Monitoring and Control of Exposure to Buprofezin in Greenhouses
Received: 15-Jul-2016 / Accepted Date: 09-Sep-2016 / Published Date: 13-Sep-2016 DOI: 10.4172/2476-2067.1000117
Abstract
The aim of this study was to assess dermal and respiratory exposure of workers to Buprofezin during spraying and during stapling of previously treated ornamental plants in greenhouses. Eight workers were monitored. A combination of hand washing and pads placed on the skin was used to evaluate actual skin contamination. The efficacy of protective clothing was evaluated placing pads on top of outer clothing. Respiratory exposure was evaluated by personal air sampling. Respiratory dose was calculated on the basis of a lung ventilation of 15 l/min for females and 20 l/min for males. Absorbed doses were calculated assuming a skin penetration of 40% and a respiratory retention of 100%. Dislodgeable foliar residues (DFRs) were evaluated during re-entry. Buprofezin was determined by gas chromatography with selective mass detection in all matrices. Respiratory dose was 1.5-12.8% and 3.6-15.4% of the total real dose during spraying and stapling, respectively. The estimated absorbed doses, 0.05-0.57 μg/kg body weight and 0.19-1.54 μg/kg body weight during spraying and stapling, respectively, were less than the acceptable operator exposure level of 40 μg/kg body weight. During stapling, a mean dermal transfer factor of 1.13 cm2/h was estimated. During spraying, the efficacy of protective clothing depends on the spraying device used. During stapling, daily replacement of cotton gloves appears to reduce actual exposure. Since proper use of equipment and protective clothing is essential, training of workers is of fundamental importance.
Keywords: Pesticides; Skin exposure; Inhalation exposure; Re-entry; Spraying
42704Introduction
Buprofezin, (Z)-2-tert-butylimino-3-isopropyl-5-phenyl-1,3,5- thiadiazinan-4-one, is an insecticide used in different cultivations including ornamental plants [1]. The compound has moderate acute toxicity: DL50 is greater than 2198 mg/kg body weight (bw) in rats, DL50 for dermal exposure is 2000 mg/kg bw in rats, LC50 for inhalation is greater than 4.57 mg/l in rats for an exposure time of 4 h [1]. An acceptable daily intake (ADI) of 0.01 mg/kg bw/day and an Acceptable Operator Exposure Level (AOEL) of 0.04 mg/kg bw/day have been defined but occupational exposure limits have not been published [1]. The pesticide has not been assessed for mutagenic effects, although neurotoxic and irritative effects on the respiratory tract, skin and eyes and endocrine disrupting activity have been reported [1].
Assessment of the carcinogenic potential of Buprofezin by the US EPA showed “evidence of carcinogenicity, but not sufficient to assess human carcinogenic potential” [2].
Dermal absorption of commercial formulations containing Buprofezin seems slow and limited, however in the absence of further studies and conclusive data, EFSA recommends a default value of 40% (similar to oral absorption) [3].
The scientific literature includes few evaluations of respiratory and dermal exposure to Buprofezin in agricultural environments: the only paper on sampling and analysis of this active ingredient in atmospheric airborne particulate shows that fibreglass filters are the best sampling material and analysis of samples after extraction is conducted by gas chromatography with mass spectrometric detection (GC/MS) [4].
The main aim of the present study was to evaluate exposure and occupational risk during treatment of ornamental plants in greenhouses with Buprofezin and during re-entry of greenhouses for stapling treated plants. Specific aims were: 1) to evaluate exposure levels in relation to different spraying devices (PulsFog thermal nebuliser and hand spraying equipment); 2) to determine the efficacy of skin protection devices, both new and constantly used for 5 months; 3) to assess respiratory and skin doses and their contribution to total estimated doses.
Respiratory exposure was evaluated by personal air sampling using binder-free fibreglass filters. Dermal exposure was measured using skin pads and hand washing. GC/MS was used to analyse Buprofezin in the various matrices. Leaf residues were determined to evaluate the influence of leaf contamination on exposure levels during re-entry of greenhouses and to determine dermal transfer factor.
Materials and Methods
Subjects and exposure conditions
Spraying: The subjects monitored were three workers (workers 1, 2 and 3, age 27-35 years) engaged in spraying ornamental plants in a greenhouse. The commercial pesticide formulation was APPLAUD 40 SC containing 40.5% pure Buprofezin, namely 430 g/l.
During the first day of sampling, workers 1 and 2 treated Shindapsus plants in the greenhouse using a PulsFog thermal nebuliser or “fogger”, which produces thermally propelled ultrafine droplets (1-50 μm). Liquids are vaporised by the device and condense as a fog on contact with cold greenhouse air. The active ingredients should be distributed uniformly, even reaching inaccessible places, without leaving large quantities of residues. The total quantity of formula used by the workers was 1 litre dispersed in 10 litres of water, equivalent to 430 g of active ingredient. The mixture also contained a litre of ethylene glycol to aid heat nebulisation. Both workers wore complete AGRY CHIMY overalls with hood in waterproof transpirable Rainfort, as well as usual underwear, rubber gloves, rubber boots, half-face mask with A2P3 filter and cape. Only worker 1 wore new latex gloves under the rubber gloves. The overalls had a double zip front and press studs and were lined with non-woven fabric. The seams were heat sealed and leg bottoms fitted with zips. Both workers wore new overalls that had never been used before for any type of activity. Workers 1 and 2 took 63 and 66 minutes, respectively, to spray the greenhouse.
