Research Article Open Access
Morphology and Prevalence of Some Helminth Parasites in Gallus domesticus from Gurez Valley of Jammu and Kashmir, India
Sofi TA, Ahmad F and Sheikh BA*
Department of Zoology, University of Kashmir, Srinagar, India
- *Corresponding Author:
- Sheikh BA
Department of Zoology
University of Kashmir
Srinagar, India, 190 006
Tel: 9797808078
E-mail: bashirzoology@gmail.com
Received Date: December 07, 2015; Accepted Date: December 10, 2015; Published Date: January 05, 2016
Citation: Sofi TA, Ahmad F, Sheikh BA (2016) Morphology and Prevalence of Some Helminth Parasites in Gallus domesticus from Gurez Valley of Jammu and Kashmir, India. J Fisheries Livest Prod 4:159. doi:10.4172/2332-2608.1000159
Copyright: © 2016 Sofi TA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
The present study was aimed to study the distribution, diversity, and prevalence of helminth parasites in domestic fowl from this valley of Gurez. A total of 137 domestic fowl were examined for helminth parasites from May 2013 to April 2015. A high rate of helminth infection (40.14%) was observed. One cestode Raillitina tetragona and two nematodes, Ascaridia galli and Heterakis gallinarum were encountered during the present study. The collected parasites were identified according to the keys and description given by Soulsby (1982) and Yamaguti (1958). High prevalence of infection was observed during summer (41.86%) followed by autumn (34.21%), spring (33.33%) and winter (30.76%). Males (36.96%) were more infected than females (34.37%). The young ones were more infected than adults. Thus, seasonal dynamics and sex of the hosts significantly influenced the prevalence of GIH infection in domestic fowl. Nematodes were more prevalent than cestodes.
Keywords
Gurez; Gallus domesticus; Raillitina tetragona; Ascaridia galli; Heterakis gallinarum; Prevalence
Introduction
Gurez is a valley located in the high Himalayas on banks of river Kishenganga, about 86 km from District Bandipora and 123 km of Srinagar in northern Jammu and Kashmir, India. In northeast of Srinagar, the main valley of Gurez extends between (34° 30ʹ to 34°41ʹ N latitudes) and (74°37ʹ to E 74°46ʹE longitudes) at an average altitude of about 2370 m.a.s.l (about 8,000 feet). It is surrounded on its north by Ladakh, by Bandipora on the south, by Ganderbal on its southeast and on the west by Kupwara with its peripheries touching Line of Control (LoC) that divides the states of India and Pakistan. The valley is nestled among high towering peaks and lofty and glaciated snowcapped mountains which are not just an unvarying landmass but show great differences in elevation aspect, rock type, ruggedness and glacial work which coalesce to make contrasting land surfaces. One has to cross the coldest and dangerous peak Razdan (Razdan pass) located above 4000 m.a.s.l. to reach the valley. The pass not only connects the region with the rest of Kashmir but also divides the two on geographical, socio-cultural and linguistic lines. The valley is drained by mighty Kishenganga (Neelam) River between Kaobal Gali in east and Kanzalwan in west while other aquamarine and crystal streams also run through it.
Birds are important for their commercial, recreational, ethical, spiritual values and form a rich protein source for humans. There is a rich diversity of birds in this area. Among birds, Poultry is of great importance in rural production system in this area. Chicken was the only poultry bird being reared for meat and egg production. Unlike rest of Bandipora district, which has a good population of duck and geese, no non-chicken poultry species was reported from the area. The average number of chicken per household varied from 5-10. During summer months, hens laid as many as 20 eggs/month whereas no egg production was reported during winter months; which may be attributed to the short day length coupled with very little scavenging feed resources. The domestic fowl and eggs provide an important source of protein for human consumption. The increased mortality and decreased productivity in chickens is mainly due to mismanagement, lack of nutritional feeding, diseases and predation.
Among the problems facing extensive types of production of chickens in Gurez are parasitic diseases. There are numerous species of helminthes that cause significant damage to the organs in which they live. Cestodes are more commonly found in warm weather when intermediate hosts are abundant. Helminthiasis was considered to be important problems in chickens [1,2]. Helminth parasites were increminated as major causes of unsoundness and lowering performance of poultry in Egypt [3]. Avian helminthiasis constitutes one of the most common endoparasitism causing serious troubles in chicken production. Chicken cestodiasis not only cause loss of body weight of the raised chickens but also may cause several problems in affected flocks such as enteritis, loss of blood, loss of production, nervous manifestations and death [4]. The prevalence and intensity of helminth infections in birds may be influenced by several factors as distribution of intermediate hosts such as beetles, ants, crustaceans, houseflies etc. and their infection rate and the number of infective parasite eggs or larvae. The free ranging management system and climatic conditions, such as temperature and humidity alter the population dynamics of parasites resulting in dramatic change in prevalence and intensity of helminth infections.
Materials and Methods
During the present study from May 2013 to April 2015, the domestic fowl were purchased from the local people at different study sites. The hosts were then taken alive to the temporary laboratory maintained at Dawar - the capital of Gurez or were brought alive to Department of Zoology, University of Kashmir for parasitic examination. For the collection of endoparsites the body of the hosts was dissected open midventrally and different organs including alimentary canal were removed and kept in separate desired size petridishes where these organs were teased and cut open to search for parasites if any. Therefore, the gastrointestinal tract was subjected to routine examination to collect the gastrointestinal parasites, according to the procedure as described by Fowler [5]. Cestodes were collected by the help of dropper and preserved in 10% formalin or cornoy’s fluid for the identification. Morphology of cestodes was studied by preparing permanent slide according the methods as described by Cable [6]. After washing, nematodes were collected by the help of curved needle and kept in glycerin alcohol. Nematodes were best killed in steaming hot 70% alcohol, and stored in the same solution. Later, a few drops of glycerin were added. Thorough morphological study of nematodes was performed by the preparation of sub-permanent slide by adding one drop of lactophenol. The other steps in this were fixation, staining, dehydration, de-alcoholisation and clearing, mounting and labeling. Parasites were identified according to the keys and description given by Soulsby [7] and Yamaguti [8]. On comparing the recovered parasites were identified as Raillitina tetragona, Ascaridia galli and Heterakis gallinarum.
