Monocytes Contribute to High Renal Allograft Rejection Rates when Alemtuzumab is Utilized as an Induction Agent: A Retrospective Study
Received: 09-Sep-2018 / Accepted Date: 23-Sep-2018 / Published Date: 01-Oct-2018 DOI: 10.4172/2475-7640.1000122
Keywords: Alemtuzumab; Monocytes; Histology; Rejection; Kidney
Introduction
Acute cellular and antibody-mediated rejections are important obstacles to long-term success in renal transplantation [1-3]. Alemtuzumab is a human monoclonal antibody classically employed in the treatment of various hematological cancers. Recently, alemtuzumab has been employed as an induction agent prior to transplantation with the advantage of a potential steroid-free regimen [4]. Alemtuzumab is an immune cell CD52-targeting monoclonal antibody that mediates lysis through several mechanisms such as cell-mediated cytolysis, complement-dependent cytolysis, and induction of apoptosis. This lymphocyte depletion effect may lead to reduced overall rates of acute cellular rejection and Antibody Mediated Rejection (AMR) [5-7].
Despite advances in immunosuppression protocols in transplant, certain groups still remain high risk for rejection. Demographic characteristics such as being under the age of 65, black ethnicity, presensitization (elevated Panel Reactive Antibody [PRA]), and Delayed Graft Function (DGF) pose higher risk of rejection [8,9]. Although alemtuzumab may aid in ameliorating disparities in transplant outcomes in high risk patients, rejection remains a primary concern [10]. Many institutions continue to report acute rejection rates in less than 15% of transplants in non-sensitized patients [7,11-19]. Even in patients who recover from acute rejection, however, there is still a negative impact on long-term graft survival.
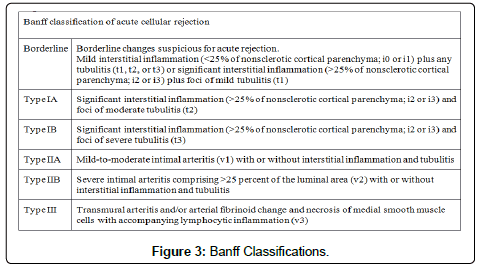
The mechanism of rejection is initiated when alloantigens, such as MHC, activate the immune system against the allograft. A defining feature of acute renal allograft rejection is tubulitis, characterized by the presence of leukocytes and inflammatory cells in the tubular wall. Thus, biopsy is the standard in determining rejection, and the Banff classification model is useful in allowing pathologists to grade the severity of rejection into mild, moderate, and severe by assessing the intensity of the infiltrate, arteritis, and tubulitis. While CD8+ T cells are classically the predominating cell type on biopsy, B cells, monocytes, NK cells, neutrophils, and eosinophils can dominate as well. There is an increasing body of evidence that the composition of cells may play an equal, if not greater, role in determining graft outcomes than the intensity of infiltrate [16,20,21]. The monocyte lineage is increasingly recognized as an indicator for worse outcomes and higher Banff scores. Monocytes are known to be less sensitive for the depleting effects of alemtuzumab than lymphocytes, possibly due to their heightened ability to evade complement-mediated cell lysis and their decreased expression of CD52 antigen [22]. However, the relationship between rejection and cellular infiltrate on histology is sparsely reported. We hypothesize that alemtuzumab is associated with more monocyte involvement in rejection within higher risk populations. Within the context of our research, we will be use the term monocyte to represent monocyte/macrophage as they share the identification marker of CD62.