On the second day of sampling, workers 1, 2 and 3 treated Ficus benjamin and Shefflera using hand spray apparatus. The total quantity of formula used by workers 1 and 2 was 400 ml dispersed in 400 litres of water, whereas worker 3 used 250 ml of formula in 250 l water. The quantity of active ingredient sprayed was therefore 172 g for workers 1 and 2 and 107.5 g for worker 3. Workers 1 and 2 wore the same overalls as the day before, rinsed externally with water after the first treatment and washed in water and detergent before the second treatment. Worker 3 wore the same type of overalls but they had been used over the previous 5 months for various treatments in greenhouses. Also in this case the overalls had been washed in water and detergent before the treatment monitored. Worker 3 wore new latex gloves under rubber gloves. Workers 1 and 2 took 40 minutes and worker 3, 55 minutes to spray the plants. In all cases the sleeves and legs of the overalls were closed with adhesive tape around the ankles and wrists (over gloves).
Re-entry: Five workers (workers 1-5, female, age 24-64 years, mean 41 ± 14 years), engaged in stapling shoots of ornamental plants (Shindapsus) to a mossy support were monitored for a week. The volume of the greenhouse was 25500 m3. The plants had been treated 47 hours before re-entry by fogging with one litre of APPLAUD 40 SC by means of the PulsFog system as described above.
The work shift was 8 am-5 pm with an hour off for lunch. On the days of monitoring, the temperature in the greenhouse was 25 ± 1.5°C (min-max 22.3-27.5°C) and relative humidity was 56 ± 13% (min-max 20-72%).
Protective clothing consisted of cotton overalls, work shoes and two pairs of gloves (cotton gloves in contact with the skin under latex gloves). Under the overalls the workers wore underwear, socks and a cotton t-shirt. Work overalls were changed two or three times a week; the cotton gloves were changed every day. The latex gloves were replaced when they broke or tore. Clothes were generally removed at home after work. The workers were responsible for washing their own clothes.
Evaluation of respiratory and skin exposure
During spraying and re-entry, personal air sampling was conducted at respiratory height to quantify the active ingredient present in inhalable airborne dust. Binder-free fibreglass membranes 25 mm in diameter mounted in IOM samplers operating at a flow of 2 l/min were used. Air sampling continued for the duration of spraying or re-entry.
Dermal contamination was determined by means of pads. The workers engaged in spraying wore 18 pads, nine of which were fixed on top of the clothing (external) and nine under the clothing in contact with skin in the positions indicated in Table 1. The skin areas monitored by the pads are also indicated as anatomical sites and as percentage of body surface area in Table 1. Pads in corresponding positions on top of and under the overalls did not overlap. The external face pad was actually under the face mask and was therefore more protected than the other external pads.
| Position | Anatomical region represented | % body surface area |
|---|---|---|
| Face | head and neck | 6.9 |
| Chest | shoulders and chest | 11.4 |
| Back | shoulders and back | 11.4 |
| Left arm | arms | 9.7 |
| Right forearm | forearms | 6.7 |
| Left anterior thigh | anterior thighs and hips | 13.55 |
| Right posterior thigh | posterior thighs and hips | 13.55 |
| Left calf | calves | 6.75 |
| Right shin | feet and ankles | 13.15 |
Table 1: Positions of pads on body.
For greenhouse re-entry, dermal contamination was determined by means of pads every day except Monday. Each worker wore 13 pads, nine of which were in contact with the skin in the positions indicated in Table 1 and four of which were worn externally on top of the overalls on the chest and back, left anterior thigh and right forearm.
The pads consisted of squares of filter paper (49 cm2 for all skin areas except the face, where they measured 16 cm2) and were maintained in position with adhesive tape.
Hand contamination was evaluated by washing. Workers rubbed their hands together while 150 ml of ethanol (95°C) was poured slowly over them and collected in a disposable aluminium tray. The worker then kept his/her hands and especially nails in the alcohol solution for 30 sec. During spraying operations, hand washing was performed before and after treatment and the two ethanol samples were placed in different containers. During re-entry operations, hand washing was performed before morning tea, before lunch and at the end of the daily work shift (i.e. when the workers would normally have washed their hands). The three ethanol samples were collected in the same container. The cotton gloves of the same workers (re-entry) were also collected at the end of each day to determine hand contamination under the latex gloves.
To avoid chemical degradation, all samples were protected from the light with aluminium foil and stored in a freezer at -18°C until analysis. Field blanks were performed to assess any contamination of the matrices used for sampling.
Determination of dislodgeable foliar residues (DFR)
To evaluate decay of the active ingredient we sampled leaves immediately before and after spraying with Buprofezin and then 48, 72, 96, 120 and 144 h after spraying. Each sample consisted of 18 discs punched from different leaves [5]. Since the discs measured 2 cm in diameter, samples represented 38.52 cm2 of leaf, counting only one side. Sampling points in the greenhouse were at the corners of a grid dividing the two sides of the greenhouse into four equal quadrants. At the different times, discs were punched from the same leaves of the plants at the corners of the grid. Leaves with intermediate development were chosen. Samples were protected from the light with aluminium foil and stored in the freezer at -18°C until analysis.
Calculation of exposure doses
Concentrations of active ingredient in personal air samples were used to obtain the potential respiratory dose for a lung ventilation rate of 20 l/min for males and 15 l/min for females.
Daily dermal contamination (excluding hands) was obtained by summing the contamination of the various anatomical regions where pads were attached. The contamination of an anatomical region was obtained multiplying the concentration detected on single pads (ng/ cm2) by the surface areas of the anatomical regions represented. Table 1 shows the percentages of total body area of the different anatomical regions [6]. Total skin area was calculated for each worker by the formula of Du Bois [7].
