Monitoring the Effects of Ligustilide on Mice with Idiopathic Pulmonary Fibrosis and Determining the Underlying Mechanism
Received: 01-Nov-2022 / Manuscript No. wjpt-23-87397 / Editor assigned: 03-Nov-2022 / PreQC No. wjpt-23-87397 (PQ) / Reviewed: 17-Nov-2022 / QC No. wjpt-23-87397 / Revised: 22-Nov-2022 / Manuscript No. wjpt-23-87397 (R) / Accepted Date: 28-Nov-2022 / Published Date: 29-Nov-2022 DOI: 10.4172/wjpt.1000170
Abstract
Idiopathic pulmonary fibrosis is a chronic interstitial lung condition that currently lacks viable treatment options. One of angelica’s key bioactive ingredients is ligustilide (LIG). The current study’s objectives were to investigate the underlying mechanism and monitor the impact of LIG on mice with advanced pulmonary fibrosis lung fibrosis. After a single BLM instillation of 14 days, the mice received daily LIG treatment for 2 weeks. Then the effect of LIG on lung fibrosis was observed then. The impact of LIG on pulmonary fibrosis was assessed using the pulmonary function test, Hematoxylin-Eosin (H&E) and Masson’s trichrome staining, immunofluorescence, and Western blot. Following in vitro therapy with transforming growth factor 1 (TGF-1), we looked into the underlying mechanism.
Keywords
Lung fibrosis; Bleomycin; Ligustilide; Fibroblasts; Oxidative stress
Introduction
Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease that causes a gradual and irreversible deterioration in lung function as a result of the excessive deposition of extracellular matrix in the pulmonary interstitium [1-3]. IPF sufferers are currently becoming more numerous each year. IPF is a chronic interstitial lung disease, and the average survival period after diagnosis was just 2 to 5 years. The pathophysiology of IPF is still unclear after intensive research. Although they have been licenced for use in the clinical treatment of pulmonary fibrosis (Nintedanib and Prfenidone, or PFD) [4,5], these medications do not have a 100% success rate. It is therefore vital to investigate novel treatment medicines for pulmonary fibrosis. A significant amount of extracellular matrix and other proteins are deposited together with a high number of fibroblasts that proliferate, migrate, activate, and accumulate in the region of lung damage under pathological circumstances [6,7], resulting in irreversible lung fibrosis. By encouraging fibroblast proliferation, differentiation, and extracellular matrix secretion, transforming growth factor-1 (TGF-1) contributes significantly to the onset of lung fibrosis [8,9]. According to research, TGF-1-induced fibrosis can activate both canonical (Smadbased) and non-canonical (non-Smad-based) signalling pathways [10]. Numerous studies have demonstrated that the TGF-1/Smad pathway dysregulation is a significant pathogenic factor in lung fibrosis [11] and that Smad3 is a crucial downstream regulator that supports tissue fibrosis brought on by TGF-1 [12]. Recent research suggests that the stimulation of fibroblasts may be mediated by cytokines and reactive oxygen species (ROS) [13,14]. When oxidative stress is produced, excessive ROS production occurs [15]. According to studies, Nuclear factor-erythroid 2-related factor 2 (Nrf2) is a crucial transcription factor that activates the transcription of several genes, including HO-1 and NQO1, protecting tissues from harm and squelching ROS generation under conditions of oxidative stress [16]. In mice treated with BLM, the modulation of Nrf2 can minimise extracellular matrix deposition and restrict myofibroblast activation [17].
Ligustilide (LIG; 3-butylidene-4,5-dihydrophthalide; Figure 1), the primary bioactive ingredient of the Umbelliferae family used in Traditional Chinese Medicine, including Rhizoma Ligustici Chuanxiong and Radix Angelicae Sinensis, has been linked to a variety of pharmacological activities, including anti-oxidant, antiinflammatory, neuroprotective, and protective effects on many diseases [18-21]. The impact of LIG on fibroblast activation following lung fibrosis is unknown, though. In this work, we investigated the potential mechanisms of LIG on fibroblast activation following in vitro exposure to TGF-1 and examined the effects of LIG on lung fibrosis in mice following BLM administration.
