Morphology of Lignin Analysis Pre-Treatment on Fiber Surfaces after Organosolv
Received: 04-Aug-2022 / Manuscript No. bsh-22-72212 / Editor assigned: 06-Aug-2022 / PreQC No. bsh-22-72212 (PQ) / Reviewed: 20-Aug-2022 / QC No. bsh-22- 72212 / Revised: 23-Aug-2022 / Manuscript No. bsh-22-72212 (R) / Published Date: 30-Aug-2022 DOI: 10.4172/bsh.1000124
Abstract
The redisposition of lignin to the fiber face after organosolv pre-treatment was studied using two different reactor types. Results from the conventional autoclave reactor suggest that redisposition occurs during the cooling down stage. Redeposited patches appeared to be globular in shape. The size and population viscosity of the patches depends on the attention of organosolv lignin in the cuisine liquor, which is harmonious with the thesis that reprecipitation of lignin occurs when the system is cooled down. The use of a relegation reactor showed that displacing the spent cooking liquor with fresh cuisine liquor helps in reducing the redisposition and the addition of a washing stage with fresh cuisine liquor reduced the reprecipitation of lignin, particularly on the external fiber shells. Redeposition of lignin was still observed on regions that were less accessible to washing liquid, similar as fiber lumens, suggesting that complete forestalment of redisposition wasn’t achieved.
Keywords
Organosolv pre-treatment; Cuisine liquor; Forestalment
Introduction
Pre-treatment is an important step in the product of biofuel (ethanol). The primary end of pre-treatment is to make the cellulose accessible to enzymes during enzymatic hydrolysis. There are several mechanical, chemical, and thermal pre-treatment styles available to make the cellulose accessible by altering the structure or composition of biomass [1]. Lignin junking is one of the primary pretensions of numerous pre-treatments. Since lignin acts as a defensive subcaste, the junking of lignin improves the enzymatic hydrolysis.
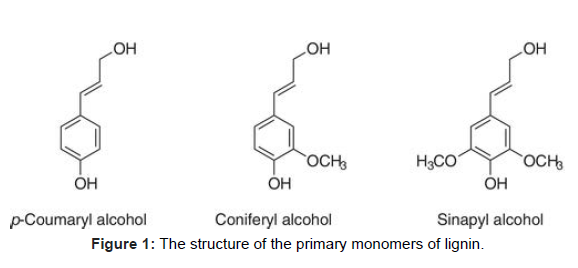
Lignin is an unformed, phenolic polymer, whose primary purpose is to strengthen the cell wall and to cover the factory from microbial attack. The primary monomers of lignin are p- coumaryl, coniferyl, and sinapyl alcohol, as shown in (Figure 1). Softwood generally contains guaiacyl lignin made of coniferyl alcohol. Several liaison are present in lignin similar as β- O- 4, α- O- 4, β- 5, and β- β’. Lignin – carbohydrate complexes (LCC) can also be present in the cell wall.
During pre-treatment, lignin is assumed to go through a phase transition from solid to liquid and back to solid via a complex medium marvels similar as phase transition, response, and solubilisation may do during the pre-treatment. Experimenters have noticed the conformation of globular structures on the biomass face after pulping processes used for paper product. Analogous structures were also reported in pre-treatment processes similar as dilute acid pre-treatment and hot water pre-treatment. These conformations are proven to be conforming substantially of lignin, but the conformation of pseudolignin from carbohydrate declination products has also been reported [2]. Experimenters have tried to explain the medium behind the drop conformation. One presumptive explanation, especially in the case of pre-treatment with water, is that temperature exceeding the glass transition temperature of lignin allows lignin to come mobile and move within the cell wall. When in contact with a waterless medium, lignin tries to reduce the face area in contact with water due to its hydrophobic nature. This will beget the lignin to form globular structures and upon cooling these driblets get deposited on the biomass face. The presence of analogous driblets in organosolv pulping or pre-treatment suggests that another medium is possible for the lignin Redeposition other that the hydrophobicity of lignin. In similar cases, the Redeposition could be due to the reduction in detergent attention during response or due to a reduction in temperature causing the solubility of lignin in the detergent to drop. Both of these can do during organosolv pretreatment.