The contaminant concentrations derived from pads in contact with the skin can be used to obtain the “real dose”. In our case the real dermal dose was obtained summing hand contamination, namely the quantity of Buprofezin in hand wash liquid (under gloves) and contamination of other anatomical regions. “Potential dermal dose” (except hands) was obtained using contaminant concentrations on pads on top of protective clothing. As explained more fully later, the total potential dermal dose was underestimated in workers engaged in spraying and re-entry (all workers), in the first case because the external face pad was in fact under the face mask and hand contamination was evaluated under gloves. In the case of greenhouse re-entry, hand contamination was again evaluated under gloves; moreover, summing the contribution of cotton gloves does not correctly estimate the potential dose on the hands.
To calculate absorbed doses we assumed 40% skin penetration and 100% lung retention of particulate, as recommended by EFSA [3]. In the case of spraying, the workers wore a mask, so calculation of the real dose had to consider minimum total filtering efficiency, which is 98% for the A2P3 filter used [8,9].
Analysis of samples
Fibreglass filters and pads: The sample was spiked with internal standard (2-amino-2',5-dichlorobenzophenone), dried for 60 min. and extracted with three 10 ml portions of ethyl acetate in a mechanical shaker for 10 min. Pooled extracts were evaporated to dryness in a rotary evaporator at 30°C. The residue was made up with hexane and analysed by GC/MS.
Hand-wash liquid: 10 ml of sample (or different volumes according to sample concentration), spiked with internal standard (2-amino-2',5- dichlorobenzophenone) was evaporated to dryness in a rotary evaporator at 30°C. The residue was made up with 1 ml hexane and analysed by GC/MS.
Gloves: The sample, spiked with internal standard (2-amino-2',5- dichlorobenzophenone), was dried for 60 min. and extracted with three portions of 200, 100 and 100 ml ethyl acetate by contact for 30 min. at room temperature. Pooled extracts were evaporated to dryness in a rotary evaporator at 30°C. The residue was made up with hexane and analysed by GC/MS.
Leaves: Dislodgeable foliar residues (DFR) were obtained washing the sample twice with 25 ml of 0.01% dioctyl sodium sulphosuccinate solution and then with 25 ml water. Pooled wash solution was spiked with internal standard (2-amino-2',5-dichlorobenzophenone) and extracted three times with 30 ml ethyl acetate. Pooled extracts were dried with anhydrous sodium sulphate and evaporated to dryness in a rotating evaporator at 30°C. The residue was made up with hexane and analysed by GC/MS.
Statistical analysis
Statistical analysis of the data was performed using the Stat View statistical package (5.0 Power PC Version, SAS Institute Inc.). Parametric tests (linear regression analysis) were applied to data obtained during re-entry to evaluate the relation between airborne concentrations of Buprofezin (personal air samples) and facial contamination (pads) or the anterior thigh contamination (pads on top of overalls). Similarly, analysis of variance of the data, in relation to variables worker and day, was applied to personal air samples, hand washing liquids, glove contamination, skin contamination (under overalls) and overalls contamination. Significance was set at alpha=0.05.
Results
Spraying
Table 2 shows the data for hand contamination after spraying, expressed as absolute values and in relation to grams of active ingredient used during treatment. Analysis of hand wash liquid before treatment showed concentrations of Buprofezin below the detection level (<0.1 μg) and 0.18 μg for workers 1 and 2, respectively, on the first day, and 0.28, 0.84 and 0.21 μg for workers 1, 2 and 3, respectively, on the second day. These are extremely low quantities but show that before spraying, the workers’ hands had come into contact with surfaces contaminated with the active ingredient.
The highest hand contamination after spraying was recorded in worker 2 on both days: the practice of wearing new latex gloves under rubber gloves proved very effective in preventing any pesticide accumulating inside rubber gloves from transferring to the hands. On the second day of spraying, contamination of both workers was lower than on the first day, showing that hand spraying devices are associated with less hand contamination than the PulsFog thermal nebuliser. The results of worker 3 on the second day sustain this hypothesis, as contamination was very similar to that of worker 1 on the same day and with the same spraying device.
The concentration of Buprofezin in personal air samples is summarised in Table 2. Airborne concentrations in the respiratory zone in relation to the quantity of active principle dispersed in the greenhouse proved practically the same for the three workers on the second day, when the hand spraying device was used. The results were, however, different for the two workers monitored on the first day. This may be because worker 2 used the PulsFog nebuliser in a different and perhaps not entirely correct way with respect to worker 1.
| Worker | First day | Second day | ||||||
| Contamination of hands | Personal air samples | Contamination of hands | Personal air samples | |||||
| µg | ng/g active ingredient sprayed | µg/m3 | ng/m3/g active ingredient sprayed | µg | ng/g active ingredient sprayed | µg/m3 | ng/m3/g active ingredient sprayed | |
| 1 | 6.33 | 14.7 | 17.9 | 41.7 | 0.598 | 3.48 | 73.5 | 427 |
| 2 | 17.9 | 41.7 | 477 | 1109 | 2.06 | 12 | 79.1 | 460 |
| 3 | - | - | - | - | 0.265 | 2.47 | 53.7 | 499 |
Table 2: Exposure to Buprofezin during spraying: contamination of hands and concentrations in personal air samples.
