Monitoring of Molecular Variation among Egyptian Faba Bean Rhizobium Isolates as Response to Pesticides Stress
Received: 26-Mar-2015 / Accepted Date: 27-May-2015 / Published Date: 29-May-2015 DOI: 10.4172/2155-6199.1000296
Abstract
Ten Rhizobium isolates from nodules of Faba bean (Vicia faba L.) plants that represent different geographic and climatic locations in Egypt were evaluated for their tolerance against different doses of Zeus and Stomp as pesticides (250, 500 and 750 mg). In general, with increasing of pesticides doses, the tolerance and the viability of isolates were decreased. All isolates did not succeed to grow at the highest dose of Zeus (750 mg), while the isolates RLA, RLG and RLI succeeded to grow at the same concentration of Stomp. The most important nodulation genes nodA, nodD and the bidirectional promoter region between them were amplified using PCR and two principals bands were detected in the most isolates. The protein banding patterns of some Rhizobium isolates were detected using SDSPAGE analysis under pesticides stress and compared with their untreated control.
Keywords: Rhizobium; Legumes; Nod D - A genes and Pesticides
22159Introduction
The symbiosis between the legumes plants and Rhizobia is very important process for agriculture that lead to fixation high rates of nitrogen and therefore increasing the fertility of soil [1,2]. In legumes specific flavonoids released from the roots interact with the NodD protein of Rhizobium to activate transcription of other nod genes for synthesizing of Nod factors [3]. Nod factors are implicated in intracellular thread and starting of nodule development. Rhizobia species and genotypes differ in their complement of nod genes and their allelic forms. These differences lead to diversity in Nod factor structure that save basis for characterization of Rhizobial isolates [4]. The population of Rhizobia collected from a pasture soil was characterized using the nod D gene as marker [5]. Moreover, RFLP was used to analyze the fragment containing nod DAB genes to discriminate Rhizobia from china legumes [6].
Previous papers showed that factors as soil temperature, pH and light can effect on the growth of nitrogen- fixing Rhizobia [7,8]. Herbicides are among the factors that may be also influence the growth of each Rhizobia and plant [1,9-11]. In this period, huge quantities of fungicides and herbicides are consumed toward control of plant pathogenic fungi and weeds that damage most of yield. Hence, it’s very important to avoid the harmful effects of these fungicides and herbicides on the various beneficial soil microorganisms like Rhizobium and as well as their biological activities related to soil fertility [12]. Herbicides are suggested to have negative effects on growth of Rhizobia [13,14]. Moreover, another study showed that the effect of herbicides on Rhizobium growth depends on the concentration and the weather conditions of its application [15]. The aims of this study were: characterization of some Egyptian Rhizobium isolates of Faba bean plants using Nod D-A specific primers; evaluation of their fungicides and herbicides tolerance in vitro and analysis their protein profiles using SDS-PAGE.
Materials And Methods
Collection and isolation of Rhizobium isolates
Ten isolates of Rhizobium leguminosarum symbiovar. Viciae (RLV) were isolated from nodules of Faba bean plants (Vicia faba L.) representing different soil types and geographic locations in Egypt [16]. The samples collection areas in Egypt and soil types are shown in Table 1.
| Isolate code | Soil type | Location |
|---|---|---|
| RLQ | Clay | Quesna city |
| RLS | Sand | Sadat city |
| RLZ | Clay | Zefta city |
| RLB | Clay | Beni suef city |
| RLT | loam | El-tor city |
| RLA | loam | El-Arish city |
| RLM | loam | El-Menia city |
| RLK | Clay | Kaha city |
| RLG | Clay | El-Giza city |
| RLI | Sand | El-Ismailia city |
Table 1: Name of isolates and its locations.
DNA isolation
Total genomic DNA of each isolate was extracted from bacterial cultures grown in yeast extract mannitol media (YEM) according to Ausubel et al. [17].
Amplification of nod D-A region
The primers used for the polymerase chain reaction (PCR) to amplify nodD-A region were: JZ3 (5’-AGGACGTCTTCAGTCCAAC-3’) and JZ5 (5’-AGAGCCGGCCATTGGACAG-3’), PCR were carried out in 25 μl reaction mixtures using the following conditions: initial denaturation at 94°C for 10 min, followed by 35 cycles of 94°C for 1 min, 68°C for 1 min, and 3 min at 72°C. A final extension was done at 72°C for 7 min. PCR products were separated on 2% agarose gels at 100 V for 1 h in TBE buffer, stained with ethidium bromide, and photographed under UV light.