Methods
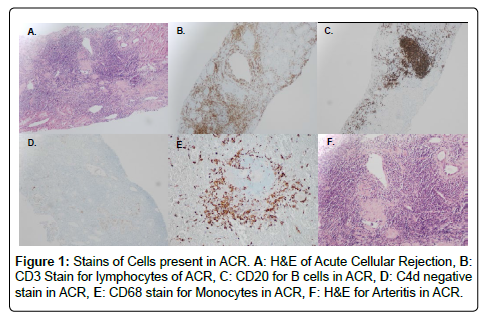
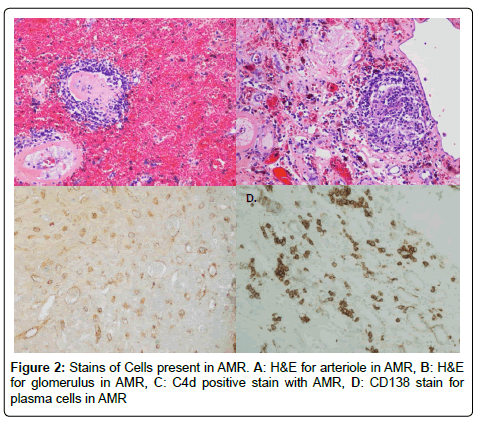
We performed an IRB approved single center retrospective analysis of 181 biopsies from recipients diagnosed with acute rejection between March 2006 and May 2015. All patients were induced with alemtuzumab. Screenings, transplants, and follow-up were performed at The University of Toledo Medical Center. Immunosuppression included: Alemtuzumab-30 mg IV, or 0.5 mg/kg if less than 60 kg, Methylprednisolone-500 mg IV, Mycophenolate sodium-720 by mouth (PO). The majority of recipients were steroid free. All subjects were negative for T and B cell cross match. All cases of rejection were biopsy proven; and were obtained percutaneously using sonographic guidance. Histological preparation of biopsies involved paraffin embedding, cutting, and staining. Specific stains included CD68 (marker for macrophages), CD138 (marker for plasma cells), CD3 (marker for lymphocytes), CD20 (marker for B cells), and C4d (for type of rejection) by immunohistochemistry with satisfactory positive and negative controls. Hematoxylin-eosin staining (H&E) was used for neutrophils and eosinophils. Particular cell type dominance was identified in multiple high-power fields, per the assessment of an experienced pathologist. Biopsies were described as having no cells if there were no principal leukocyte population present. Figures 1 and 2 demonstrate images of stain biopsies for each corresponding stain described above. Each is a representative sample image used for cellular identification and quantification. Severity of rejection was determined using Banff 2013 classification consistent with the most recent guidelines (Figure 3) [23].
Patient demographics were divided based on met criteria. Elderly patients were defined as age of 65 years or greater. High PRA was defined as panel reactive antibody 20% or greater upon time of testing. Patients suffering from Delayed Graft Function (DGF) required dialysis within 7 days post-transplant. Other recipient characteristics included ethnicity, and retransplant status. Those suffering from chronic rejection, determined by presentation, biopsy phenotype, medications, or outcome, were excluded. For analysis, recipients were not isolated to a single focus group. For example, if a patient was found to have both a high PRA and be of elderly age, this patient was examined in both the elderly and PRA categories independently. Demographic characteristics, together with the histological profiles of the biopsy itself, were assessed in concordance with clinical outcomes.
Statistical analyses were performed using SPSS 21.0 (Armonk, IBM Corp). Data were assessed using two-sided Student’s t-test or Mann– Whitney U test for continuous variables and Pearson’s Chi-squared Test or Fisher’s Exact Test for categorical variables. Survival was calculated using Kaplan-Meier analysis.
Results
A total of 661 patients underwent renal transplantation between March 2006 and May 2015. Baseline characteristics for recipients and donor kidney types are outlined in Table 1. A total of 181 patients suffered rejection (27%), with a median time 82 ± 516.3 days. Rejection diagnoses included 124 (68.5%) ACR, 24 (13.3%), AMR, and 33 (18.2%) combined ACR/AMR. Banff scores included 24 (13.4%) grade Ia/Ib, 141 (78.8%) grade IIa/IIb, and 14 (7.8%) grade III (Table 2).
| Median age (S.D.) | 52.3 years (13.8) |
| Median BMI | 28.1 (5.0) |
| Diabetes Mellitus | 253 (38.4%) |
| Sex (male) | 419 (63.5%) |
| Ethnicity | |
| White | 470 (71.2%) |
| Black | 148 (22.4%) |
| Hispanic | 29 (4.4%) |
| Asian | 13 (2%) |
| Donor Status | |
| Deceased | 490 (74.2%) |
| Extended Criteria | 55 (8.3%) |
| Deceased after Cardiac Death | 48 (7.2%) |
| Related | 83 (12.6%) |
| Living | 138 (20.9%) |
Table 1: Study demographics.