The results obtained with pads on top of and under overalls are shown in Table 3. Low skin contamination was recorded for the three workers monitored on both days. By contrast, contamination on overalls was not negligible, especially on the first day when spraying was done with the PulsFog nebuliser. Summary analysis of the data already shows good effectiveness of the AGRY CHIMY overalls under the various conditions of use. To assess this aspect more fully we calculated percentage penetration through the overalls in the various anatomical sites.
| Position of pads | Worker | First day | Second day | ||
| Range (ng/cm2) | Range (ng/cm2/g active ingredient sprayed) | Range (ng/cm2) | Range (ng/cm2/g active ingredient sprayed) | ||
| On overalls | 1 | 1.69-1443 | 0.0039-3.36 | 3.06-59.0 | 0.0178-0.343 |
| On skin | 0.204-4.56 | 0.0005-0.0106 | 0.122-14.4 | 0.0007-0.0839 | |
| On overalls | 2 | 138-857 | 0.321-1.99 | 4.10-309 | 0.0238-1.80 |
| On skin | 1.43-9.18 | 0.0033-0.0214 | 0.0612-14.9 | 0.0004-0.0865 | |
| On overalls | 3 | - | - | 6.81-4890 | 0.0634-45.5 |
| On skin | - | - | 0.204-0.633 | 0.0019-0.0059 | |
Table 3: Exposure to Buprofezin during spraying: contamination of skin assessed with pads attached on top of and under overalls in the same positions.
On the first day of the study, when workers 1 and 2 sprayed the green house with the PulsFog nebuliser and wore new overalls, percentage penetration never exceeded 1.2%: in other words, the overalls kept out more than 98% of the active ingredient (mean penetration 0.5%). On the second day, when hand spraying devices were used, the percentage penetration was much higher than on the first day, reaching a peak of 54% on the right posterior thigh of worker 2. The capacity of the overalls to keep out the active principle averaged 94%, but declined to about 90% on the chest and about 70% on the posterior thigh. This comparison is particularly interesting because it shows an opposite trend to that of skin contamination.
Comparing mean penetration on the second day for workers 1 and 2 who wore new overalls and worker 3 who wore overalls that had been used for 5 months, it seems possible to state that although external contamination of the overalls was high for worker 3, penetration was not much different from the case of worker 1, and therefore five months of use had not modified the protective properties of the overalls.
Table 4 shows total potential dermal doses (excluding hands) of individual workers. The data confirms that contamination of overalls was greater for workers 1 and 2 when the PulsFog system was used. The practically identical values of the two workers show that on the first day, skin contamination averaged 23 μg/g of active ingredient sprayed in the greenhouse. A similar estimate is not possible for the case of the hand spraying device, because the contamination levels recorded for the three workers varied more widely. Table 4 shows also the real and absorbed doses of Buprofezin for the different workers on the two days of monitoring. Both doses were below the AOEL and ADI, which are 40 and 10 μg/kg bw, respectively.
| Worker | First day | Second day | ||||||
| Potential dermal doses | Real dose | Absorbed dose | Potential dermal doses | Real dose | Absorbed dose | |||
| µg | µg/g active ingredient sprayed | µg/kg bw | µg/kg bw | µg | µg/g active ingredient sprayed | µg/kg bw | µg/kg bw | |
| 1 | 9624 | 22.4 | 0.456 | 0.186 | 306 | 1.78 | 0.352 | 0.151 |
| 2 | 10023 | 23.3 | 1.21 | 0.571 | 900 | 5.23 | 0.486 | 0.203 |
| 3 | - | - | - | - | 17822 | 166 | 0.102 | 0.049 |
Table 4: Dermal doses of Buprofezin during spraying: potential (excluding hands), real and absorbed doses.
The percentage composition of real and absorbed doses is shown in Table 5. For real dose, the greatest fraction came from skin contamination for all workers on both days, though hand and respiratory intake were not negligible. The respiratory route contributed even more to the absorbed dose, namely 27% and 25% for worker 3 and 2, respectively, on the first day.
| Real doses | Absorbed doses | ||||
| % respiratory dose | % hand contamination | % body contamination | % respiratory dose | % dermal dose | |
| worker 1 day 1 | 1.5 | 20.4 | 78.1 | 3.6 | 96.4 |
| worker 2 day 1 | 11.8 | 16.8 | 71.4 | 25.0 | 75.0 |
| worker 1 day 2 | 4.9 | 2.5 | 92.6 | 11.4 | 88.6 |
| worker 2 day 2 | 3.0 | 4.8 | 92.2 | 7.1 | 92.9 |
| worker 3 day 2 | 12.8 | 2.9 | 84.3 | 26.9 | 73.1 |
Table 5: Percentage composition of real and absorbed doses of Buprofezin during spraying.