Determination of fungicide and herbicide resistance
Two pesticides are commonly used in Egypt; Zeus (propyl 3-(dimethyl amino) propylcarbamate hydrochloride) is a fungicide (72.2 % active substrate) against fungal diseases as late blight and downy meldiew and Stomp (N-(1-ethyl propyl)-2,6- dinitro-3,4-xylidine) is strong herbicide (45.5% active substrate). These pesticides were tested for their in vitro inhibitory effects on the growth of Rhizobial isolates. Zeus and Stomp were added at doses of 250, 500 and 750 mg/L-1 to Yeast Extract Manitol medium (YEM) [18]. The plates were inoculated with 80 μl of the tested cultures (106 cell/ml-1) and incubated at 28°C for 72 h. Number of colonies were counted and compared with control.
Protein banding patterns of rhizobial isolates
The cultures of RLV isolates growing on Broth YEM medium were pelleted and resuspended in 40 μl of Laemmli Sample Buffer, 5 μl of 10%SDS and 5 μl of β-mercaptoethanol. The mixture was then boiling for 8 min and centrifugation to obtain the supernatant which contains protein fractionations. Sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli [19].
Results And Discussion
Amplification of nod D-A region
The PCR was used to amplify nodD-A region which contain nodD, nodA genes and the bidirectional promoter region between them using JZ couple primers as previously described by Zafran-Novak, et al. [20] (Figure 1). The amplification products gave two principles bands, the first band was found in all isolates with molecular weight about 500 bp, while the second band was found in most of isolates that was about 1200bp. According to previous studies, it was expected to obtain one fragment about 1680 bp including the nodulation genes D, A and the bidirectional promoter region between them. According to Zafran- Novak et al. [20], the first band of 500bp is related to nod A gene, while the second band was less than the expected band and found in all tested isolates except RLM, RLG and RLK isolates. Appearance of two bands in most of isolates may be as result of different mutations in primer binding site(s). The mutations can modify the target binding site of primers and synthesizing another binding sites in the region and this may explain the different sizes of bands. This explanation is compatible with conclusions of Zafran-Novak, et al. [20] who detected several mutations, (including deletions and insertions of 1-2 bp) which resulted in shorter regions in both autochthonous strains (201ZG and 301ZG). These mutations caused more differences in nucleotide sequences and therefore variation in NodD proteins length between different strains.
Pesticides tolerance
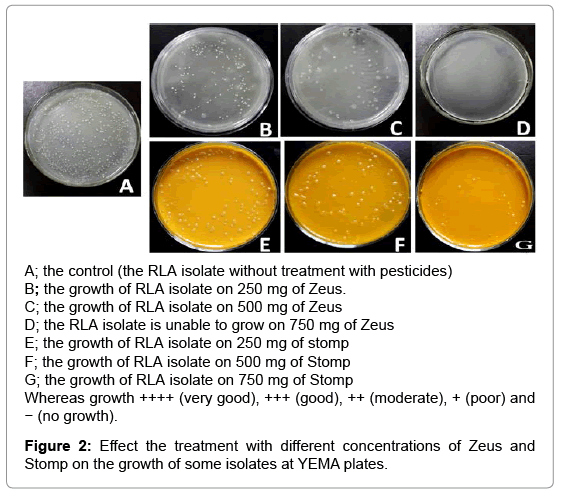
In this study, the RLV isolates were evaluated for their tolerance against two pesticides, Zeus as fungicide and stomp as strong herbicide. The isolates were grown in YEM medium supplemented with different concentration of pesticides (250, 500 and 750 mg) and the growth of colonies was considered as an indicator of tolerance (Table 2). For Zeus treatments, all isolates gave colonies on YEM medium supplemented with 250 and 500 mg of fungicide except the isolates RLQ and RLK that failed to grow. The isolate RLK of Kaha City failed to grow in presence of any fungicide doses and considered as the most sensitive one with this fungicide. Moreover, all isolates did not succeed to grow at the highest dose of fungicide (750 mg).