| Factor | Rejected (% of total) | Non-rejected population | Significance |
|---|---|---|---|
| Elderly (>65 yoa) | 31 (17.1%) | 107 (22.3%) | 0.163 |
| Black race | 45 (24.9%) | 103 (21.5%) | 0.349 |
| Retransplant | 63 (34.8%) | 115 (24.0%) | .006* |
| DGF | 31 (17.5%) | 32 (6.8%) | <0.001* |
| PRA>20% | 41 (22.9%) | 78 (16.5%) | 0.069 |
| Rejection Type | Incidence (% of total) | Banff Score | Incidence (% of total) |
| -ACR | 124 (68.5%) | -Ia/Ib | 24 (13.4%) |
| -AMR | 24 (13.3%) | -IIa/IIb | 141 (78.8%) |
| -ACR/AMR | 33 (18.2%) | -III | 14 (7.8%) |
Table 2: Rejection populations of interest.
Recipients diagnosed with rejection were stratified according to certain high risk characteristics (Table 2). No statistical significant association with rejection was found for elderly (22.5% vs. 28.7% for control, p=0.163), black race (30.4% vs. 26.60%, p=0.349), deceased donation (27.8% vs. 26.5%, p=0.766), ECD (27.3% vs. 27.7%, p=0.942) or high PRA status (34.5% vs. 25.9%, p=0.069). Significant findings included DGF (49.2% vs. 24.8%, p<0.001) and retransplantation (35.4% vs. 24.5%, p=0.006).
Biopsy results were analyzed as they pertained to the demographics of the recipients previously discussed. Predominant inflammatory cell type on biopsy included 11 (6.1%) eosinophils, 19 (10.5%) neutrophils alone, 21 (11.6%) neutrophils and monocytes, 23 (12.7%) plasma cells, 52 (28.7%) monocytes alone, and 55 (30.4%) lymphocytes (Table 3). Monocytes alone, along with neutrophils in combination with monocytes, as the principal cell type were more prevalent over lymphocytes for our patient population.
| Risk Factor | Neutrophils | Eosinophils | Plasma Cells | Monocytes | Neutrophils and Monocytes | Lymphocytes |
|---|---|---|---|---|---|---|
| Elderly | 9 (29%)1 | 2 (6.5%) | 1 (3.2%) | 9 (29%) | 4 (12.9%) | 6 (19.4%) |
| DGF | 5 (16.1%) | 1 (3.2%) | 2 (6.5%) | 11 (35.5%) | 4 (12.9%) | 8 (25.8%) |
| Retransplant | 6 (9.5%) | 7 (11.1%)2 | 8 (12.7%) | 18 (28.6%) | 6 (9.5%) | 18 (28.6%) |
| Black Race | 4 (8.9%)3 | 1 (2.2%) | 6 (13.3%) | 9 (20%) | 5 (11.1%) | 20 (44%) |
| PRA (>20) | 3 (7.3%) | 1 (2.4%) | 4 (9.8%) | 13 (31.7%) | 5 (12.2%) | 15 (36.6%) |
| Banff Ia/Ib | 1 (5.3%) | 1 (9.1%) | 2 (8.7%) | 9 (17.3%) | 3 (14.3%) | 8 (15.1%) |
| Banff IIa/IIb | 16 (84.2%) | 8 (72.7%) | 21 (91.3%) | 42 (80.8%) | 16 (76.2%) | 38 (71.7%) |
| Banff III | 2 (10.5%) | 2 (18.2%) | 0 | 1 (1.9%) | 2 (9.5%) | 7 (13.2%) |
| AMR | 1 (5.3%) | 3 (27.3%) | 6 (26.1%) | 5 (9.6%) | 3 (14.3%) | 6 (10.9%) |
| ACR | 13 (68.4%) | 4 (36.4%) | 15 (65.2%) | 37 (71.2%) | 14 (66.7%) | 41 (74.5%) |
| ACR/AMR | 5 (26.3%) | 4 (36.4%) | 2 (8.7%) | 10 (19.2%) | 4 (19%) | 8 (14.5%) |
| Total (pop) | 19 | 11 | 23 | 52 | 21 | 55 |
Due to double-counting patients between groups, numbers are not summative. Significant differences (p<0.05) of note: 1control=6.7%, 2control=3.4%, 3control=25.7%
Table 3: Histological presentation and diagnostic features.