Re-entry
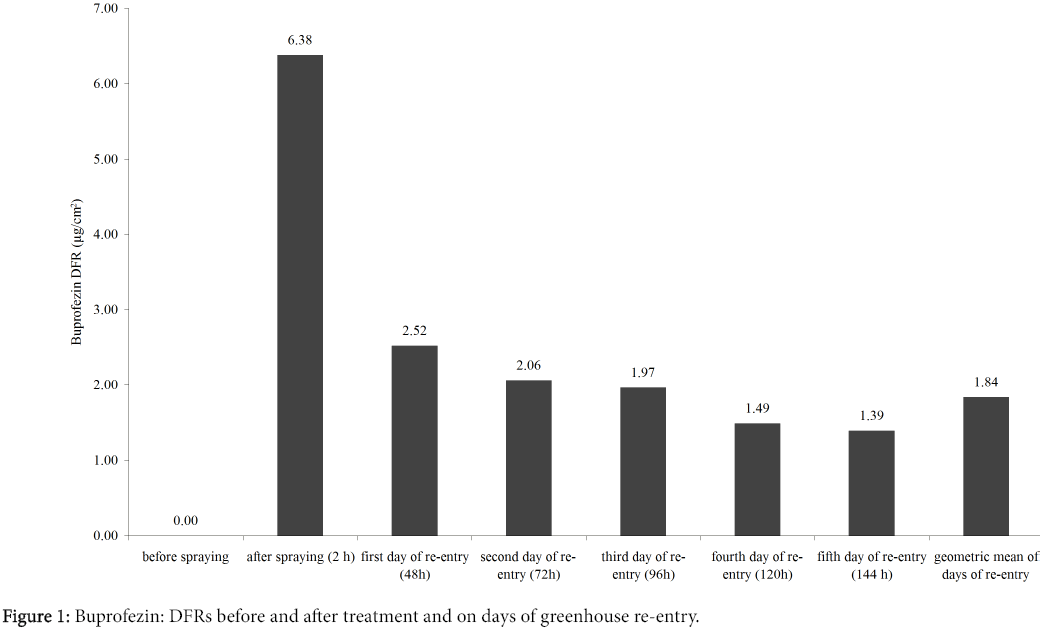
Figure 1 shows DFR of Buprofezin immediately before and after treatment and on the days of greenhouse re-entry. The results provide indications for worker exposure.
Table 6 shows descriptive statistics of Buprofezin concentrations in personal air samples and on pads, as well as quantities of active ingredient in hand wash liquid and on gloves during the working week monitored. Concentrations of the pesticide in personal air samples varied in the range 0.16-2.18 μg/m3 and the maximum value recorded was that of worker 3 on the third day of re-entry. Analysis of variance of the data in relation to the variables worker and day did not show any statistically significant fit to the model and neither variable emerged as significant. The result was expected because the DFR was similar on the different days of re-entry.
| Sample | Mean±SD | CV% | Median | GM | Min-Max |
|---|---|---|---|---|---|
| Personal air samples (µg/m3) | 0.801±0.469 | 59 | 0.616 | 0.693 | 0.172-2.18 |
| Hand wash liquid (µg) | 25.0±30.6 | 122 | 17.7 | 16.6 | 4.03-159 |
| Cotton gloves (µg) | 50.8±34.0 | 69 | 39.5 | 41.3 | 13.4-123 |
| Hand wash liquid + gloves (µg) | 75.8±54.4 | 72 | 57.2 | 60.9 | 19.7-242 |
| Face pads (ng/cm2) | 5.94±11.4 | 193 | 2.63 | 3.08 | 0.438-53.3 |
| Left arm pads (ng/cm2) | 2.22±1.86 | 84 | 1.58 | 1.68 | 0.347-7.41 |
| Right posterior thigh pads on skin (ng/cm2) | 3.35±2.88 | 86 | 1.89 | 2.35 | 0.490-10.5 |
| Left calf pads on skin (ng/cm2) | 5.20±4.28 | 82 | 3.84 | 3.67 | 0.531-17.8 |
| Right shin pads on skin (ng/cm2) | 5.55±3.90 | 70 | 5.21 | 4.2 | 0.469-16.5 |
| Chest pads on skin (ng/cm2) | 0.718±0.674 | 94 | 0.531 | 0.469 | 0.102-2.71 |
| Chest pads on overalls (ng/cm2) | 11.6±10.3 | 89 | 6.69 | 8.7 | 2.96-39.5 |
| Back pads on skin (ng/cm2) | 0.572±0.632 | 110 | 0.418 | 0.361 | 0.0408-2.71 |
| Back pads on overalls (ng/cm2) | 2.29±1.31 | 57 | 1.83 | 2 | 0.980-6.04 |
| Left anterior thigh pads on skin (ng/cm2) | 5.79±3.94 | 68 | 4.21 | 4.63 | 1.80-14.0 |
| Left anterior thigh pads on overalls (ng/cm2) | 23.4±18.5 | 79 | 16.8 | 18.7 | 7.80-80.0 |
| Right forearm pads on skin (ng/cm2) | 3.61±1.93 | 54 | 3.12 | 3.2 | 1.00-9.00 |
| Right forearm pads on overalls (ng/cm2) | 17.2±30.6 | 178 | 5.96 | 7.84 | 2.02-128 |
| GM= geometric mean | |||||
Table 6: Exposure to Buprofezin during stapling.
The quantity of active ingredient in hand wash liquid varied in the range 4.03-159 μg. The quantity of Buprofezin recorded on cotton gloves was on the average about double that measured on hands, showing that cotton gloves under the conditions studied here and/or in the manner used here do not provide good protection. This is also sustained by the fact that in at least one case, glove contamination was less than hand contamination: the maximum value recorded for hands (159 μg) was on the first day of re-entry of worker 1, when only 83.2 μg Buprofezin was detected on the gloves. Analysis of variance of the data in relation to worker and day did not show any statistically significant fit to the model, but worker emerged as significant, showing that individual behaviour affects hand contamination. This result is even more evident if we consider the sum of hand and glove contamination: the data significantly fits the model and worker emerged as a highly significant variable. As already observed for air samples, the variable day did not have a significant effect on the results.
For pads in contact with skin, most contamination was detected in the lower half of the body and on the forearms and arms (Table 6). Mean values from these protected areas of skin were of the same order of magnitude as those from the face, a part of the body not covered by clothing. The chest was the least contaminated part of the body, probably because workers wore another garment, such as a t-shirt, which reduced contamination, under their overalls. Analysis of variance of the skin contamination data (under overalls) in relation to the variables worker and day showed statistically significant fit to the model, with worker as significant, sustaining what we wrote above about the sum of hand and glove contamination. The situation was different for the face pad data, which like that of air samples did not fit the model and neither variable emerged as significant.