Methods
Animals: Purchased from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd. were male C57BL/6 mice weighing 20 2 g. All animals were reared in colony cages with unrestricted access to food and water at a constant temperature of 23 °C and humidity of 50% throughout 12-hour light/dark cycles. The Laboratory Animal Care and Use Committee at Southeast University (20210106011) gave its approval to all animal operations, which were carried out strictly in line with the National Institutes of Health’s guide for the care and use of laboratory animals.
Drugs and administration: Chengdu herbpurify, Co., Ltd. was the seller of LIG (HPLC > 98%). Six groups of eight mice each were created using a random number generator: sham, BLM, BLM plus PFD (300 mg/kg), BLM plus 10 mg/kg of LIG, BLM plus 30 mg/kg of LIG, and BLM plus 90 mg/kg of LIG. In all groups save the sham group, the mice received 100 L of intratracheal bleomycin (2 mg/kg) following anaesthesia with pentobarbital sodium (1%, 50 mg/kg). The equal amount of saline was administered to the mice in the sham group. Mice received continuous treatment with LIG, PFD, or 0.5% CMC-Na solution for 14 days following BLM injection. Every day, the mice’s body weight was recorded.
The mice were killed with pentobarbital sodium following a continuous 14-day treatment period in order to perform the pulmonary function tests as previously mentioned. The trachea of the mice was then exposed after they had been put to death. A tracheal catheter had been implanted and secured to the trachea. The Forced Manoeuvres System was then used to examine the IC (Inspiratory Capacity, Volume Inspired During Slow Inspiration), ERV (Expiratory Reserve Volume), FVC (Forced Vital Capacity, Volume Exhausted During Fast Expiration), and TLC (Total Lung Capacity, FRC+IC) parameters (EMMS, Hants, UK). Each mouse was used three times for measurements. The last step was killing the mice and collecting lung samples for future investigation. Following their removal, the lung samples were immediately fixed in 4% paraformaldehyde, cryoprotected, and then cut into 8-mm frozen sections using a freezing microtome. The sections were then stained as directed with Masson’s trichrome (Biyun Tian, China) and hematoxylin-eosin to assess for lung damage. The pictures were seen using a microscope.
ScienCell was paid for the mouse lung fibroblast cell line (MLG). Fetal bovine serum (10%), penicillin-streptomycin solution, and Dulbecco’s Modified Eagle Medium were added to the medium during cell culture, which took place at 37°C in an incubator with 5% CO2. Every two days the medium would be changed. Cells were treated to recombinant mouse TGF-1 to mimic fibrosis conditions in a test tube (Kingsley Biotechnology, China). Cells were treated with LIG (3, 10, 30 M) following 80-90% confluency, then TGF-1 was given for 24 hours. Cells were then utilised in a number of subsequent research.
Results
LIG reduced the lung fibrosis that BLM-induced in mice:
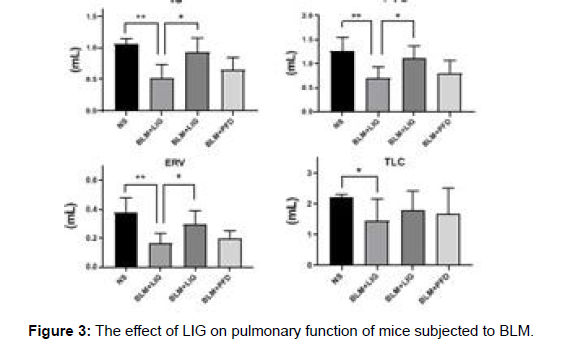
First, the toxicity of LIG was assessed to assure pharmacological safety. H&E staining revealed that no obvious lung histological damages were seen in either the Con- or LIG-treated animals, as shown in Supplementary Figure 2. Mice were stimulated with BLM (2 mg/ kg) and then given the therapy depicted in figure to test the hypothesis that treatment with LIG at the late stage of pulmonary fibrosis reduces the effects of BLM-induced lung damage. The LIG treatment groups (10, 30 or 90 mg/kg, for example), the PFD group, the vehicle group, and the sham group were the six treatment groups that were created. LIG therapy given two weeks after BLM infusion enhanced the survival rate of mice that had received BLM. In addition, LIG kept mice’s body weight stable in comparison to the vehicle group. Measurements of lung function revealed that LIG enhanced pulmonary function in comparison to the vehicle group (Figure 3).