Banff scores were also assessed in comparison to cell types. Lymphocytes were found to have the highest frequency of Banff III (n=7, 50%). Neutrophils (n=16, 11.3%), eosinophils (n=8, 5.7%), plasma cells (n=21, 14.9%), monocytes (n=42, 30.0%), and neutrophils with monocytes (n=16, 11.3%) were all present in higher frequency on Banff II biopsies. We report 24 cases of Banff I rejection; cell predominance was as follows: neutrophils=1, eosinophils=1, Plasma cells=2, monocytes=9, neutrophils with monocytes=3 and lymphocytes=8.
In terms of overall death-censored graft loss, all 181 cases of rejection were analyzed according to cell type. The results of predominant cell type for each case of rejection with graft loss included: 3 (5.2% of total) eosinophils, 3 (5.2%) neutrophils and monocytes, 7 (12.0%) neutrophils alone, 8 (13.8%) plasma cells, 17 (29.3%) monocytes, and 22 (34.4%) lymphocytes. Monocytes predominated in terms of frequency in patients with a high Banff classification and were second only to lymphocytes in terms of its association with death-censored graft loss (Table 4).
| Cell Type | Death-Censored Graft Loss |
|---|---|
| Neutrophils | 7 (12.1%) |
| Eosinophils | 3 (5.2%) |
| Plasma Cells | 8 (13.8%) |
| Monocytes | 17 (29.3%) |
| Neutrophils+Monocytes | 3 (5.2%) |
| Lymphocytes | 20 (34.5%) |
Percent shown is of total death-censored graft loss.
Table 4: Death-censored graft survival by cell type.
In addition, cell types present on biopsy were compared in each high risk focus group. Significant histological findings, at p<0.05, include neutrophils predominating for elderly (9, 29% for elderly vs. 10, 6.7% in control), retransplant showing eosinophils (7, 11.1% vs. 4, 3.4% in control), and lymphocytes being present in black race patients (20, 44.4% vs. 35, 25.7% in control). All other cell type/groups comparisons were not significant. Common, yet non-significant, cells seen in each group include monocytes for the elderly (9, 29%), monocytes for DGF patients (11, 35.5%), monocytes and lymphocytes in retransplantation patients (18, 28.6% each), monocytes and lymphocytes for black race patients (9, 20% and 20, 44.4%, respectively) and monocytes and lymphocytes for high PRA patients (13, 31.7% and 15, 36.6%, respectively) (Table 3).
Discussion
Alemtuzumab has a successful history of reducing acute rejection post transplantation [5, 24-26]. Despite this success, we uncovered a high rate of rejection with a reported frequency of 27%, greater than other institutions utilizing alemtuzumab. In the face of these findings, our institution is experimenting with long-term steroid maintenance in individuals determined to be at greater risk for rejection, as early steroid withdrawal is suspected to play a larger role in certain high risk populations. Our data, however, identifies monocytes as a likely contributor to rejection in general as their presence was highly correlated with rejection rates and graft failure for each high risk patient group examined, as well as higher Banff classification discussed later. Thus, we suggest that use of alemtuzumab contributes to a shift in circulating immune cells that may alter outcomes in those who experience acute rejection.
Monocytes, peripheral blood macrophage precursors, are members of the innate immune system that play several roles in the promotion of inflammation and in tissue healing [23]. Monocytes have been implicated in allograft rejection in a number of ways. Schinstock et al. [27] revealed that transplant glomerulopathy develops in the wake of chronic AMR and is likely driven by complement-independent pathways that include NK cells and monocytes. Recent discoveries relating to the biological function of monocytes have identified novel roles for these cells in the establishment and regulation of inflammation within the context of transplantation. However, the role of monocytes contains complexities that require additional research to delineate their precise contribution to the development of inflammation responsible for inhibition of allograft function [20]. Our identification of monocyte residence within the majority of rejection allografts supports the hypothesis that monocytes are indeed correlated with rejection. Further research is imperative to determine what role, and by what mechanism, monocytes play within the context of promoting allograft damage and renal rejection.