Some studies suggest that these driblets have a negative effect on enzymatic hydrolysis due to non-specific list of enzymes to the lignin and steric interference. These driblets increase the Klason lignin content of the pre-treated biomass and hence the understanding of how different reactor setup affects the lignin Redeposition could be a useful tool in designing the pre-treatment process in the assiduity. In the current composition, two batch reactor setups are compared, one an autoclave reactor where the biomass is cooled down with the liquor and a relegation reactor system where the spent liquor is displaced with fresh liquor before cooling down. The authors are comparing how these two styles affect the lignin redeposit ion and other factors impacting the drop size and population [3].
Different reactor setups to ameliorate the delignification process have been employed ahead. Flow- through processes have shown to ameliorate the delignification in processes similar as alkaline and organosolv pre-treatment and to help the condensation of dissolved lignin. The reactor system used in this work is a design called Rapid Heating Displacement Pre-treatment Reactor, designed and developed by RISE PFI. The reactor has a detergent heating section which can toast up the detergent previous to mixing with the substrate enabling rapid-fire heating (30°C/min). The “High- pressure Rapid Heating Displacement Pre-treatment Reactor” is an inflow- through reactor, albeit with the possibility of veritably rapid-fire heating of the cuisine liquor. Due to heat transfer limitations in the rather large biomass patches, the rapid-fire heating point wasn’t used [4]. The crucial point of the reactor system is to displace the spent liquor without reducing the process temperature or pressure. This crucial point will help in understanding the goods the cooling stage has on the pre-treatment process.
Materials and Methods
Raw accoutrements and chemicals
The wood chips used in this study were artificial Norway improve (Picea abies) chips from Norske Skog Skogn, Norway. The chips were dried and fractionated to different flyspeck sizes at RISE PFI. The − 8/ 7 mm bit was stored at room temperature until farther use. Absolute ethanol was attained from VWR chemicals. Sulphuric acid (ACS reagent, 95 – 98), potassium permanganate (ACS reagent ≥ 99 pure), and mannitol (ACS reagent, ≥ 98) were entered from Sigma- Aldrich. All the chemicals were used as entered.
Pre-treatment procedure
63(w/ w) ethanol – water admixture was used as the detergent in all the responses and the liquid to biomass rate was7.51. 1 wt H2SO4 (roaster- dried wood base) was used as a catalyst. The choice of 63 ethanol in water was grounded on results attained by Agnihotri etal [5]. Who attained excellent delignification of Norway improve (Picea abies) at this ethanol attention, showing that Norway improve needed a kindly advanced ethanol attention than the non-wood substrate sugarcane bagasse. After the response, the chips were washed completely, first with fresh detergent and also with water, and were stored in a watertight bag until farther use.
Conventional autoclave reactor
The reactor used in these trials was a custom- made autoclave reactor system with six resemblant autoclaves from TOP diligence, France. Each autoclave has an internal volume of 1 L and the system uses electrical heating. The maximum operating pressure and temperature are 50 bar and 220 °C. The reactor cap is fitted with a thermocouple which is extended to the inside to measure the internal temperature. The reactor is seated in an inclined position and is agitated throughout the response for proper mixing and indeed toast distribution. After response, the reactor was cooled down in a water bath before transferring the content.
High- pressure rapid-fire heating relegation pre-treatment reactor
The relegation reactor is a state- of- the- art reactor setup with the provision of displacing the cuisine liquor with fresh detergent without cooling down the reactor. It’s an inflow- through reactor with oil painting heating [6]. The biomass is placed in the reactor and the cuisine liquor is pumped to the reactor from the liquid storehouse tank. Indeed though the reactor is able of rapid-fire heating, a heating rate of 2 °C per nanosecond was used to match the heating rate of the autoclave reactor and to reduce temperature slants in the chips during heat up. A rotation of cooking liquor was employed throughout the response to assure proper mixing and an indeed temperature distribution. After the response, the cuisine liquor was displaced by fresh liquid and the displaced effluent was collected for farther analysis. The reactor is equipped with a provision to wash the pre-treated biomass inside the reactor before cooling down. The washing liquid can be pumped from the force tank and pass through the biomass the same way as the relegation is done.