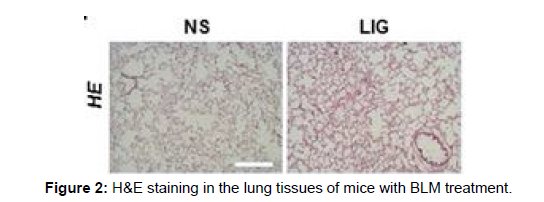
LIG reduced BLM-induced widespread alveolar collapse and wall thickening in the lung tissue, according to Hematoxylin-Eosin (H&E) staining. Additionally, LIG decreased collagen accumulation in the lungs of mice treated with BLM, according to Masson’s trichrome staining. The findings demonstrated that LIG increased mice’s survival rates and lung function after receiving BLM infusions and protected against BLM-induced lung damage, suggesting that LIG may be a potential medication for the treatment of pulmonary fibrosis.
LIG decreased extracellular matrix synthesis in mice treated with BLM:
Extracellular matrix is deposited together with the development of pulmonary fibrosis, hence the impact of LIG on this process was assessed. LIG reduced the deposition of collagen I and -SMA as compared to the vehicle group. Additionally, a Western blot demonstrated how LIG affected the protein levels of collagen I and –SMA. LIG inhibits the formation of extracellular matrix in mice after BLM induction, which may be connected to how it affects pulmonary fibrosis.
LIG’s impact on the TGF-1 pathway in BLM-induced pulmonary fibrosis:
TGF-1 plays a crucial part in the development of pulmonary fibrosis by encouraging fibroblast differentiation, proliferation, and secretion of extracellular matrix. Therefore, the impact on the TGF-1 pathway in the lung of BLM-exposed mice was evaluated and demonstrates that BLM dramatically raised TGF-1 levels in mouse lungs relative to the vehicle group, but LIG attenuated this effect. Smad 3 is a crucial component of the TGF-1 pathway and becomes active after being phosphorylated [12,22]. LIG reduced the phosphorylation of Smad 3 brought on by BLM. These findings suggested that LIG might control TGF-1 pathway activation in lung fibrosis following BLM therapy.
LIG lessens fibroblast activation brought on by TGF-1:
A key step in the development of pulmonary fibrosis is the activation of fibroblasts. We initially measured the protein level of the cells following TGF-1 exposure to determine if LIG had a protective effect against the TGF-1-induced fibroblast activation. Prior to that, we established that LIG (3, 10 and 30 M) incubation of fibroblasts for 24 hours had no deleterious impact on their viability (data not shown). LIG decreased the elevated protein level of collagen I following TGF-1 treatment, as seen in. To gauge the ability of the treated cells to migrate, a 2D scratch test was used. LIG prevented cell migration in response to TGF-1. We investigated if LIG therapy may prevent fibroblast proliferation following TGF-1 injection because cell proliferation is necessary for fibroblast activation. The LIG-treated group’s fibroblast proliferation was reduced according to the data. The protective effect of LIG on pulmonary fibrosis may be connected to the reduction of fibroblast activation following TGF-1 exposure, according to our research, which indicated that LIG decreased TGF-1 induced fibroblast activation.
LIG’s impact on the Nrf2 pathway and the generation of ROS in fibroblasts:
We examined whether LIG’s effect on fibroblast activation involves the antioxidant action of LIG in light of the possibility that oxidative stress is the cause of fibroblast activation. We anticipated the route associated to oxidative stress triggered by TGF-1 based on a combined investigation of online public databases and literature publications. According to the molecular docking results, LIG fits reasonably into the cavity of the Keap1 protein, and their strong connection is indicated by their binding energy of -6.7 kJ/mol. However, Nrf2 was insufficient to engage with LIG (data not shown). The Nrf2 pathway, which plays a key role in transcription under oxidative stress, was studied, along with the expression of its target antioxidant genes, NQO1 and HO- 1. As demonstrated, upon TGF-1 exposure, LIG increased the protein levels of Nrf2, HO-1, and NQO1. The impact of LIG on ROS synthesis was then studied. According to data, LIG decreased ROS generation in fibroblasts exposed to TGF-1. LIG’s impact on the generation of ROS was diminished by the Nrf2 inhibitor ML385, indicating that LIG’s impact on oxidative stress may be influenced by the control of the Nrf2 pathway.