Alemtuzumab’s use as an induction agent in transplant remains off-label. As a humanized monoclonal antibody, alemtuzumab targets the cluster of differentiation 52 that is highly expressed on T and B cell populations [28]. CD52 is also expressed on a number of cells of the innate immune system, including eosinophils, plasma cells, and, to a lesser extent, neutrophils and monocytes [29-31]. However, the mechanism of alemtuzumab includes potent complement stimulation, which allows for effective ablation of members of both the innate and adaptive immune system through complement-mediated cellular lysis via direct antibody/epitope binding and complement activation [32]. Yet, when monocytes are considered, our understanding of this particular mechanism becomes less clear.
Fabian et al. [33] demonstrated that monocytes clearly express CD52, justifying the use of alemtuzumab for their ablation. Nonetheless, monocytes continue to be associated with renal rejection in presence of alemtuzumab induction. Lenihan et al. report monocytic infiltration with alemtuzumab induction, postulating that properties of alemtuzumab permit the infiltration of monocytes leading to subsequent contributions to transplant glomerulitis [21]. The contradictory expression of CD52 by monocytes in conjunction is mechanistically puzzling. Sambasiva et al., utilizing flow cytometry and peripheral blood of donors, demonstrated that while monocytes do express CD52, they also expressed a relatively greater quantity of complement inhibitory proteins (CIPs) as compared to CD52 expression. This property potentially renders monocytes far less susceptible to complement mediated cell lysis precipitated by alemtuzumab, rendering monocytes the ability to promote future rejection.
Given that our patient population was treated solely with alemtuzumab pre-operatively, an explanation is offered as to why monocytes are the predominant cell type present post-induction and stand to contribute to allograft rejection. Indeed, we report that monocytes were the most commonly observed cell type seen on biopsy irrespective of associated risk factors and were correlated with adverse outcomes including graft failure as indicated by their high Banff classification. This supports the notion of potential contributions by monocytes to heightened rejection severity. Zhang et al. [34] found that monocytes were associated with acute cellular rejection with alemtuzumab induction and further demonstrated that these rejections responded well to steroid treatment. This suggests that the steroid free properties of alemtuzumab may be blunted in higher-risk patients, and that these groups may benefit from the addition of corticosteroids to their regimen.
We report a rejection frequency of 27% with 18.7% being Acute Cellular Rejection (ACR) (69.3% of rejection) and 3.6% being AMR (13.3% of rejections) at 5 years of follow up. While this confirms the trend that alemtuzumab decreases the risk of AMR, our overall rejection rate is considerably higher than the national average. By comparison, other institutions report frequencies of ACR varying from 4.4% to 14.4% depending on follow up, with most studies achieving a primary end point of 5 years [11-17]. For AMR, rejection rates have been reported to range from 6.39% to 15%, where a five year follow up was attained in the majority of studies [7,18,25]. Therefore, we have amended our protocol to include long-term maintenance steroids in high risk patients as well as also encouraging a full dosage of mycophenolate.
The benefits of alemtuzumab and minimal steroid usage in kidney transplant have been well documented. However, few have acknowledged the potential consequences of alemtuzumab induction should the complication of rejection arise. Our research provides meaningful insight into the inflammatory intricacies of rejection as well as the correlations between recipient populations and induction agent. Furthermore, uniform pre- and post-operational transplant protocols were used throughout, therefore minimizing confounding factors. Although it must be included that our investigation has a number of limitations. As a single center, results may not be applicable to all regions. Secondly, the retrospective nature of this study lends itself to the difficulties of inadequate follow-up data. A relatively small sample size of recipients experiencing rejection proved difficult for statistical analysis in some instances. Lastly, there was also no control group.
In conclusion, we highlight the complexities of using alemtuzumab as an induction agent and suggest that monocytes are more likely to play a significant role in rejection when this agent is used. Given that Zhang et al. demonstrated that monocytes were responsive to steroids, it is very likely that our postoperative immunosuppression protocols lack sufficient implementation of steroids in a capacity which would inhibit the rejection promoting function of monocytes. Monocytes are capable of evading this induction and potentially promote deleterious allograft outcomes. Our results, as well as previous studies, also suggest that aggressive treatment is justified in the post-rejection setting should monocytes be seen on renal biopsy, as monocytes were present in a significant number of patients with adverse outcomes. Future investigations are needed in order to determine the mechanism by which monocytes damage allograft tissue within this context, and more importantly, the aim of future studies is to ultimately identify a therapeutic agent that can combat monocyte-dominant rejection.