Light microscopy was conducted under Olympus Research microscope with lens combination of 7X, 10X, and 15X eye pieces and 4X, 10X, 20X, 40X and 100X objectives. The drawings for identification purposes were made to scale with the help of prism type camera- Lucida. The Photographs were taken with the help of Sony Digital SLR Camera Model Number (DSLR – A200). Photomicrography was conducted with DP – 12 Digital Camera attached to Olympus Research Microscope in the department of Zoology.
The prevalence of Helminthiasis was recorded as per formulae described as
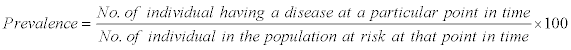
Definitions
The ecological terms used in this study are
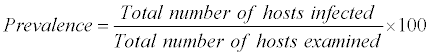
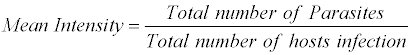
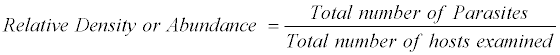
The above nomenclature is followed by that given by Morgolis et al. [9]
Data analysis
The most common measurements of parasite population levels in hosts are prevalence, mean intensity and mean abundance [10]. Prevalence refers to the percentages of organisms infected by a particular species of parasite. Mean intensity is the number of parasites of a given species per infected host. Mean abundance refers to the number of parasites of a given species per host examined, infected and uninfected. The nomenclature used to define ecological parameters is in consistency with that of Margolis et al. [9].
Results
As mentioned above three different helminth parasites belonging to two classes; cestoda and nematode were observed during the present study. The description of these recovered parasites is as follows:
Cestoda
Raillietina tetragona
Generic Diagnosis: Numerous proglottids. Rostellum with hammer shaped hooks, suckers armed with minute deciduous or persistant hooks. Testes numerous. Cirrus pouch small. Genital pores unilateral or bilateral. Ovary bilobed. Vitelline gland compact. Egg capsule with one to several eggs. Parasites of birds and mammals.
Species Diagnosis: Rostellar hooks in circular row. Genital pores unilateral and the egg capsules with several onchospheres. Testes numerous; ovary median.
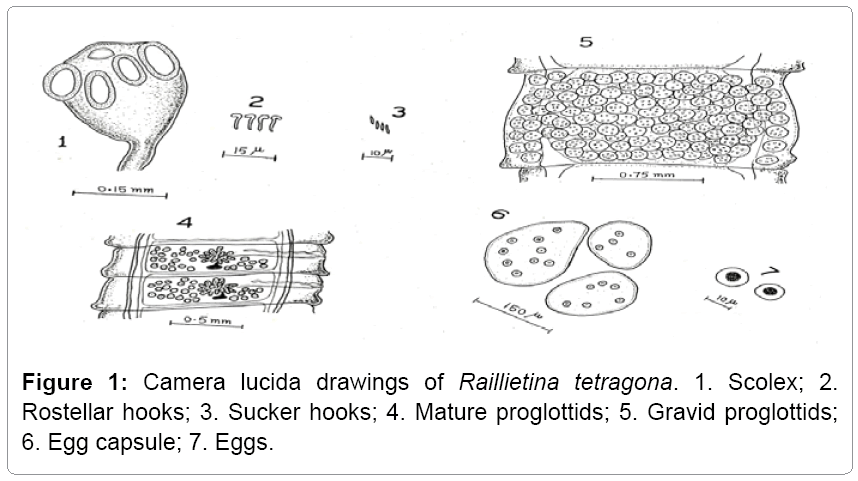
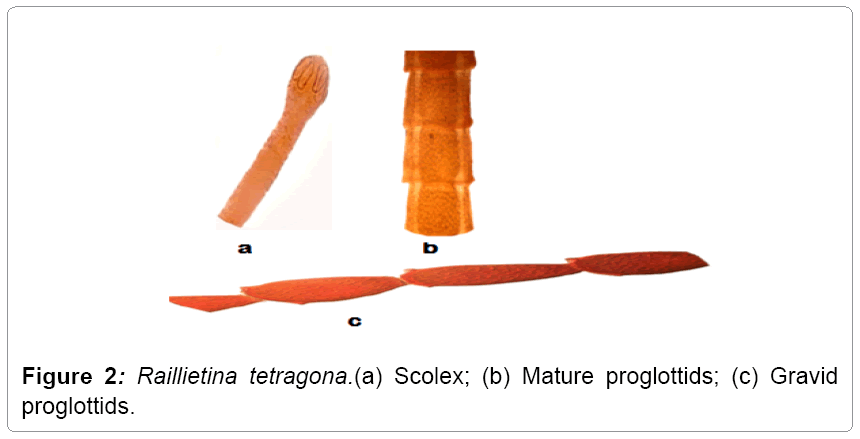
Description: Raillietina tetragona found in small intestines of domestic fowls and in case of heavy infection its location to large intestine also. Its size varies from 6-17 cm. The scolex is smaller that measures 0.17 mm in width. The rosetellum is armed with one or two rows of hooks and suckers, which are oval and armed. Eggs are found in egg capsule, each containing one or more eggs (Figures 1 and 2).