As expected, pads worn on top of overalls recorded much greater contamination than the corresponding pads in contact with the skin. The variability of the data as percentage variation (CV%) was generally very high, exceeding 100% for face pads, pads on the back in contact with skin and on the forearm on top of overalls. Analysis of variance of the contamination data for overalls in relation to the variables worker and day did not significantly fit the model and neither variable emerged as significant. This result was expected because the DFR values varied very little from day to day.
Percentage penetration of overalls, measured in the various skin regions of the five workers during the working week monitored, averaged 17.7% (geometric mean), with 5.4% for the chest and 18.1%, 24.7% and 40.8% for the back, thigh and forearm. The low penetration in the chest area was probably due to the fact that workers wore underwear, which while light, could reduce the passage of active ingredient towards the skin. It also seems likely that contamination of forearms is not only due to penetration of overall fabric but also to entry of pesticide at the wrists or direct contamination caused by rolling up the sleeves.
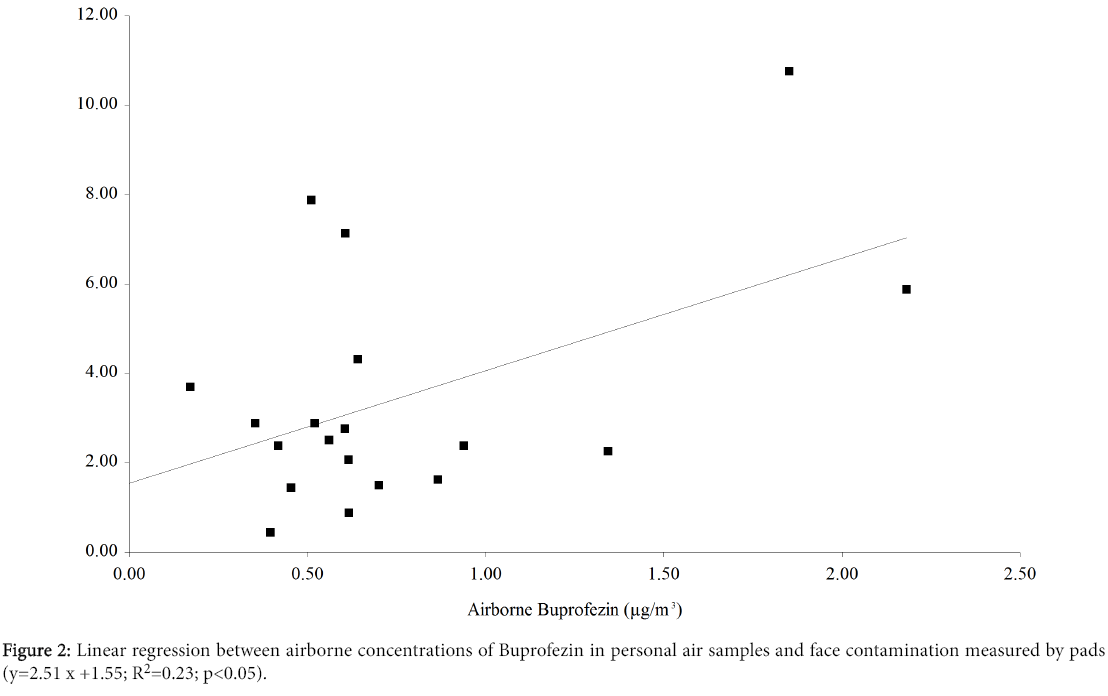
Our hypothesis was that a major determinant of facial exposure (facial skin not covered by clothing) was deposition of airborne particulate. Figure 2 shows the results of linear regression analysis between airborne concentrations of Buprofezin (personal air samples) and facial contamination (pads). The regression was significant, although the variance explained by the model was only about 23%, confirming that other mechanisms also contribute to contamination of facial skin. To obtain a significant correlation we eliminated the facial contamination of 53.31 ng/cm2 recorded on the Wednesday for worker 4.
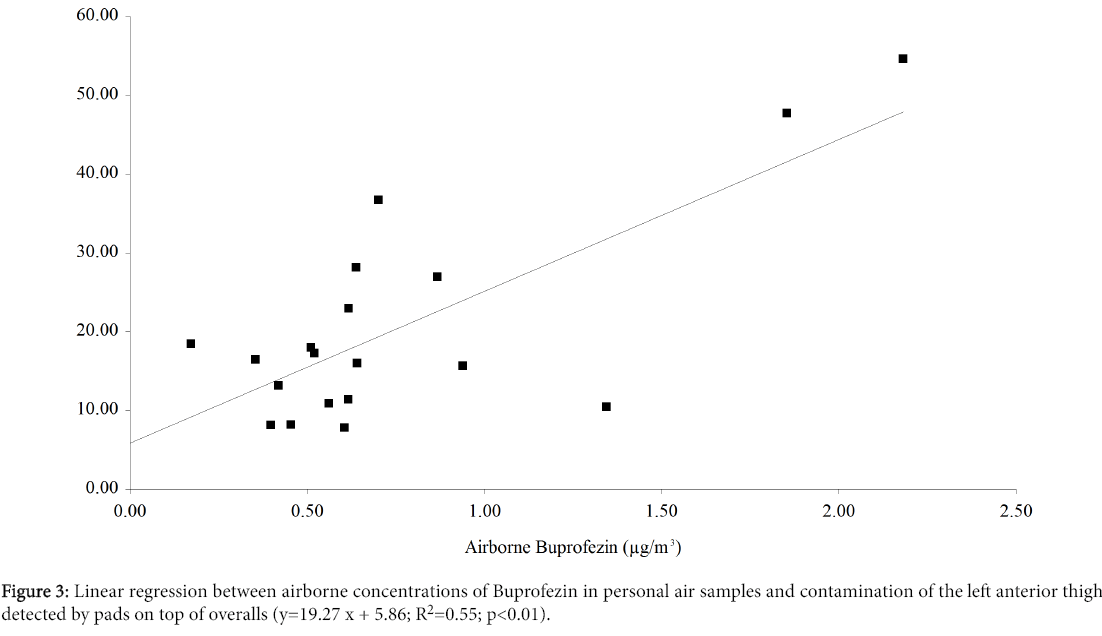
Similarly, Figure 3 shows the results of linear regression analysis between airborne concentrations of Buprofezin and contamination of the anterior thigh (pads on top of overalls). The regression was highly significant and the variance explained by the model was about 55%. Again we eliminated the reading of 80.04 ng/cm2 recorded on the Wednesday for worker 1. The same regression analyses applied to the forearm, chest and back were not significant.