Discussion
In this study, we discovered that LIG demonstrated survival rate and lung function following BLM infusion for two weeks, and it also provided protection against lung injury and collagen deposition. LIG also decreased TGF-1-induced ROS formation, fibroblast activation, including cell migration, proliferation, and collagen I protein levels, as well as activation of the Nrf2 pathway. Thus, LIG stimulated the Nrf2 pathway to lessen the oxidative stress response, which helped to diminish the activation of myofibroblasts in pulmonary fibrosis.
Pulmonary fibrosis is characterized by the differentiation of fibroblasts to myofibroblasts followed by excessive ECM deposition. After pulmonary fibrosis, respiratory function is substantially compromised, as evidenced by a dry cough and growing dyspnea. As the illness and lung damage worsen, patients’ respiratory function continues to decline. Idiopathic pulmonary fibrosis is becoming more common and has a higher death rate each year, however there are currently no viable treatments available. Finding novel medications to treat pulmonary fibrosis is so crucial. BLM is a chemotherapy drug, one of which side effects is to lead pulmonary fibrosis [23]. As a result, BLM is a frequently utilised inducer for the development of pulmonary fibrosis in animal models. Being a profibrotic cytokine, TGF-1 was shown to be much higher following BLM therapy, indicating that it plays a role in the pathophysiology of the pulmonary fibrosis brought on by BLM [24]. Numerous cells, including alveolar macrophages, fibroblasts, and activated alveolar epithelial cells, generate TGF-1 in the lungs [11,25]. According to research, preventing TGF-1-induced fibroblast activation may lessen lung fibrosis.
Conclusion
Numerous studies have demonstrated the physiological effects of natural bioactive compounds, which are found in a broad variety of plants, animals, marine creatures, and microbes. These chemicals also have anti-inflammatory, anti-cancer, antioxidant, and other physiological effects. The primary active ingredient of the volatile oil of the Chinese umbrella plant Angelica sinensis is LIG, commonly referred to as Angelica phthalide. According to reports, LIG has a wide range of pharmacological properties, including antioxidant and anti-inflammatory properties, and it also acts as a preventative against cardiovascular disease. For instance, LIG reduced inflammatory pain that was mediated by microglia as well as the generation of proinflammatory cytokines. By inhibiting the SIRT1/NF-B signalling pathways, LIG reduced podocyte damage. LIG also reduced apoptosis and the breakdown of extracellular matrix in nucleus pulposus cells, as well as intervertebral disc degeneration. By controlling the GPR30/ EGFR pathway, LIG promoted bone growth. LIG altered the PI3K/ Akt pathway’s activity, reducing the ischemia-reperfusion-induced death of hippocampus neurons. In HUVECs, LIG controlled Nrf2/ HO-1 activation and no production. In this study, we discovered that LIG stimulated the Nrf2 pathway to decrease the oxidative stress response, which helped to suppress the activation of myofibroblasts in pulmonary fibrosis. As a result, our study showed that LIG might lessen the development of lung fibrosis in mice given BLM. The Nrf2 pathway’s activation may be responsible for LIG’s protective effects. This discovery could offer a therapeutic option for pulmonary fibrosis.
Acknowledgement
Not applicable
Conflict of Interest
Author declares no conflict of interest
References
- King TE, Pardo A, Selman M (2011) Idiopathic pulmonary fibrosis. The Lancet 378:49-61.
- Heukels P, Moor CC, von der Thusen JH, Wijsenbeek MS, Kool M (2019) Inflammation and immunity in IPF pathogenesis and treatment. Respir Med 147:79-91.
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, et al. (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8:532.