| Isolates | Control | The growth at presence of Zeus (mg/ L-1) |
The growth at presence of Stomp (mg/ L-1 ) |
||||
|---|---|---|---|---|---|---|---|
| 250 | 500 | 750 | 250 | 500 | 750 | ||
| RLQ | ++++ | +++ | − | − | ++ | + | − |
| RLS | ++++ | ++ | + | − | ++ | + | − |
| RLZ | ++++ | +++ | ++ | − | +++ | ++ | − |
| RLB | ++++ | +++ | ++ | − | +++ | ++ | − |
| RLT | ++++ | +++ | +++ | − | +++ | +++ | − |
| RLA | ++++ | ++++ | +++ | − | ++++ | ++ | + |
| RLM | ++++ | +++ | + | − | +++ | ++ |
|
| RLK | ++++ | − | − | − | +++ | ++ | − |
| RLG | ++++ | ++ | + | − | +++ | ++ | + |
| RLI | ++++ | +++ | ++ | − | +++ | ++ | + |
Whereas growth ++++ (very good), +++ (good), ++ (moderate), + (poor) and − (no growth).
Table 2: Effects of different concentrations of Zeus and Stomp on the growth of tested isolates at YEMA Plates.
On the other side, all tested isolates can grow at different degrees on YEM medium supplemented with 250 and 500 mg of Stomp. Only, the isolates RLA, RLG and RLI to grow at the highest concentration 750 mg of Stomp. From the above results, we can conclude that with increasing of pesticides doses, the tolerance and the viability of isolates were decreased. The negative effect of the fungicide and the herbicide on Rhizobial isolates may be due to their suppression the effectiveness of Rhizobia [21-23]. Another report showed that the effect of Herbicides on Rhizobium growth depends on the concentration and the conditions of its application [15]. In general, the tolerance of isolates against herbicide was better than fungicide, one of explanations that the herbicide is directed basicly to the weeds while the fungicide is directed to fungi as microorganisms and therefore can affect Rhizobium also. The ability of isolates like RLA, RLI to grow well and tolerate both the fungicide and herbicide at different concentrations, may be as a result of adaptation of these isolates under these conditions (Figure 2). These results are compatible with previous studies shown that Rhizobia can tolerate many fungicides [24-28].
Characterization of Rhizobium isolates by SDS-PAGE
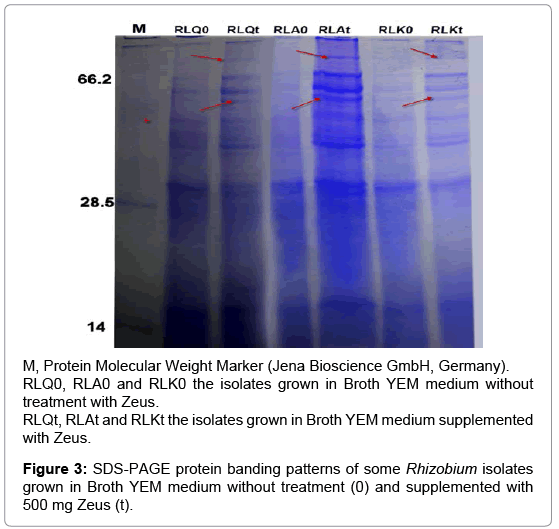
Since the SDS-PAGE analysis of protein banding patterns is important in identification and differentiation among the isolates within the same group [29-30]. Hence, the protein banding patterns for some Rhizobium isolates were detected using SDS-PAGE analysis under pesticides stress and compared with their untreated control. Firstly for Zeus treatment, the isolates RLQ and RLK were selected as sensitive isolates while the isolate RLA was selected as tolerant isolate. The treatment by Zeus induced new bands and also increased the density of the other bands (Figure 3). Comparative analysis of the lanes showed production of two new protein bands (about 90 and 50 kDa) by the treated isolates and these bands did not detected with their control isolates. In general, the isolate RLA treated with Zeus have bands number more than other isolates and this may be explain their tolerance against pesticides.