Remarks: While comparing the present form under study with the different species described under the sub genus Raillietina Stiles and it closely relates to Raillietina (R.) tetragona reported from various avian hosts such as Gallus gallus domesticus, Meleagris gullopavo, Lagopus lagopus, L. mutus, Pavo cristatus, P. muticus etc. The present form shows its resemblance to Raillietina (R.) tetragona Molin in number and size of rostellar hooks, numbers of testes, position of genetal pore, etc., (Table 1) besides some minor variations which are of intra specific nature and have no such taxonomic significance. Thus the present form is assigned to species Raillietina (R.) tetragona and forms the first record of its nature from the Gurez valley.
| Particulars | Tanveer, 1989 | Aziz, 1992 | Present study |
|---|---|---|---|
| Strobila size | ------- | 155-240 x 2.85-3.0 | 122-220 x 2.70-2.95 |
| Scolex | 0.234-0.242 x 0.266-0.284 | 0.125-0.168 x 0.16-0.21 | 0.145-0.170 x 0.16-0.17 |
| Rostellum | --------- | 0.33-.36 x 0.24-0.26 | ----------- |
| Rostellar hooks: Number Size |
94-110 5-6 |
90-110 4-7 |
90-100 4-6 |
| No. of testes | 30-36 | 26-38 | 24-32 |
| Egg capsule number | --------- | 70-130 | ---------- |
| Host | Fowl | Fowl | Fowl |
Table 1: Comparative characteristics (measurements in mm) of Raillietina tetragona.
Nematoda
Ascaridia galli [11]
Generic diagnosis: Mouth with three well-developed lips, one dorsal and two sub-ventral, long oesophagus, club shaped without posterior bulb. Lateral alae often present. Male: Caudal alae poorly developed or absent. Spicules almost equal in size. Caudal papillae relatively large. Gubernaculum absent. Female: Valva near middle of the body. Uterine branches divergent, oviparious, eggs thick shelled. Adults as intestinal parasites of birds (Table 2).
| Particulars | Kates and Colglazier, 1970 | Tanveer, 1989 | Aziz Mir, 1992 | Ramadan and Znada, 1992 | Ayesha, 2007 | Present Study |
|---|---|---|---|---|---|---|
| Body length | 60-65 (M); 80-100 (F) | 10-12 (M); 15-17 (F) | 45.3-68.4 (M); 80-109 (F) | 42-76 (M); 72-108 (F) | 22-38 (M); 270 -75.3 (F) | 45-80 (M); 60-90 (F) |
| Max. Width | --------- | 0.4-0.7 (M); 0.7-0.9 (F) | 0.79-1.18 (M); 1.38-1.50 (F) | 0.56-0.91 (M); 0.90-1.80 (F) | 0.41-1.03 (M); 0.6–0.89 (F) | 0.60-1.07 (M); 1.25-1.45 (F) |
| Esophagus length | ----------- | 1.5-2.4 | 3.89-4.34 (F) | 2.48-5.32 (M); 2.88-4.24 (F) | 1.77–3.25 (M); 2.49–3.0 (F) | 3.40-4.2 (M); |
| Esophagus width | ----------- | ------- | 0.45-0.55 (F) | 0.28-0.59 (M); 0.38-0.49 (F) | 0.31–0.41 (M); 0.31–0.41 (F) | 0.30-0.42 (M); 0.35-0.44 (F) |
| Tail length | ----------- | 0.32-0.53 (M); 1.5 (F) | 0.68-0.86 (M); 1.35-1.4 (F) | 0.57-0.78 (M) | 0.51–0.60 (M); 0.91–1.05 (F) | 0.6-0.7 (M); 0.82-1.00 (F) |
| Spicule length | 1.5-2.4 | 1-2.7 and above | 2.20-2.52 | ---------- | 2.88, 2.02, 1.94, 2.13 (M); 2.88, 2.02, 1.94, 2.13 (F) | 2.3-2.55 |
| Egg size | -------- | 0.04-0.08 | 0.056-0.076 x 0.044-0.052 | ----------- | 0.06 x 0.04 | 0.55-0.65 x 0.04-0.47 |
Table 2: Comparative characteristics (measurements in mm) of Ascaridia galli.
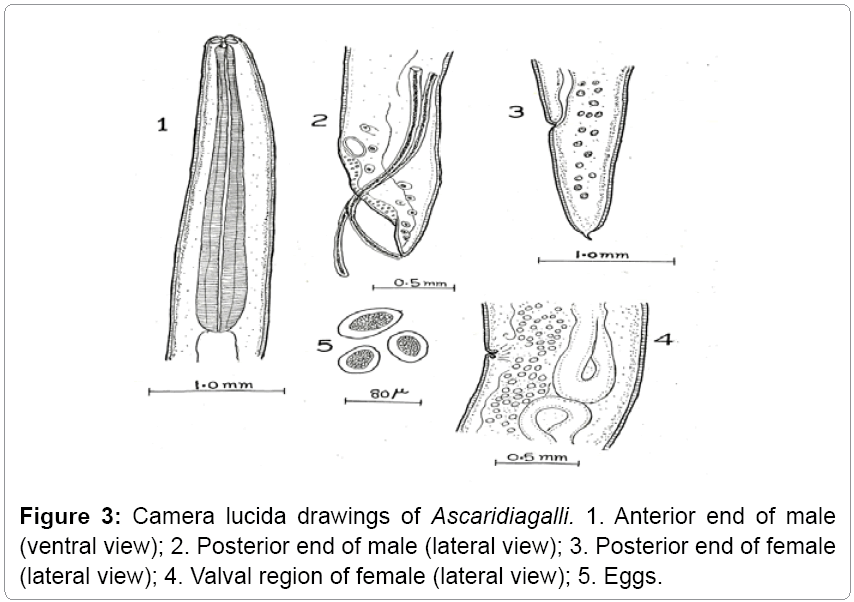
Species diagnosis: First pair of ventral caudal papillae anterior to precloacal sucker; fourth pair of ventral papillae widely separated just posterior to second pair of lateral papillae. Spicules about equal; average length 1.9 mm, with marked membranous structure over about the distal half; distal ends typically blunt with slight indentation, 24-28 μ wide.