Acknowledgements
The authors of the enclosed manuscript would like to acknowledge the efforts and contribution of the department of Pathology at the University of Toledo, Medical Center in the preparation and analysis of the renal biopsies and for providing the imagines supplied herein. The authors would particularly like to acknowledge the efforts of Neha Varshney for her contributions in this capacity.
Contributors
• Alexander Stanton: Manuscript author, article drafting, editor, interpretation of data
• Merna Naji: Manuscript author, article drafting, editor, interpretation of data
• Luke Mugge: Article drafting, project design, and concept
• Graham Mitro: Statistical analysis
• Amira Gohara: Analysis of biopsies, pathology description, and manuscript review
• Michael Rees: Editor
• Jorge Ortiz: Project PI, concept inception, project oversight, and editing
Conflict of Interest
All authors of the enclosed manuscript have affirmed that they have no financial or personal relationship with any private or public company to disclose.
References
- Â Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, et al. (2014) Diagnosis and management of antibody-mediated Rejection: Current status and novel approaches. Am J Transplant 14: 255-271
- Â Girlanda R, Kleiner DE, Duan Z, Ford EAS, Wright EC, et al. (2008) Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant 8: 600-607.
- Â Joosten SA, Sijpkens YWJ, Van Kooten C, Paul LC (2005) Chronic renal allograft rejection: pathophysiologic consideration. Kidney Int 68: 1-13.
- Â Weissenbacher A, Hautz T, Kimelman M, Oberhuber R, Ulmer H, et al. (2015) Lymphocytes as an indicator for initial kidney function: A single center analysis of outcome after alemtuzumab or basiliximab induction. J Immunol Res 2015: 98546.
-  Morgan RD, O’Callaghan JM, Knight SR, Morris PJ (2012) Alemtuzumab induction therapy in Kidney Transplantation: A Systematic review and meta-analysis. Transplantation 93: 1179-1188
- Sidaway P (2014) Transplantation: Alemtuzumab induction reduces acute rejection risk. Nat Rev Nephrol 10: 542.
- Zhang X, Huang H, Han S, Fu S, Wang L (2012) Alemtuzumab induction in renal transplantation: A meta-analysis and systemic review. Transpl Immunol 27: 63-68.
- Â Smith A, John MM, Dortonne IS, Paramesh AS, Killackey M, et al. (2015) Racial disparity in renal transplantation: Alemtuzumab the great equalizer? Ann Surg 262: 669-674.
- Â Hamed MO, Chen Y, Pasea L, Watson CJ, Torpey N, et al. (2015) Early graft loss after kidney transplantation: Risk factors and consequences. Am J Transplant 15: 1632-1643
- Â Naji M, Stanton AD, Ekwenna O, Mitro G, Rees M, et al. (2017) Alemtuzumab equalizes short term outcomes in high risk PRA patients: Long term outcomes suffer. J Clin Exp Transplant 2: 117.
- Â Noureldeen T, Albekioni Z, Machado L, Muddana N, Marcus RJ, et al. (2014) Alemtuzumab induction and antibody-mediated rejection in kidney transplantation. Transplant Proc 46: 3405-3407
- Â Sutherland AI, Akhtar MZ, Zilvetti M, Brockmann J, Ruse S, et al. (2014) Alemtuzumab and sirolimus in renal transplantation: six-year results of a single-arm prospective pilot study. Am J Transplant 14: 677-684
- Â Zachariah M, Nader ND, Brar J, Singh N, Venuto R, et al. (2014) Alemtuzumab and minimization immunotherapy in kidney transplantation: long-term results of comparison with rabbit anti-thymocyte globulin and standard triple maintenance therapy. Transplant Proc 46: 94-100.
- Â Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, et al. (2014) Randomized trial of three induction antibodies in kidney transplantation: long-term results. Transplantation 97: 1128-1138.
- Â Khalafi-Nezhad A, Sagheb MM, Amirmoezi F, Jowkar Z, Dehghanian AR (2015) Comparison of the effect of alemtuzumab versus standard immune induction on early kidney allograft function in Shiraz transplant center. Int J Organ Transplant Med 6: 150-156.