Biomass yield after pre-treatment
The biomass yield after pre-treatment was calculated gravimetrically. The mass of biomass after pre-treatment was recorded [7]. The humidity content of the sample was calculated gravimetrically. A pre-weighed quantum of the sample was dried in a roaster at 105 °C overnight and also counted again. The weight and humidity content were used to calculate the dry mass of the pre-treated sample. Sot mass was also used to calculate the biomass yield after pre-treatment as the chance of biomass taken for pre-treatment.
Lignin analysis
Lignin analysis of pre-treated biomass was carried out grounded on the NREL system for structural carbohydrate and lignin analysis in biomass. The sample was air- dried to humidity content lower than 10. The sample was also pulverized and 300 ± 10 mg of the sample was transferred to a response tube. Three millilitres of 72 H2SO4 was added to the sample and mixed completely with a Teflon rod. The tube was also placed in a Lab shake at 30 °C and incubated for 60 min with stirring the sample every 5 – 10 min. After incubation, the tube was removed and the contents of the tube were transferred to a 100 ml Pyrex glass bottle and adulterated with 84 mL distilled water to make the acid attention be 4 [8]. The sample was also autoclaved at 121 °C for 1 h after which it was removed, cooled to room temperature, and filtered through a pre-weighed sludge paper. The liquid was collected, annulled using Ca (OH) 2, and anatomized using HPLC. The sludge paper and the un dissolved content were dried in a roaster at 105 °C overnight and the Klason lignin was anatomized gravimetrically. The organosolv effluent lignin (OEL) was measured by gravimetric. Ten millilitres of effluent was taken and the lignin in the effluent was rained by adding three times the volume of water (30 ml). The rained lignin was also filtered using a pre-weighed sludge paper and the sludge cutlet with the sludge paper was dried overnight. The dried sludge cutlet was counted to calculate the quantum of lignin and the attention of effluent lignin.
Lignin staining and indurate- drying
The samples were stained with KMnO4 as per the system of Gregersen etal. The biomass samples were soaked in 4 KMnO4 result for roughly 5 min. After soaking, the sample was completely washed with water to remove redundant KMnO4. The samples were firmed in a rotavap using liquid nitrogen and dried in a snap drier under vacuum [9].
Scanning electron microscopy
Samples were sputter carpeted with 20 nm gold or platinum using a Cressington 208 HR B sputter fleece. Chat- carpeted samples were also transferred to a Helios G4 UX FIB/ SEM. Images were recorded using between 3 and 5 keV acceleration voltage, as indicated, with current and working distance as indicated. The sensor used was an in- chamber Ion Conversion and Electron (ICE) sensor, set to descry secondary electrons. For conventional electron microscopy, the sample wasn’t listed. For sampling imaging/ imaging of fiber lumen, the sample was listed to 52 °, and the sample was cut into using the instrument’s Ga ion ray at high ray currents. Ion milling was performed using high currents. After milling, the mulled area was imaged using the electron ray.
Image analysis
The images from SEM were anatomized using Image J software. Images included in the handwriting have had brilliance/ discrepancy acclimated automatically using Image J’s erected- in algorithm. Lumen micrographs in rotated to align the lumen horizontally in the image, also cropped [10]. Driblets were linked by visual examination, manually named in Image J, and measured using the erected- in algorithm. The size of the lignin driblets and the number of driblets per unit area were measured manually through area selection. Once named, the patches were measured using Image J’s erected- in measure function. We assumed Feret’s periphery was an accurate representation of the flyspeck periphery. Image J reports the major Feret periphery, which for anon-circular blob is the largest end- to- end distance across the flyspeck [11].