Table 7 shows the total potential dermal doses (excluding hands) recorded for the workers during the week monitored. The data was processed as a whole and divided on the basis of day of re-entry and worker. The highest doses were found for Wednesday after the plants had been watered, which facilitated transfer of pesticide. The highest potential dose was recorded by worker 1, followed by workers 3 and 4. The greater contamination of these workers could be due to more intense working activity (greater number of plants handled per day). The geometric mean of the coefficient of dermal transfer from leaves to hands, namely the ratio of hand contamination (μg/h) to DFR (μg/cm2), was 1.13 cm2/h (1.68 ± 1.71 cm2/h; min-max 0.26-7.88 cm2/h). The similar coefficient of transfer from leaves to cotton gloves had a geometric mean of 4.13 cm2/h (5.15 ± 3.53 cm2/h; min-max 1.25-13.48 cm2/h).
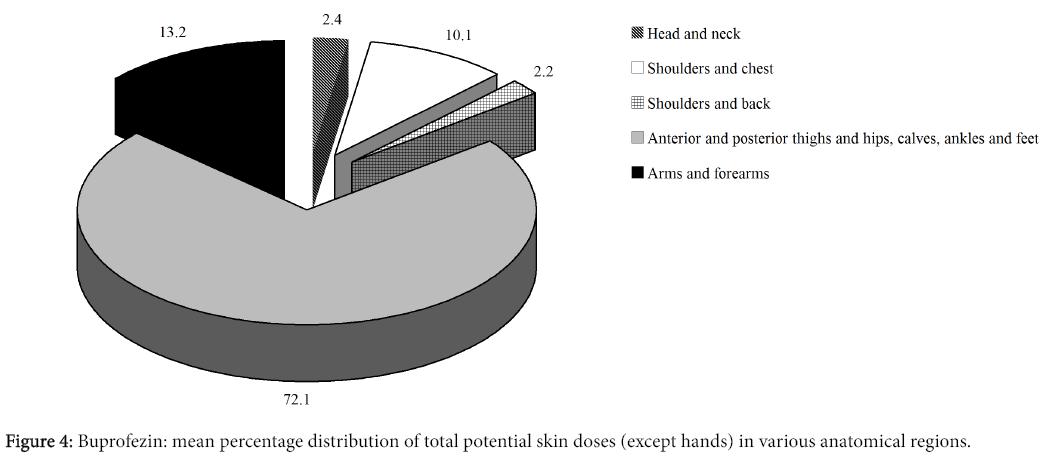
Figure 4 shows the mean percentage distribution of total potential dermal doses (excluding hands) of active ingredient in the various anatomical regions. It can be seen that on average, contamination of the lower part of the body contributed in a preponderant way to potential dermal dose (excluding hands), whereas the head, neck and back contributed in a minor way (about 2%).
Table 7 shows also the real and absorbed doses of Buprofezin for workers engaged in stapling during the working week monitored. The data was processed all together and on an individual worker basis and is expressed in μg/kg bw. Perusal of Table 7 shows that real and absorbed doses were both below the AOEL and ADI which are 40 and 10 μg/kg bw, respectively.
| Mean±SD | CV% | Median | GM | Min-Max | |||||||||||
| P | R | A | P | R | A | P | R | A | P | R | A | P | R | A | |
| AWAD | 250 ± 210 | 1.4 ± 0.77 | 0.61 ± 0.32 | 84 | 55 | 53 | 165 | 1.2 | 0.53 | 199 | 1.2 | 0.54 | 77/944 | 0.41/3.5 | 0.19/1.5 |
| W1 | 482 ± 359 | 2.5 ± 0.86 | 1.1 ± 0.38 | 74 | 35 | 36 | 433 | 2.4 | 1 | 372 | 2.4 | 1 | 120/944 | 1.6/3.5 | 0.66/1.5 |
| W2 | 127 ± 51.0 | 1.4 ± 0.43 | 0.61 ± 0.16 | 40 | 31 | 26 | 118 | 1.4 | 0.59 | 120 | 1.4 | 0.6 | 77/197 | 0.98/1.9 | 0.48/0.80 |
| W3 | 255 ± 149 | 0.79 ± 1.1 | 0.39 ± 0.07 | 59 | 14 | 17 | 204 | 0.83 | 0.39 | 227 | 0.79 | 0.39 | 143/467 | 0.64/0.89 | 0.31/0.47 |
| W4 | 230 ± 135 | 1.5 ± 0.35 | 0.63 ± 0.14 | 59 | 23 | 22 | 192 | 1.6 | 0.65 | 205 | 1.5 | 0.62 | 114/424 | 1.1/1.8 | 0.47/0.75 |
| W5 | 155 ± 51.9 | 0.80 ± 0.38 | 0.35 ± 0.16 | 33 | 48 | 46 | 152 | 0.73 | 0.32 | 148 | 0.73 | 0.32 | 95/221 | 0.41/1.3 | 0.19/0.57 |
| T | 150 ± 40.1 | - | - | 27 | - | - | 152 | - | - | 146 | - | - | 108/211 | - | - |
| WE | 437 ± 315 | - | - | 72 | - | - | 424 | - | - | 354 | - | - | 151/944 | - | - |
| TH | 145 ± 88.2 | - | - | 61 | - | - | 114 | - | - | 129 | - | - | 77/297 | - | - |
| F | 268 ± 175 | - | - | 65 | - | - | 221 | - | - | 233 | - | - | 127/569 | - | - |
AWAD=All workers, all days; W=Worker; P=potential dose; R=real dose; A=absorbed dose; T=Tuesday; WE=Wednesday; TH=Thursday; F=Friday; GM= geometric mean
Table 7: Dermal doses of Buprofezin during stapling: potential (excluding hands) (μg), real and absorbed doses (μg/kg bw).