- Heilman RL, Khamash HA, Smith ML, Chakkera HA, Moss AA, et al. (2013) Delayed allograft inflammation following alemtuzumab induction for kidney transplantation. Clin Transplant 27: 772-780
- Â Umber A, Killackey M, Paramesh A, Liu Y, Qin H, et al. (2016) A comparison of three induction therapies on patients with delayed graft function after kidney transplantation. J Nephrol 30: 289-295.
- Â Willicombe M, Roufosse C, Brookes P, Galliford JW, Mclean AG, et al. (2011) Antibody-mediated rejection after alemtuzumab induction: incidence, risk factors, and predictors of poor outcome. Transplantation 92: 176-182.
-  LaMattina JC, Mezrich JD, Hofmann RM, Foley DP, D’Alessandro AM, et al. (2012) Alemtuzumab as compared to alternative contemporary induction regimens. Transpl Int 25: 518-526.
- Kwan T, Wu H, Chadban SJ (2014) Macrophages in renal transplantation: Roles and therapeutic implications. Cell Immunol 291: 58-64.
- Lenihan CR, Tan JC, Kambham N (2013) Acute transplant glomerulopathy with monocyte rich infiltrate. Transpl Immunol 29: 114-117
- Â Rao SP, Sancho J, Campos-Rivera J, Boutin PM, Severy PB, et al. (2012) Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS One 7: e39416.
- Â Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, et al. (2011) Alemtuzumab induction in renal transplantation. N Engl J Med 364: 1909-1919
- Â Van Den hoogen MWF, Hesselink DA, Van Son WJ, Weimar W, Hilbrands LB, et al. (2013) Treatment of steroid-resistant acute renal allograft rejection with alemtuzumab. Am J Transplant 13: 192-196
- 3C Study Collaborative Group, Haynes R, Harden P, Judge P, Blackwell L, et al. (2014) Alemtuzumab-based induction treatment versus basiliximab base induction treatment in kidney transplantation (the 3C Study): A randomized trial. Lancet 384: 1684-1690.
- Schinstock CA, Stegall M, Cosio F (2014) New insights regarding chronic antibody-mediated rejection and its progression to transplant glomerulopathy. Curr Opin Nephrol Hypertens 23: 611-618
- Â Turner MJ, LaMorte MJ, Chretien N, Havari E, Roberts BL, et al. (2013) Immune status following alemtuzumab treatment in human CD52 transgenic mice. J Neuroimmunol 261: 29-36.
-  Elsner J, Höchstetter R, Spiekermann K, Kapp A (1996) Surface and mRNA expression of the CD52 antigen by human eosinophils but not by neutrophils. Blood 88: 4684-4693
- Â Kumar S, Kimlinger TK, Lust JA, Donovan K, Witzig TE (2003) Expression of CD52 on plasma cells in plasma cell proliferative disorders. Blood 102: 1075-1077
- Ambrose LR, Morel AS, Warrens AN (2009) Neutrophils express CD52 and exhibit complement-mediated lysis in the presence of alemtuzumab. Blood 114: 3052-3055
- Â Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, et al. (1998) Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk Res 22: 185-191.
- Fabian I, Flidel O, Gadish M, Kletter Y, Slavin S, et al. (1993) Effects of CAMPATH-1 antibodies on the functional activity of monocytes and polymorphonuclear neutrophils. Exp Hematol 21: 1522-1527.
- Â Zhang PL, Malek SK, Prichard JW, Lin F, Yahya TM, et al. (2014) monocyte-mediated acute renal rejection after combined treatment with preoperative campath-1h (alemtuzumab) and postoperative immunosuppression. Ann Clin Lab Sci 34: 209-213
Citation: Stanton A, Naji M, Mugge L, Mitro G, Gohara AF, et al. (2018) Monocytes Contribute to High Renal Allograft Rejection Rates when Alemtuzumab is Utilized as an Induction Agent: A Retrospective Study. J Clin Exp Transplant 3: 122. DOI: 10.4172/2475-7640.1000122
Copyright: © 2018 Stanton A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9103
- [From(publication date): 0-2018 - Dec 15, 2025]
- Breakdown by view type
- HTML page views: 8142
- PDF downloads: 961