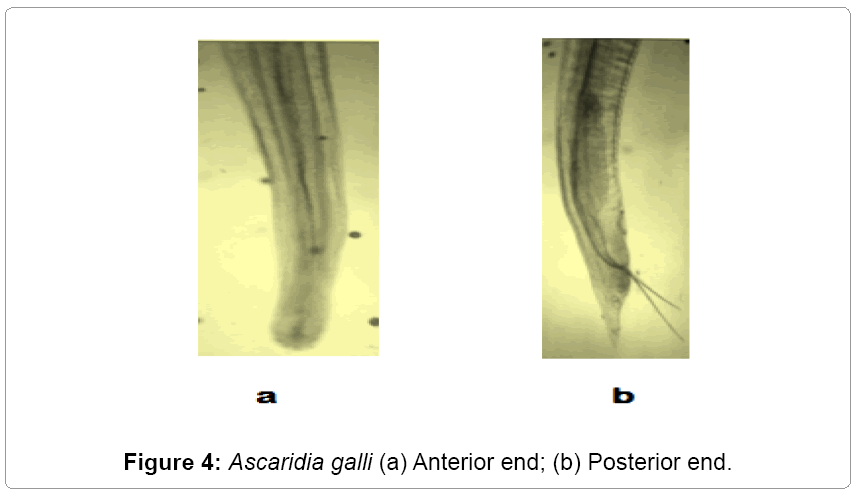
Description: The parasites are elongated, rounded, semitransparent, and creamy white. Mouth is surrounded by three lips. Esophagus has no posterior bulb. It occurs in small intestines of domestic fowl (Figures 3 and 4).
Remarks: The various characteristics of the present form under study like oesophagus, genital suckers, body size, length of tail, length of spicules, egg size etc, are in agreement with that of Ascaridia galli Schrank [11] Freeborn [12]. Hence the present form is assigned to Ascaridia galli Schrank [11] Freeborn [12]. This also makes the first record from the study area (Gurez).
Generic diagnosis: Lips without cordons, body usually with well developed lateral alae. Anterior end usually curved dorsally, oesophagus with a short pharynx and a posterior bulb containing a vulvular apparatus. Male caudal end with a round preanal sucker. Spicules two equal, sub equal or markedly unequal in length. Vulva near middle of the body or anterior to it. Branches of uterus opposite. Eggs with thick shells. Adult worms parasitic in the intestine of birds and mammals.
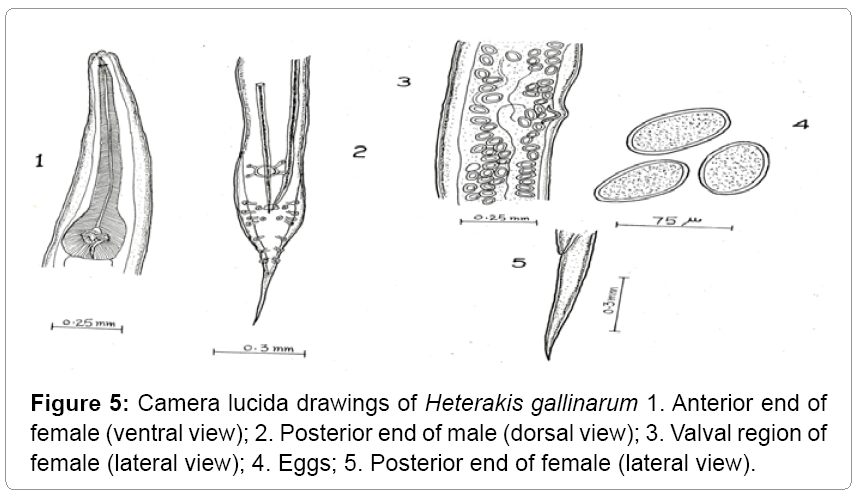
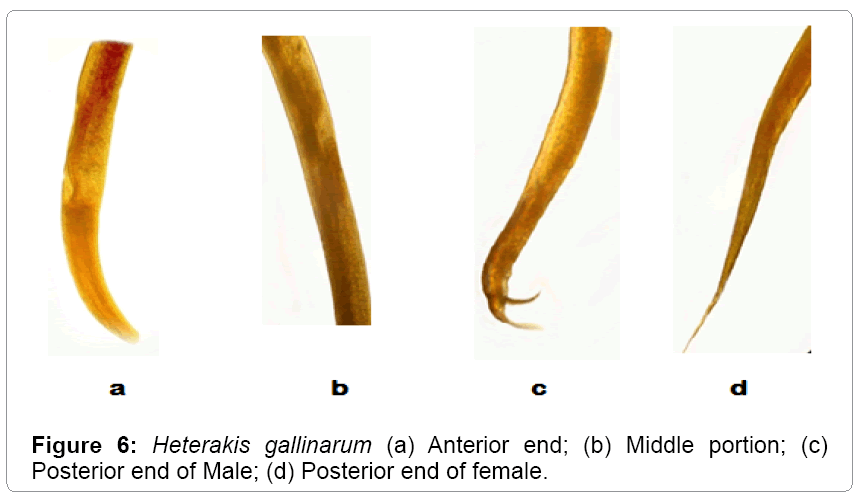
Description: These parasites are commonly called as ceacal worms. They are small, round and white. The anterior extremity is slightly bent (Table 3). The esophagus is provided with posterior bulb. The body length of male ranges from 5-7 mm. The length of esophagus ranges from 0.90-1.05 mm. The spicule length ranges from 1.25-2.05 mm. The tail measures 0.33-0.50 mm. Females are larger than male worms. Their body length measures from 7-10 mm and their maximum width is 0.36 mm. The tail is straight, long; narrow and pointed (Figures 5 and 6).