- Saito S, Alkhatib A, Kolls JK, Kondoh Y, Lasky JA (2019) Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac Dis 11:1740-1754.
- Ogura T, Taniguchi H, Azuma A, Inoue Y, Kondoh Y, et al. (2015) Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 45:1382-1392.
- Coward WR, Saini G, Jenkins G (2010) The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis 4: 367-388.
- Xie T, Wang Y, Deng N, Huang G, Taghavifar F, et al. (2018) Single-Cell Deconvolution of Fibroblast Heterogeneity in Mouse Pulmonary Fibrosis. Cell Rep 22:3625-3640.
- MacKinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, et al. (2012) Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med 185:537-546.
- Liu RM, Desai LP (2015) Reciprocal regulation of TGF-beta and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol 6:565-577.
- Juan FS, Miguel Q, Carmelo B (2011) TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci 121:233-51.
- Hu HH, Chen DQ, Wang YN, Feng YL, Cao, G, et al. (2018) New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact 292:76-83.
- Walton KL, Johnson KE, Harrison CA (2017) Targeting TGF-beta Mediated SMAD Signaling for the Prevention of Fibrosis. Front Pharmacol 8:461.
- Kliment CR, Oury TD (2010) Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 49:707-717.
- An L, Peng LY, Sun NY, Yang YL, Zhang XW, et al. (2019) Tanshinone IIA Activates Nuclear Factor-Erythroid 2-Related Factor 2 to Restrain Pulmonary Fibrosis via Regulation of Redox Homeostasis and Glutaminolysis. Antioxid Redox Signal 30:1831-1848.
- Cheresh P, Kim SJ, Tulasiram S, Kamp DW (2013) Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta 1832:1028-1040.
- Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 73:3221-3247.
- Liu R, Chen H, Bai H, Zhang W, Wang X, et al. (2013) Suppression of nuclear factor erythroid 2-related factor 2 via extracellular signal-regulated kinase contributes to bleomycin-induced oxidative stress and fibrogenesis. Toxicol Lett 220:15-25.
- Wang K, Chen T, Ying X, Zhang Z, Shao Z, et al.(2019) Ligustilide alleviated IL-1beta induced apoptosis and extracellular matrix degradation of nucleus pulposus cells and attenuates intervertebral disc degeneration in vivo. Int Immunopharmacol 69:398-407.
- Zhou Y, Ming J, Li Y, Deng M, Chen Q, et al. (2019) Ligustilide attenuates nitric oxide-induced apoptosis in rat chondrocytes and cartilage degradation via inhibiting JNK and p38 MAPK pathways. J Cell Mol Med 23:3357-3368.
- Wu Q, Mao Z, Liu J, Huang J, Wang N (2020) Ligustilide Attenuates Ischemia Reperfusion-Induced Hippocampal Neuronal Apoptosis via Activating the PI3K/Akt Pathway. Front Pharmacol 11:979.
- Luo Z, Deng H, Fang Z, Zeng A, Chen Y, et al. (2019) Ligustilide Inhibited Rat Vascular Smooth Muscle Cells Migration via c-Myc/MMP2 and ROCK/JNK Signaling Pathway. J Food Sci 84:3573-3583.
- Feng M, Tang PMK, Huang XR, Sun SF, You YK, et al. (2018) TGF-beta Mediates Renal Fibrosis via the Smad3-Erbb4-IR Long Noncoding RNA Axis. Mol Ther 26:148-161.
- Della Latta V, Cecchettini A, Del Ry S, Morales MA (2015) Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol Res 97:122-130.
- Qian W, Cai X, Qian Q, Zhang W, Wang D (2018) Astragaloside IV modulates TGF-beta1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med 22:4354-4365.
- Frangogiannis N (2020) Transforming growth factor-beta in tissue fibrosis. J Exp Med 217:103.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Wang J (2022) Monitoring the Effects of Ligustilide on Mice withIdiopathic Pulmonary Fibrosis and Determining the Underlying Mechanism. WorldJ Pharmacol Toxicol 5: 170. DOI: 10.4172/wjpt.1000170
Copyright: © 2022 Wang J. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1456
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1125
- PDF downloads: 331