Results and Discussion
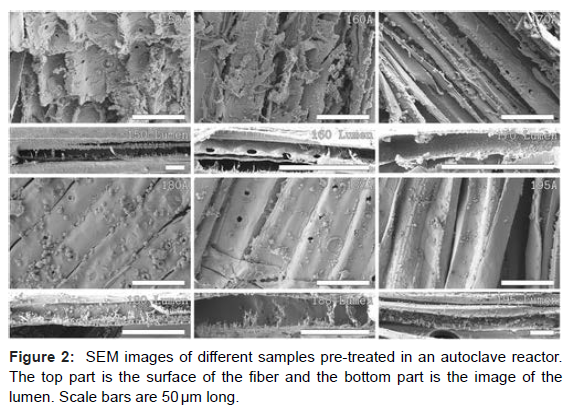
Lignin analysis of all the pre-treated samples. Detailed response parameters of some of the autoclave reactor runs are reported away. (Figure 2) shows the SEM images of the samples from the autoclave reactor. No visible Redeposition of lignin on the first two samples (150A and 160A) was observed. From sample 170A and over (A liquor lignin attention of15.8 g/ L and advanced), lignin Redeposition was observed, and a presumptive explanation for this is super saturation. The Redeposition of lignin happens in the cooling stage of pretreatment and when the temperature goes down, the solubility of lignin in the detergent reduces causing the lignin to precipitate. For samples 150A and 160A, it can be seen that the attention of lignin in the effluent is low. It’s assumed that indeed after cooling, the lignin doesn’t reach super saturation and there’s no visible Redeposition when the effluent lignin attention is low. Once the pre-treatment inflexibility goes up, the attention of lignin in the effluent increases [12]. The high attention of lignin in the effluent results in the result reaching super saturation while cooling causing the lignin to redeposit back onto the fiber face. The face of the wood fiber is rougher in the least pre-treated samples compared to the other samples. It appears that the face becomes smoother as lignin is removed.
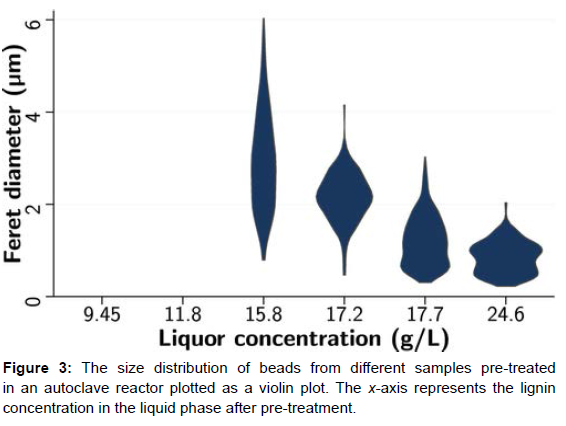
The image analysis of the rest of the samples from the autoclave reactors showed an intriguing trend; the size of the driblets is dwindling as the temperature or the inflexibility of pre-treatment is adding. At the same time, the quantum of globules per unit area increased. This observation supports the thesis of super saturation being the cause of Redeposition. Super saturation is the driving force for nucleation and crystal clear growth. As per super saturation proposition, both the nucleation rate and the growth are dependent on super saturation. At lower super saturation, the crystal clear growth rate will be faster than the nucleation rate, and hence, the chargers grow bigger. At advanced super saturation, the nucleation rate dominates leading to advanced population viscosity with lower chargers [13]. It can be seen that at a liquor attention of15.8, the driblets are larger in size, but the population viscosity is lower. As the lignin attention goes up, the drop size is reduced, but the number of driblets per unit area increases. Since the attention of lignin in the effluent is advanced at high inflexibility, the cooling stage creates an advanced number of nucleation spots. This could be the reason for the high drop viscosity at advanced rigidness. Since the number of nucleation spots is high, the driblets will only grow a little. But at lower temperature or inflexibility, the lignin attention in the effluent is lower, and hence, the number of nucleation spots will be reduced relative to advanced inflexibility. This leads to further quantum of lignin accumulating on to smaller nucleation spots causing the driblets to grow bigger. (Figure 3) shows this effect.