| Particulars | Gram, 1921 | Qadri, 1982 | Tanveer, 1989 | Aziz Mir, 1992 | Ayesha, 2007 | Present Study |
|---|---|---|---|---|---|---|
| Body length | 8.3-10.9 | 5.25-10.91 | 5-7 (M); 6-8 (F) | 7.65-8.4 (M); 8.6-12.5 (F) | 5.85-8.6 (M); 8.21–10.00 (F) | 5-7 (M); 7-10 (F) |
| Width | 0.32-0.39 | 0.216-0.407 | 0.21-0.32 | 0.26-0.32 (M); 0.32-0.38 (F) | 0.24-0.33 (M); 0.16–0.25 (F) | 0.20-0.36 |
| Oesophagus | 1.06-1.12 | 0.742-1.072 | 0.90-1.10 | 0.89-0.97 x 0.16-0.23 (M); 0.97-1.02 x 0.18-0.23 (F) | 0.94-1.05 x 0.31-0.52 (M) | 0.90-1.05 |
| Spicule length | 2-2.17; 0.7-1.1 | 1.206-20.412-0.566.296; | 1.0-1.3 | 1.75-2.64 (M) | 1.37-1.99 | 1.25-2.05 |
| Tail length | 0.45, 1-1.14 | 0.309-0.481;0.711-0.1.01 | 0.30-0.40 (M); 0.4 (F) | 0.41-0.65 (M); 0.52-0.68 (F) | 0.30-0.33 | 0.33-0.50 |
| Egg size | --------- | ---------- | -------- | 0.069-0.076 x 0.038-0.046 | 0.05-0.06 x 0.03-0.04 | ---------- |
Table 3: Comparative characteristics (measurements in mm) of Heterakis gallinarum.
Remarks
Heterakis gallinarum has been recorded from a variety of gallinaceous birds from Jammu and Kashmir. The present observations are in conformity with those of Tanveer [14], Aziz Mir [15] except for minor deviations with regard to the length of the body and tail. Hence asigned to the species Heterakis gallinarum. Moreover the same makes it as the first record of its nature from Gurez valley.
Helminth parasite dynamism in avian host (Fowl)
Prevalence: A total of 137 specimens of fowl were examined during the present study which revealed 40.14% (55/137) of infection by helminthes in this beautiful valley. Three different types of helminth parasites recovered during the study include two nematodes (Heterakis gallinarum and Ascaridia galli) and one cestode i.e., Raillietina tetragona. Heterakis gallinarum showed a highest prevalence of 35.76% followed by Ascaridia galli (32.11%) and Raillietina tetragona (27.00%) (Tables 4 and 5).
| Host | NE | Un-infected | Infected | Percentage | Trematode | Cestode | Nematode |
|---|---|---|---|---|---|---|---|
| Fowl | 137 | 82 | 55 | 40.14% | ------ | 37 (27.00%) | 49 (35.76%) |
Table 4: Overall prevalence of helminthes collected from fowl.
| Host infected with | No. examined | No. infected | Percentage |
|---|---|---|---|
| Raillietinatetragona | 137 | 37 | 27.00% |
| Ascaridiagalli | 44 | 32.11% | |
| Heterakisgallinarum | 49 | 35.76% | |
| T-Value = 26.91 P-Value = 0.001 | |||
Table 5: Prevalence of helminthes.
Seasonal prevalence: The study showed that the prevalence of parasites in fowl was throughout the year but the prevalence varied from season to season. The highest prevalence was observed during summer followed by autumn and least in winter. During summer 43 fowl were examined, out of which 15 (34.88%), 17 (39.53%) and 18 (41.86%) were found infected with Raillietina tetragona, Ascaridia galli and Heterakis gallinarum respectively. Similarly during autumn out of 38 specimens examined, 9 (23.68%), 12 (31.57%) and 13 (34.21%) were infected with Raillietina tetragona, Ascaridia galli and Heterakis gallinarum respectively. However a lowest prevalence of these helminth parasites was observed during winter. Out of 26 specimens examined 5 (19.23%); 6 (23.07%) and 8 (30.76%) were infected with Raillietina tetragona, Ascaridia galli and Heterakis gallinarum respectively (Table 6). Thus a decreasing order of prevalence was summer > autumn > spring > winter.
| Host | Season | NE | Infected | ||
|---|---|---|---|---|---|
| Raillietina tetragona | Ascaridia galli | Heterakis gallinarum | |||
| Fowl | Spring | 30 | 8 (26.66%) | 9 (30.00%) | 10 (33.33%) |
| Summer | 43 | 15 (34.88%) | 17 (39.53%) | 18 (41.86%) | |
| Autumn | 38 | 9 (23.68%) | 12 (31.57%) | 13 (34.21%) | |
| Winter | 26 | 5 (19.23%) | 6 (23.07%) | 8 (30.76%) | |
| Total | ------- | 137 | 37 (26.11%) | 44 (31.08%) | 49 (35.04%) |
| T-Value = 12.25 P-Value = 0.001 | T-Value = 14.52 P-Value = 0.001 | T-Value = 12.36 P-Value = 0.001 | |||
Table 6: Seasonal prevalence of helminthes.
Age-wise prevalence: Fowl specimens of different age groups were examined. Out of 49 examined fowl specimens of 0-6 months age, 14 (28.57%), 17 (34.69%) and 18 (36.73%) were infected with Raillietina tetragona, Ascaridia galli and Heterakis gallinarum respectively. Similarly out of 54 fowl specimens of 6 months - 2 years age group, 15 (27.77%), 18 (33.33%) and 19 (35.18%) were infected with Raillietina tetragona, Ascaridia galli and Heterakis gallinarum respectively. However out of 34 hosts from 2 years and above age group only 8 (23.52%), 9 (26.47%) and 12 (35.29%) were infected with Raillietina tetragona, Ascaridia galli and Heterakis gallinarum respectively. The results indicate that the hosts have maintained a moderate resistance against cestodes with advancement of age. However there is no significant age resistance shown by the hosts against nematode infection. Thus the hosts of any age group may be exposed to helminth infections with a slight resistance developing during the advancement of age (Table 7).
| Host | Age group | NE | Trematode | Cestode | Nematode | |
|---|---|---|---|---|---|---|
| Raillietina tetragona | Ascaridia galli | Heterakis gallinarum | ||||
| Fowl | <6 month | 49 | ------ | 14 (28.57%) | 17 (34.69%) | 18 (36.73%) |
| 6 months-2 years | 54 | ----- | 15 (27.77%) | 18 (33.33%) | 19 (35.18%) | |
| >2 years | 34 | ------ | 8 (23.52%) | 9 (26.47%) | 12 (35.29%) | |
| Total | 137 | ------- | 37 (26.62%) | 44 (31.49%) | 49 (35.73%) | |
| T-Value = 8.67 P-Value = 0.013 |
T-Value = 9.64 P-Value = 0.011 |
T-Value = 7.63 P-Value = 0.017 |
||||
Table 7: Age-wise prevalence of helminthes.