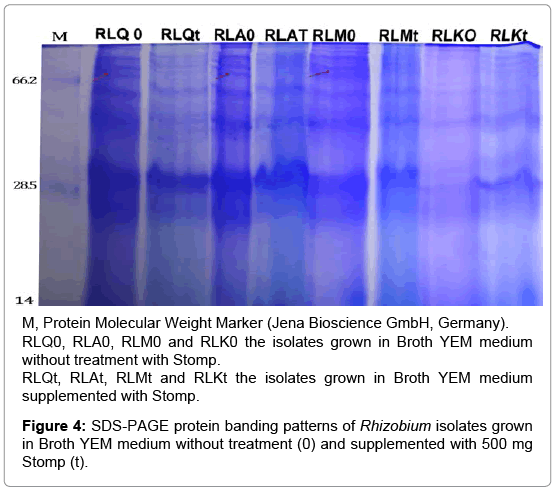
Secondly for Stomp treatment, the isolates RLQ, RLM and RLK were selected as sensitive isolates while the isolate RLA was selected as tolerant isolate. Surprisingly, the treatment by stomp reduced number of bands as compared with control (Figure 4). Moreover, a protein band (about 70 kDa) can be detected in the control isolates did not detected with their treated isolates. Detection of new protein bands in tolerant isolates is due to the differential response of these isolates to stress effects. These results are in compatible with previous studies on salt stress shown that some bands disappeared and new bands appeared after treatment by salt due to alterations in gene expression [31-33].
References
- Singh G, Wright D (2002) In vitro studies on the effects of herbicides on the growth of rhizobia. Lett Appl Microbiol 35: 12-16.
- Agrawal PK, Agrawal S, Singh U, Katiyar N, Verma SK (2012) Phenotypic characterization of rhizobia from legumes and its application as a bioinoculant. Journal of Agricultural Technology 8: 681-692.
- Abdel-Lateif K, Bogusz D, Hocher V (2012) The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signalling & Behavior 7: 1-6
- Moschetti G, Peluso A, Protopapa A, Anastasio M, Pepe O et al. (2005) Use of nodulation pattern, stress tolerance, nodC gene amplification, RAPD-PCR and RFLP-16S rDNA to discriminate genotypes of Rhizobium leguminosarum biovar viciae. Syst Appl Microbiol 28: 619-631
- Adolphe Zeze , Lesley A Mutch, Peter W Young J (2001) Direct amplification of nodD from community DNA reveals the genetic diversity of Rhizobium leguminosarum in soil. Environmental Microbiology 3: 363-370.
- Tan ZY, Wang ET, Peng GX, Zhu ME, MartÃnez-Romero E, et al. (1999) Characterization of bacteria isolated from wild legumes in the north-western regions of China. Int J Syst Bacteriol 49: 1457-1469.
- Dart P (1977) Infection and development of leguminous nodules. In: Hardy (eds.) A Treatise on Dinitrogen Fixation, John Wiley, New York.
- Gibson AH, Jordan DC (1983) Ecophysiology of nitrogenfixing systems. In: Lange OL, Nobel PS, Osmond CB, Zeigler H (eds.) Physiological Plant Ecology III. Responses to the Chemical and Biological Environment Berlin,Springer-Verlag pp. 301-390.
- Sharma P, Khanna V (2011) In vitro sensitivity of Rhizobium and phosphate solubilising bacteria to herbicides. Indian J Microbiol 51: 230-233.
- Zobiole LHS, Oliveira JR, Tormena C, Constantin J, Cavalieri SD, et al. ( 2007) Effect of soil compaction and sulfentrazone on soybean under two soil moisture conditions. Planta Daninha 25: 537-545.
- Moorman TB (1989) A review of pesticides on microorganisms and soil fertility. J Prod Agric 2: 14-22.
- Amer (2008) Monitoring of Variation among Faba Bean Rhizobium Isolates: 2. Biodegradation of Herbicide, 3(3,4 Dichlorophenyl) -1-methoxy-1-methylurea. Australian Journal of Basic and Applied Sciences 2: 540-548.
- MÃ¥rtensson AM (1992) Effects of agrochemicals and heavy metals on fast-growing rhizobia and their symbiosis with small-seeded legumes. Soil Biology and Biochemistry 24: 435-445
- Clark SA, Mahanty HK (1991) Influence of herbicides on growth and nodulation of white clover, Trifolium repens. Soil Biology and Biochemistry 23: 725-730.
- Shankar PV, Shaikh NR, Vishwas PS (2012) Effect of different herbicides on the nodulation property of rhizobial isolates. Universal J. Environ. Res. Technol 2: 293-299.