Since the liquid lignin attention is assumed to be the main parameter affecting the lignin reprecipitation, its worth noticing that there appeared to have some anomalies in the effluent lignin computation. For sample 180A, the lignin attention is lower than that of sample 170A indeed though the Klason lignin content of 180A is lower.
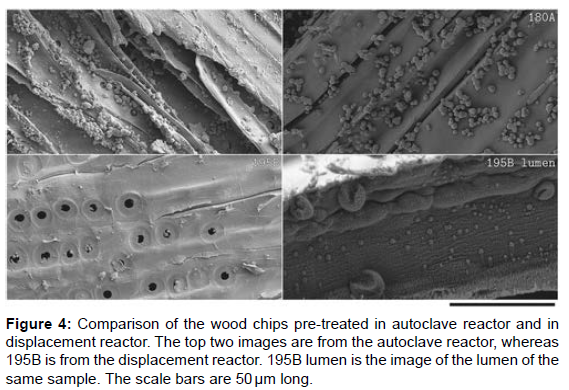
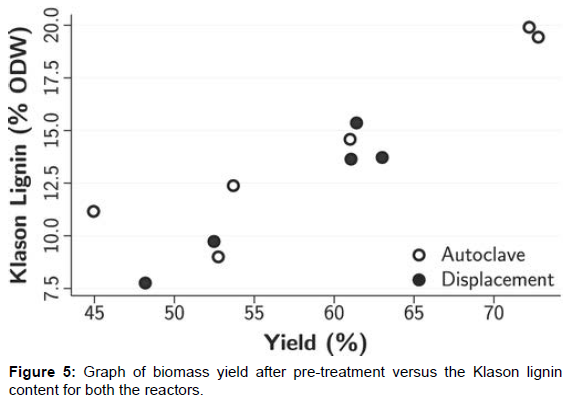
A clear reduction in Redeposition of lignin driblets was observed on samples pre-treated in the relegation reactor as shown in (Figure 4). This was an anticipated result since the washing stage is done before cooling down in the relegation reactor, in agreement with the Redeposition thesis. Since utmost of the effluent containing lignin was displaced at a temperature near to the response temperature, the possibility of lignin Redeposition to the fiber face was excluded. A comparison of Klason lignin content versus biomass yield after pretreatment on both reactors agrees with this observation. As shown in (Figure 5), the Klason lignin content for the relegation reactor samples is lower compared to the bones from the autoclave reactor for analogous total biomass yield [14]. The anomaly to this general observation can be seen as one of the relegation reactor samples has advanced Klason lignin content at the same biomass yield. This sample was one of the relegation samples which were washed with water and not organosolv. The reason for the high lignin content isn’t clear to the authors at this point but assume that it might have happed due to a poor relegation caused by poor mass transfer or the preface of water might have caused rush of lignin due to reduced solubility. Farther disquisition is needed to confirm the reason for the observed anomaly. One of the autoclave samples also appeared to have analogous Klason lignin content as the relegation reactor. The chip size used for this sample was different from the other samples, and the authors assume that it might have caused the anomaly. Since the main focus of this composition is the Redeposition of lignin due to effluent lignin attention, the authors believe that indeed though the mass and heat transfer might have changed due to flyspeck size, the sample is eligible for the comparison.
Indeed though the face of the wood chips treated in the relegation reactor appears to be cleaner than the bones from the autoclave, there was still lignin Redeposition inside the lumen. This observation suggests that the inner wall of the fiber is less accessible during the washing stage and the trapped cuisine liquor in these regions could beget Redeposition during cooling. These observed driblets on the face pre-treated in the relegation reactor, indeed though was important smaller compared to the autoclave samples, suggest that one- stage relegation was shy to fully displace the cuisine liquor. The remaining cuisine liquor or the presence of lignin might have caused Redeposition during the cooling stage when pure water was introduced.