Sex-wise prevalence: Out of 137 specimens of Gallus domesticus examined during the present study, 73 were males and 64 were females. A prevalence of 28.76% (21/73), 32.87% (24/73) and 36.96% (27/73) in males, and 25% (16/64), 31.25% (20/64) and 34.37% (22/64) in females of Raillietina tetragona, Ascaridia galli and Heterakis gallinarum respectively was observed during the present study. The results show that there is no marked but a slight resistance shown by females as compared to males (Tables 8 and 9).
| Host | Sex | NE | Trematode | Cestode Raillietina tetragona | Nematode | |
|---|---|---|---|---|---|---|
| A. galli | H. gallinarum | |||||
| Fowl | Male | 73 | ----- | 21 (28.76%) | 24 (32.87%) | 27 (36.96%) |
| Female | 64 | ----- | 16 (25.00%) | 20 (31.25%) | 22 (34.37%) | |
| Total | ------ | 137 | ----- | 37 (27.16%) | 44 (32.25%) | 49 (35.76%) |
| T-Value = 47.40 P-Value = 0.013 |
T-Value = 57.50 P-Value = 0.011 |
T-Value = 45.00 P-Value = 0.014 |
||||
Table 8: Sex-wise prevalence of helminthes.
| NE | Parasite | NI | %age | |
| 137 | Raillietina tetragona | 21 | 15.32 | |
| Ascaridia galli | 19 | 13.86 | ||
| Heterakis gallinarum | 26 | 18.97 | ||
| Multiple type | Raillitina + Heterakis | 5 | 3.64 | |
| Raillitina + Ascaridia | 7 | 5.10 | ||
| Ascaridia + Heterakis | 14 | 10.21 | ||
| Raillitina+Heterakis+Ascaridia | 4 | 2.91 |
Table 9: Single and multiple species infection in domestic fowl.
Discussion
After extensive study of the bird host, Gallus domesticus for helminth parasitism in Gurez valley during the present study, different species of helminth parasites were recovered with a moderately high prevalence (40.14%). These results when compared with those of other researchers around the globe indicate that this small, however isolated, valley does not figure out of the helminth infestation; but is an endemic area for helminth parasites in fowl. Permin and Nansen [16] after studying Danish organic poultry farming reported increased infestation of internal parasites including Heterakis gallinarum. The author further stated that species of nematodes like Heterakis and Ascaridia are widely distributed, causing non-specific clinical signs of infection, such as loss of appetite and growth, a general in condition and on occasions death. Permin et al. [17] in their survey in Denmark concluded that there was a high risk of helminth infection in free range/ organic poultry systems and that prevalence may also be high in deep litter systems. Permin et al. [18] reported that prevalence of GIH are high whether in tropical or temperate climate. Oyeka [19] found 54.5% of chickens to be infected with helminth parasites in Anambra state in Nigeria. Yadav and Tandon [20] revealed 90.9% of helminth infections in subtropical high rainfall area of India. Mpoame and Agbede [21] found 93.55% of domestic fowl infected with gastrointestinal helminthes. Eshetu et al. [22] found 91.01% chickens infected with gastrointestinal helminthes from Amhara region Ethiopia. Nokana et al. [23] during their survey of helminth parasites in backyard flocks in Michigan by litter examination also showed relatively high contamination rates. Edger [24] reported the presence of a wide range of helminthes in chickens including A. galli. Wilson et al. [25] revealed the prevalence of A. galli was in the range of 40% on commercial farms in the state of Arkansas. Long [26] reported that majority of broiler chickens were infected with A. galli at the age of 23-29 days. Konanenko and Khaizade [27] while working on helminth fauna of Charadriiformes and Anseriformes observed a prevalence of 50%, 45%, 13.3% and 16% of cestodes, nematodes, trematodes and acanthocephalans respectively. Luka and Ndams [28] reported that 62% of domestic fowl were infected with helminth parasits in Samaru, Zaria Nigeria. Phiri et al. [29] reported 95.2% free range chickens infected with GIH in central Zambia. Wahid reported a prevalence of 30.75%, 31.25% of Raillitina spp. and Heterakis spp. in domestic ducks, and a prevalence of 50% of Ascaridia galli in goose from Srinagar Kashmir. Ayesha reported a prevalence of 51.42%, 31.42% and 30.71% of Raillitina tetragona, Heterakis gallinarum and Ascaridia galli in domestic fowl from Doda district of Jammu and Kashmir.
The remarkable prevalence of infection observed in domestic fowl from Gurez valley can be attributed to a number of factors like the type of management and production system, exposure to intermediate hosts, inadequate or no use of anthelmintics, the climatic conditions which alter the population dynamics of the parasite. As the environment and climatic conditions of the study area (Gurez valley) seem to be unfavourable for such a high intensity of infection that could have as such resulted in a lowest level of infection, a major ecological factor like dispersal and emigration plays a significant role in maintaining such a high prevalence of helminth infection in this beautiful valley of Gurez. During severe winters a small fraction of human population along with their poultry birds migrate from this valley to Kashmir valley until the onset of spring. Thus a chance of transmission of infection (even if minimum) might have occurred which shows a good proliferation while the birds are back in their native land. During pleasant summers, the shepherds, Bakerwals along with their poultry birds, visit the high altitude pastures of Gurez for grazing their sheep and goats where the possible chances of transmission of helminth infection do occur. As the summers are very favourable for helminth proliferation, a high prevalence of infection was observed during summers.
In the present study, a good number of domestic fowl were harbouring more than one type of helminth species which is in agreement with the work of many other researchers found in following references [16,17,21,22,24-35].