- Abdel-Lateif K, Hewedy O, El-Zanaty AF (2014) Molecular screening of some Egyptian Rhizobium isolates under salt stress. International Journal of Scientific Research 3: 11-15.
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman GJ, et al. (1987) Current protocols in molecular biology. John Wiley & Sons, Inc., New York.
- Jordan DC (1984) Family III. Rhizobiaceae Conn 1938, 321AL In: Kreig INR and Holt JG (eds) Bergey's Manual of Systematic Bacteriology, The Williams & Wilkins Co. Baltimore: p 964.
- Laemmli UK (1970) Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4 Â Nature 227: 680-685.
- Zafran-Novak J, Redzepovi S, Cetkovic H (2010) Genetic analysis of a nodA-nodD region of autochthonous strains of Rhizobium leguminosarum biovar viciae that showed effective nodulation of host plants. PERIODICUM BIOLOGORUM 12: 459-467.
- Graham PH, Ocampo G, RuizL D, Duque A (1980) Survival of Rhizobium phaseoli in contact with chemical seed protectants. J Agron 72: 625-627.
- Borges A, Guimarães W, muchovej R, Gonzales G (1990) Resistance of Bradyrhizobium japonicum strains to different fungicides and herbicides. World J Microbiol Biotechnol 6: 428-430.
- Revellin P, Leterme G, Catroux (1993) Effect of some fungicide seed treatments on the survival of Bradyrhizobium japonicum and on the nodulation and yield of soybean [Glycine max. (L) Merr.] J Biol Fertil Soils 16: 211-214.
- Allievi L, Gigliotti C (2001) Response of the bacteria and fungi of two soils to the sulfonylurea herbicide cinosulfuran. J Environ Sci Healt 36: 161-175.
- Castro S, Vinocur M, Permigiani M, Halle C, Taurian T et al. (1997) Interaction of the fungicide mancozeb and Rhizobium sp. In pure culture and under field conditions. Biol Fertil Soils 25: 147-151.
- Lennox LB, Alexander M (1981) Fungicide enhacement of nitrogen fixation and colonization of Phaseolus vulgaris by Rhizobium phaseoli. Appl Environ Microbiol 41: 404-411.
- Milosevic NA, Govedarica MM (2002) Effect of herbicides on microbiological properties of soil. Proc Nat Sci 102: 5-21.
- Ramadoss S, Sivaprakasam K (1991) Effect of fungicides and insecticides on Rhizobium and Rhizobium-cowpea symbiosis. Soil Fertil 54: 282-286.
- Fabriano E, Arias A (1990) Identification of inoculants strains and naturalized population of Rhizobium leguminosarum bv. trifolii using complementary methodologies. World J Bacteriol Biotechnol 6: 127-133.
- Broughton WJ, Samrey U, Stanely (1987) Ecological genetics of Rhizobium meliloti: Symbiotic plasmid transfer in the Medicago sativa rhizosphere. FEMS Microbiol Let 40: 251-255.
- Soussi M, Santamarıa M, Ocana A, Lluch C (Castro) Effects of salinity on protein and lipopolysaccharide pattern in a salt-tolerant strain of Mesorhizobium ciceri. J Appl Microbiol 90: 476-481.
- Pereira SIA, Lima AIG, Figueira EAMP (2006) Heavy metal toxicity in Rhizobium leguminosarum biovar viciae isolated from soils subjected to different sources of heavy-metal contamination: Effects on protein expression. Appl Soil Ecol 33: 286-293.
- Shoukry AA, Khattab AA, Abou-Ellail M, El-shabrawy H (2013) Molecular and biochemical characterization of new Rhizobium leguminosarum sv. viciae strains isolated from different located of Egypt. J Appl Sci Res 9: 5864-5877.
Citation: Abdel-lateif KS, El-Zanaty AFM, Hewedy OA, El Sobky MA (2015) Monitoring of Molecular Variation among Egyptian Faba Bean Rhizobium Isolates as Response to Pesticides Stress. J Bioremed Biodeg 6: 296. DOI: 10.4172/2155-6199.1000296
Copyright: © 2015 Abdel-lateif KS, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15513
- [From(publication date): 7-2015 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 10872
- PDF downloads: 4641