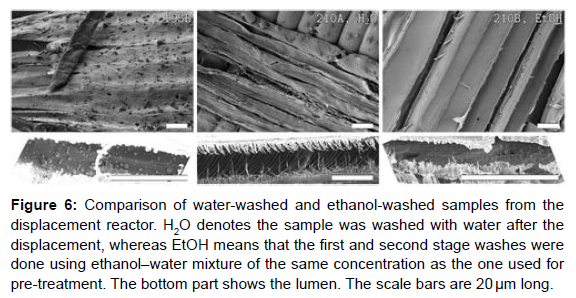
In order to see the effect of relegation, two samples (210B and 210C) were washed with an ethanol – water admixture of the same attention as the pre-treatment liquor. (Figure 6) shows a comparison of the water- washed and the ethanol- washed samples [15]. The ethanolwashed samples appeared to have coming to no Redeposition on the face, and the lumen was cleaner than for the water- washed samples. This shows that an alternate and third stage washing with the same detergent increased the relegation effect and averted utmost of the lignin Redeposition
Figure 6: Comparison of water-washed and ethanol-washed samples from the displacement reactor. H2O denotes the sample was washed with water after the displacement, whereas EtOH means that the first and second stage washes were done using ethanol–water mixture of the same concentration as the one used for pre-treatment. The bottom part shows the lumen. The scale bars are 20 μm long.
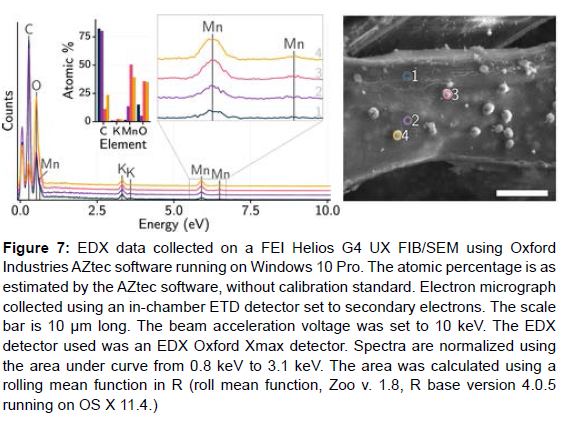
The observed correlation between the drop size and population viscosity and the effluent lignin content suggests that the Redeposited driblets substantially contain lignin. Being literature also suggests that the driblets are lignin redeposit ions. An EDX- SEM analysis was done on the KMnO4- stained samples where the diapasons were taken from different regions on the biomass face (clean face and driblets). The data are shown in (Figure 7). The diapason attained from driblets shows advanced quantum of Mn than the clean face. Since the KMnO4 staining was targeted for lignin, this increase in the quantum of Mn on driblets indicates that the driblets contain substantially lignin.
Figure 7: EDX data collected on a FEI Helios G4 UX FIB/SEM using Oxford Industries AZtec software running on Windows 10 Pro. The atomic percentage is as estimated by the AZtec software, without calibration standard. Electron micrograph collected using an in-chamber ETD detector set to secondary electrons. The scale bar is 10 μm long. The beam acceleration voltage was set to 10 keV. The EDX detector used was an EDX Oxford Xmax detector. Spectra are normalized using the area under curve from 0.8 keV to 3.1 keV. The area was calculated using a rolling mean function in R (roll mean function, Zoo v. 1.8, R base version 4.0.5 running on OS X 11.4.)
Conclusion
The cooling stage of the pre-treatment process causes part of the dissolved lignin to redeposit on to the fiber face in the form of globular globules. The size and population viscosity of these globules depend on the attention of lignin in the liquid phase. The use of a relegation reactor, where the cuisine liquor is displaced with fresh liquor previous to the cooling down stage, was shown to help this Redeposition of lignin to a great extent. The relegation stage was further bettered by employing a two- stage washing process with the fresh cuisine liquor previous to the cooling stage. Redeposition was still observed on regions which are less accessible to the displacing liquid.