The most prevalent helminth parasite recorded during the present study from Gurez was Heterakis spp. (35.76%) followed by Ascaridia galli (32.11%) and Raillitina spp. (27.00%). The present study is in agreement with the work of many others like; Qureshi (1950) reported a high prevalence of Ascaridia galli (31.02%) and Raillitina tetragona (18.7%) in Desi adult fowls in U.P India. Wilson et al. [25], Permin et al. [16] in deep litter and backyard system, Eshetu et al. [22] from Amhara region also reported similar prevalence in their studies. However Mpoame and Agbede [19], Schou et al. [13], Luka and Ndams [28] reported a higher prevalence of Ascaridia galli. Since the present results are in agreement with those of many others, still the variations can be attributed to the environmental conditions in the area and inadequate availability of intermediate hosts. The environmental conditions like temperature and moisture do favour the larval development and facilitate transmission and ingestion of infested droppings.
The present studies indicate that with the advancement of age there was a decrease in the prevalence of infection which makes it in agreement to the reports made by the researchers in the references [7,21,29,32,36-38]. It is evident that it can be attributed to the increased immune status in adults than in young ones against the helminth parasites. To accommodate with the environmental conditions and thus traditionally the chicks do hatch out during late spring and as such they do enjoy the summer and autumn in their young age when these get exposed to helminth infections. These could be the possible reasons for decrease in prevalence of helminth infection with increase in age.
As is evident from the observations made during the present study that males do show a slightly higher rate of infection than females, which can be related with the physiological influence of hormones on the susceptibility of host animals to infection [32]. Similar trend was observed by Fakae and paul-Abiade [33] in fowl where male fowls carried significantly (p<0.05) more parasite burden than females. Magwish et al. [32] also observed that prevalence of Heterakis gallinarum was higher in males than females. Similar results were observed by Ayesha in domestic fowl from Doda district of Jammu and Kashmir.
Although helminth parasites were prevalent throughout the year, but the observations made in the present study show higher prevalence of infection during the warm summer months followed by autumn, spring and winter respectively. As the study area is far-off from University of Kashmir and along the Line of control, monthly investigations were not possible. Thus, seasonal investigations were made to study the helminth infestation throughout the year. The higher prevalence observed during summer and lower during cold winters can be due to the impact of many factors like geographical location of the area, environmental conditions prevailing in the area. Low temperature inhibits the development and survival of infective larval stages and as such decreases the access to intermediate hosts or final hosts. On the other hand, the enough availability of intermediate hosts and favourable temperature for larval development favours the chances of helminth proliferation in summers. It can be suggested that the seasonal fluctuations in the abundance of infective stages in the environment may also play a contributing role in the differences observed. These findings are in accordance to the reports made by many workers from other parts of the world. Threlfall [37] examined herring gulls in north-wales and found seasonal diversity of helminth parasites and attributed the differences to changes in the diet of the gulls over the course of the year caused by an altered availability of the intermediate hosts. Busher [10] and Mclaughlin and Burt [39], reported that the density and the magnitude of helminth infection increases to peak in late summer. Wallace and Pence [40] stated that there is no recruitment of replacement species during winters due to changes in diet and limited availability of infective stages in intermediate hosts. Fedynich and Pence [41] reported that mallards had higher mean abundance of helminth in summer than in winter. Mpoame and Agbede [21] reported that the parasitic prevalence and the worm burdens were generally higher during April to October. Magwisha et al. [32] observed that helminth infection varied in the months of rainy season.
The present study reveals that single type infections were more prevalent than multiple type infections. Multiple type infections with helminthes in domestic fowl was also observed by researchers in the references [18,20,21,29,32]. In this study, majority of the host birds harboured multiple type of infection of helminthes which suggest that the prevailing environmental conditions and free range management systems are favourable to many species of helminth parasites [42-44].
Acknowledgement
The authors are greatly thankful to the Department of Zoology, University of Kashmir for the facilities they provided. BAS is also thankful to those people of Gurez valley who make them host available for research purpose.
References
- Jansen J, Pandey VS (1989) Observations on Helminth Parasites of Domestic Fowls in Zimbabwe. Zimbabwe Veterinary Journal 20: 15-17.
- Abebe W, Asfaw T, Genete B, KassaB,DrochiesP (1997) Comparative Studies Of External Parasites and Gastrointestinal Helminthes of Chickens Kept Under Different Management System in and Around Addis Ababa. Revue de Medicine veterinaire148: 497-500.
- Khater HF (1993) Studies on enteric helminthparasitites in domestic birds. MVSc Thesis Fac Vet Med BanhaZagazig University.
- Calneck BW, Barnes HJ, Beard CW, McDougald LR, Saif YM (1997) Diseases of Poultry (10th edn.). Editorial Board for the American Association of Avian Pathologists. Mosby – Wolfe.
- Fowler NG (1990) How to carry out a field Investigation In: Poultry Diseases, FTW (2ndedn.),BailliereTindall, Londan: 370-400.
- Cable RM (1957) An Illustrated Laboratory Manual of Parasitology, (4thedn.) Burgess Publishing Co, 426, South Sixth Street, Minneapolis 15, Minnesota.
- SoulsbyEJL (1982) Helminthes, Arthropods and Protozoa of domesticated animals. Bailliere Tindall, London.
- Yamaguti S (1958) SystemaHilminthum. Vol. I, II, III and V, Intersience Publishers, Inc. New York, USA.
- Margolis L, Esch W, Holmes JC, Kuris AM,Schad GA (1982) The use of ecological terms in parasitology (Report of an Ad Hoc Committee of the American Society of Parasitologists). J Parasitol 68: 131-133.
- Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis etal.revisited. J Parasitol83: 575-583
- Kates KC, Colglazier ML (1970) Differential Morphology of Adult Ascaridiagalli(Schrank 1788) and Ascaridiadissimilis(Perez Vigueras 1931). ProcHelmintholSoc Washington37: 80-84.
