Munns R, Husain S, Rivelli AR, James RA, Condon AG, et al. (2002) Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant Soil 247: 93-105.
- Khan MSA, Hamid A, Karim MA (1997) Effect of sodium chloride on germination and seedling characters of different types of rice (Oryza sativa L.). J Agron Crop Sci 179: 163-169.
- Heenan DP, Lewin LG, McCaffery DW (1988) Salinity tolerance in rice varieties at different growth stages. Aust J Exp Agric 28: 343-349.
- Anil VS, Krishnamurthy H, Mathew MK (2007) Limiting cytosolic Na+ confers salt tolerance to rice cells in culture: a two-photon microscopy study of SBFI-loaded cells. Physiol Plant 129: 607-621.
- Feng G, Zhang FS, Li XL, Tian CY, Tang C, et al. (2002) Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12: 185-190.
- Hirayama M, Wada Y, Nemoto H (2006) Estimation of drought tolerance based on leaf temperature in upland rice breeding. Breed Sci 56: 47-54.
- Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1-14.
- Munns R (1993) Physiological processes limiting plant-growth in saline soils – some dogmas and hypotheses. Plant Cell Environ 16: 15-24.
- Anil VS, Krishnamurthy P, Kuruvilla S, Sucharitha K, Thomas G, et al. (2005) Regulation of the uptake and distribution of Na+ in shoots of rice (Oryza sativa) variety Pokkali: role of Ca2+ in salt tolerance response. Physiol Planta 124: 451-464.
- Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25: 239-250.
- Yeo AR, Flowers TJ (1982) Accumulation and localisation of sodium ions within the shoots of rice (Oryza sativa) varieties differing in salinity resistance. Physiol Plant 56: 343-348.
- Chen HZ, Natalia L, Zhu DF, Lin XQ, Mang YP, et al. (2007) Absorption and distribution of Na+ and K+ in rice seedling under salt stress. Chines J Plant Ecol 31: 937-945.
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247-273.
- Aslam M, Muhammad N, Qureshi RH, Ahmad Z, Nawaz S, et al. (2003) Calcium and salt-tolerance of rice. Commun Soil Sci Plant Anal 34: 3013-3031.
- Song JQ, Fujiyama H (1996) Difference in response of rice and tomato subjected to sodium salinization to the addition of calcium. Soil Sci Plant Nutr 42: 503-510.
- Flowers TJ, Dalmond D (1992) Protein synthesis in halophytes: the infuence of potassium, sodium and magnesium in vitro. Plant and Soil 146: 153-161.
- Leidi EO, Silberbush M, Lips SH (1991) Wheat growth as affected by nitrogen type, pH and salinity. II. Photosynthesis and transpiration. J Plant Nutr 14: 247-256.
- Krause H, Weis W (1991) Chlorophyll Fluorescence and Photosynthesis: The Basics. Ann Rev Plant Physiol Plant Mol Biol 42: 313-349.
- Lutts S, Kinet JM, Bouhrmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78: 389-398.
- Schroeter B, Green TGA, Seppelt RD, Kappen L (1992) Monitoring photosynthetic activity of crustose lichens using a PAM-2000 fluorescence system. Oecologica 92: 457-462.
- Van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynthesis Res 25: 147-150.
- Whitlow TH, Bassuk NL, Ranney T G, Reichert DL (1992) An improved method for using electrolyte leakage to assess membrane competence in plant tissues. Plant Physiol 98: 198-205.
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. The Plant J 45: 523-539.
- Asch F, Dingkuhn M, Dörffling K, Miezan K (2000) Leaf K/Na ratio predicts salinity induced yield loss in irrigated rice. Euphytica 113: 109-118.
- Nguyen HTT, Shim IS, Kobayashi K, Usui K (2005) Effects of salt stress on ion accumulation and antioxidative enzyme activities of Oryza sativa L. and Echinochloa oryzicola Vasing. Weed Biol Management 5: 1-7.
- Babu S, Sheeba A, Yogameenakshi P, Anbumalarmathi J, Rangasamy P (2007) Effect of salt stress in the selection of salt tolerant hybrids in rice (Oryza sativa L.) under in vitro and in vivo condition. Asian J Plant Sci 6: 137-142.
- Moradi F, Ismail AM (2007) Responses of photosynthesis, chlorophyll fluorescence and ROS-Scavenging systems to salt stress during seedling and reproductive stages in rice. Annu Bot 99: 1161-1173.
- Yamane K, Kawasaki M, Taniguchi M, Miyake H (2008) Correlation between chloroplast ultrastructure and chlorophyll fluorescence characteristics in the leaves of rice (Oryza sativa L.) grown under salinity. PPS 11: 139-145.
- Tsuchiya M, Miyake M, Naito H (1994) Physiological-response to salinity in rice plants. 3. A possible mechanism for Na+ exclusion in rice root under NaCl-stress conditions. Japanese J Crop Sci 63: 326-332.
- Lutts S, Kinet JM, Bouharmont J (1995) Changes in plant response to NaCl during development of rice (Oryza sativus L.) varieties differing in salinity resistance. J Exp Bot 46:1843-1852.
- Kim DW, Shibato J, Agrawal GK, Fujihara S, Iwahashi H, et al. (2007) Gene transcription in the leaves of rice undergoing salt-induced morphological changes (Oryza sativa L.). Molecules and Cells 24: 45-59.
- Dionisio-Sese ML, Tobita S (2000) Effects of salinity on sodium content and photosynthetic responses of rice seedlings differing in salt tolerance. J Plant Physiol 157: 54-58.
- Hernandez JA, Jimenez J, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long term stress is associated with induction of antioxidant defences. Plant Cell Environ 23: 853-862.
- Demiral T, Turkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247-257.
- Mandhania S, Madan S, Sawhney V(2006) Antioxidant defense mechanism under salt stress in wheat seedlings. Biol Plant 227: 227-231.
- Alamgir ANM, Musa M, Ali MY (2007) Some aspects of mechanisms of NaCl stress tolerance in the seedlings of four rice genotypes. Bangladesh J Bot 36: 181-184.
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651-681.
- Roy SJ, Negrao S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26: 115-124.
- Nath M, Yadav S, Sahoo RK, Passricha N, Tuteja R, et al. (2016) PDH45 transgenic rice maintain cell viability through lower accumulation of Na+, ROS and calcium homeostasis in roots under salinity stress. J Plant Physiol 191: 1-11.
- Hille R (2013) The molybdenum oxotransferases and related enzymes. Dalton Trans 42: 3029-3042.
- Kneip C, Lockhart P, Voss C, Maier UG (2007) Nitrogen fixation in eukaryotes-new models for symbiosis. BMC Evol Biol 7: 55.
- Bittner F (2014) Molybdenum metabolism in plants and crosstalk to iron. Front Plant Sci 5: 28.
Citation: Siahpoosh MR, Ghamer M (2019) Morpho-Physiological Responses and Nutrient Profile of Rice Cultivars to Salinity. J Rice Res 7:210.
Copyright: © 2019 Siahpoosh MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3144
- [From(publication date): 0-2019 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2352
- PDF downloads: 792