References
- Trajano H L, Engle N L, Foston M, Ragauskas A J, Tschaplinski T J et al.( 2013) The fate of lignin during hydrothermal pre-treatment , Biotechnol Biofuels 6:110-115.
- Selig M J, Viamajala S, Decker S R, Tucker M P (2007) Deposition of lignin droplets produced during dilute acid pre-treatment of maize stems retards enzymatic hydrolysis of cellulose. Biotechnol Prog 23:1333-1336.
- Kristensen J B, Thygesen L G, Felby C, Jørgensen H, Elder T (2008) Welcome to biotechnology for biofuels. Biotechnol Biofuels 1: 1-5.
- Pingali S V, Urban V S, Heller W T, McGaughey J, O'Neill H et al. (2010) Breakdown of cell wall nanostructure in dilute acid pre-treated biomass. Biomacromolecules 11: 2329-2331.
- Donohoe B S, Decker S R, Tucker M P, Himmel M E, Vinzant T B (2008) Visualizing lignin coalescence and migration through maize cell walls following thermochemical pre-treatment. Biotechnol Bioeng 101: 913-916.
- Pu Y, Hu F, Huang F, Davison B H, Ragauskas A J (2013) Characterization and genomic analysis of kraft lignin biodegradation by the beta-proteobacterium Cupriavidus basilensis B-8. Biotechnol Biofuels 6: 1-5.
- Kumar R, Hu F, Sannigrahi P, Jung S, Ragauskas A J et al. (2013) Carbohydrate derived-pseudo-lignin can retard cellulose biological conversion. Biotechnol Bioeng 110: 737-739.
- Xu Y, Li K, Zhang M (2007) Simultaneous determination of endogenous and orally administered (15) N-labelled polyamines in rat organs. Anal Biochem 301: 255-256.
- He J, Huang C, Lai C, Huang C, Li X et al. (2018) Elucidation of structure-inhibition relationship of monosaccharide’s derived pseudo-lignin in enzymatic hydrolysis. Ind Crops Prod 113: 368-369.
- Pihlajaniemi V, Sipponen M H, Pastinen O, Nyyssölä A, Laakso S (2016) The effect of direct and counter-current flow-through delignification on enzymatic hydrolysis of wheat straw, and flow limits due to compressibility. Biotechnol Bioeng 113: 2605-2608.
- Kumar R, Wyman C (2010) 3 - Key features of pre-treated lignocelluloses biomass solids and their impact on hydrolysis. Bioalcohol Production, Sawston, England, Ch. 3.
- Sewalt V J, Ni W, Jung H G, Dixon R A (1997) Lignin Impact on Fiber Degradation: Increased Enzymatic Digestibility of Genetically Engineered Tobacco (Nicotiana tabacum) Stems Reduced in Lignin Content. J Agric Food Chem 45: 1977-1999.
- Fengel D, Wegener G (1984) Wood: Chemistry, Ultrastructure, Reactions; Walter 289 de Gruyter, Walter de Gruyter, Berlin, New York.
- Chang H M, Cowling E B, Brown W (1975) Comparative Studies on Cellulolytic Enzyme Lignin and Milled Wood Lignin of Sweet gum and Spruce. Holzforschung 29: 153-155.
- Agnihotri S, Johnsen I A, Bøe M S, Øyaas K, Moe S (2015) Ethanol organosolv pretreatment of softwood (Picea abies) and sugarcane bagasse for biofuel and bio refinery applications. Wood Sci Technol 49: 881-885.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Vegar O (2022) Morphology of Lignin Analysis Pre-Treatment on Fiber Surfaces after Organosolv. Biopolymers Res 6: 124. DOI: 10.4172/bsh.1000124
Copyright: © 2022 Vegar O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1692
- [From(publication date): 0-2022 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1346
- PDF downloads: 346