- Freeborn SB (1923) Nicotine as a poultry vermifuge. Science 57: 692-693.
- Schou TW, PerminA, Madsen JHR, Sorexsen P, Labouriau R, et al. (2006) Gastrointestinal helminthes in indigenous and exotic chickens in Vietnam: association of the intensity of infection with the major histocompatibility complex. Parasitology 21: 1-13.
- Tanveer S (1989) Parasitism in Poultry birds of Kashmir. M. Phil. Thesis, University of Kashmir.
- Aziz M (1992) Studies on the Parasitoid complex of Galliform, Anseriform and Podicipediform birds of Kashmir.Thesis, University of Kashmir.
- PerminA, Nansen P (1996) Parasitological Problems in organic poultry production.Beretning Fra StatensHusdyrbrugsforsog 729: 91-96.
- PerminA, Bisgaard M, FrandsenF, Pearman, Kold J, et al. (1999) Prevalence of gastrointestinal helminthes in different poultry production system. Br PoultSci 40: 439-443.
- PerminA, Bojesen M, Nansen P, Bisgaard M, Frandsen F, et al. (1997) Ascaridiagalli populations in chickens following single infections with different dose levels. Parasitol Res83: 614-617.
- Oyeka CA (1989) Prevalence of intestinal helminthes in poultry farms in Anambra State Nigeria. Bulletin of Animal Health and Production in Africa 37: 217-220.
- Yadav AK, TandonV (1989) Helminth Parasitism of Domestic fowl (Gallus domesticus L) in a subtropical high rainfall area of India. Br Vet J 145: 57-61.
- Mpoame M, Agbede G (1995) The Gastrointestinal helminth infection of domestic fowl in Dschang, Western Cameroon. Rev Elev Med Vet Pays Trop48: 147-151.
- EshetuY, Mulualem E, Ibrahim H, Berhanu A, Aberra K (2001) Study of Gastrointestinal helminthes of scavenging chickens in four rural districts of Amhara region Ethiopia. Rev Sci Tech off IntEpiz 20: 791-796.
- Nonaka N, Donoghue AR, Manzoni AM, Van VTWS (1991) A survey of helminth parasites in backyard flocks in Michigan by litter examination. Avian Diseases 35: 554-558.
- Edgar SA (1953) A Preliminary checklist of Parasites of Some Domestic fowls of Alabama. Poultry Science 32: 949-952.
- Wilson YI, Yazwinski TA, Tucker CA, Johnson ZB (1994) A survey into the prevalence of poultry helminthes in northwest Arkanasas commercial broiler chickens. Avian Dis 38: 158-60.
- Long PL (1977) Ascaridiagalli in broiler chickens.TheVeteranary Record 100: 342
- Kononenko AF, Khaizade N (1983) The helminth fauna of some birds, aquatic and living near water, on the south-eastern shore of the Caspian Sea. BiologicheskieresursyKaspiiskogoMorya: 186-199
- Luka SA, Ndams IS (2007) Gastrointestinal Parasites of domestic chickens Gallus gallusdomesticus Linnaeus 1758 in Samaru Zaria Nigeria. Science World Journal 2: 27-29.
- Phiri IK, Phiri AM, Ziela M, Chota A, Masuku M, et al. (2007) Prevalence and distribution of gastrointestinal helminthes and their effects on weight gain in free range chickens in Central Zambia. Tropical Animal Health and Production 39: 309-315.
- Qureshi SH (1950) Incidence of helminthic infection in fowls in the Uttar Pradesh (UP). Indian Journal of Helminthlogy2: 57-62.
- Islam MR, Shaikh H, Baki MA (1988) Prevalence and Pathology of helminth Parasites in domestic duck of Bangladesh. Vet Parasitol 29: 73-77.
- Magwisha HB, Kassuka AA, Kyvsgaard NC, Permin A ( 2002) A comparison of the prevalence and burden of helminth infections in growers and adults free range chickens. Tropical Animal health and Production 34: 205-214.
- Fakae, Paul A (2003) Rainy season period prevalence of helminthes in the domestic fowl (Gallus domesticus) in Nsukka Eastern Nigeria. Nigerian Veterinary Journal 24: 21-27.
- Hassouni T, Beighyti D (2006) Distribution of Gastrointestinal helminthes in chickens farms in the Gharb region Morocco. Parasitology Research 99: 181-183.
- Negesse T (1991) Survey of Intestinal Parasites of local chickens of Southern Ethiopia. Indian Journal of Poultry Science 26: 128-129.
- Sanders JE, Schwartz RD (1994) Evaluation of three water susceptible formulation of fenbendazole against Ascaridiagalli infection in broiler chickens. Avian Diseases 38: 350-353.
- Threlfall W (1967) Studies on the helminth parasites of the herring gull, LarusargentatusPontopp in northern Caernarvonshire and Anglesey. Parasitology57: 431-453.
- Buscher HN (1965) Dynamics of the Intestinal Helminth Fauna in Three Species of Ducks. JWildlManage29: 772-781.
- MclaughlinJD ,Burt MDB (1979) Studies on the hymenolepidcestodes of water fowl from New Brunswick, Canada. Can J Zool 57: 34-79.
- Wallace BM, Pence DB (1986) Population dynamics of the helminth community from migrating blue-winged teal: loss of helminths without replacement on the wintering grounds. Canadian Journal of Zoology64: 1765-1773.
- FedynichAM, Pence DB (1994) Helminth Community Structure and Pattern in a Migratory Host (Anasplatyrhynchos). Canadian Journal of Zoology 72: 496-505.
- FAO (2000) Food and Agricultural Organization of the United Nations Statistical data bases. FAO Rome.
- Ramadan HH, Znada NYA (1992) Morphology and life history of Ascaridiagalli in the domestic fowl that are raised in Jeddah4: 87 -99.
- WATT (1996) Poultry Statistical Yearbook. Poultry International 35:8.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 22718
- [From(publication date):
March-2016 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 21000
- PDF downloads : 1718