To better define real and absorbed doses, Table 8 indicates the percentage contributed by hand and body contamination as well as by respiratory input (means of individual workers and global mean). With regard to real doses, the greatest contribution came from body and hand contamination for all workers, although respiratory dose was not negligible (about 15%), and was particularly high for worker 3. The respiratory route contributed even more to absorbed dose, averaging about 30% for worker 3. The greater respiratory contribution in this worker could be due to more intense working activity (a greater number of plants handled during the working shift) which raises more dust.
| Real doses | Absorbed doses | ||||
| % respiratory dose | % hand contamination | % body contamination | % respiratory | % dermal | |
| worker 1 | 4.3 | 28.4 | 67.3 | 10.1 | 89.9 |
| worker 2 | 6.7 | 35.7 | 57.7 | 14.4 | 85.6 |
| worker 3 | 15.4 | 14.3 | 70.4 | 30 | 70 |
| worker 4 | 3.6 | 19 | 77.4 | 8.4 | 91.6 |
| worker 5 | 6.7 | 23.1 | 70.2 | 15.2 | 84.8 |
| mean | 7.3 | 24.1 | 68.6 | 15.6 | 84.4 |
Table 8: Percentage composition of real and absorbed doses of Buprofezin during stapling.
Discussion and Conclusions
The methods of sampling airborne Buprofezin in the present study have also been used by other authors for airborne atmospheric particulate [4]. Evaluation of skin exposure by means of filter paper pads and hand washing with 95% ethanol has likewise been reported in other studies [10-16]. The methods used to extract samples are fast and give good recovery of analyte and good precision within and between series. Gas chromatography with mass detection is sufficiently specific to dispense with the need for purification of extract to eliminate interfering compounds. Limits of detection are sufficiently low to enable detection of analyte in all samples, even under conditions of low exposure such as those investigated in the present study. Sample storage conditions ensure good stability of the active ingredient in all matrices for the time necessary to perform the analyses.
Biological monitoring of exposure to Buprofezin was not performed because no validated biological indicators are yet available for this active ingredient.
Personal respiratory exposure to Buprofezin was evaluated for airborne particulate because a vapour pressure of 1.25 mPa at 15°C [17] made it possible to exclude the possibility of significant amounts of vapour in the work place. The data obtained cannot be compared with other studies on Buprofezin because none has been performed in the occupational environment.
Under the working conditions investigated during spraying and stapling, the pesticide dispersed as aerosol is a source of respiratory exposure and dermal contamination. Contamination of overalls and exposed skin can mostly be ascribed to deposition of airborne particulate, and to a lesser degree to contact with contaminated surfaces in the case of workers engaged in spraying. The presence of active ingredient on pads worn under protective clothing and/or work clothing can be due to penetration of fabric or entry through pores, seams and imperfections.
For workers engaged in spraying, the data demonstrates that penetration of overalls depends on the type of spraying device: although the PulsFog system led to greater external contamination, penetration was greater with individual spray devices. This could be because the micro-aerosol formed by the PulsFog nebuliser does not wet the overalls and little active ingredient penetrates the fabric. On the other hand, larger aerosol droplets from conventional spray devices wet the overalls, making them less protective. We can therefore confirm that the AGRY CHIMY overalls are very effective when pesticide is distributed by the PulsFog system: protection exceeds 98% in all skin areas. The protection afforded by the overalls is much less (about 94%) when conventional spray devices are used. No differences in penetration were found between new and five-month-old overalls. The period of 6 months normally observed by the firm for replacement of overalls is therefore appropriate. The hands of workers engaged in spraying were regularly contaminated by Buprofezin at the end of treatment, but contamination levels were not high and it can therefore be said that hand protection is adequate.
For workers engaged in stapling, dermal exposure was determined by airborne concentrations of Buprofezin and contamination of leaves and other surfaces with which the workers came into contact. Leaves and contaminated surfaces enable mass transfer of pesticide. Similarly, contact with non-contaminated surfaces can lead to decontamination of the corresponding skin region. Clearly these two processes cannot be separated and both influence the dynamic process of dermal exposure [18]. Areas of skin less subject to contact with surfaces are the face and thighs. The women worked between pallets of plants about one metre high and mainly the chest and forearms brushed against the leaves. This was confirmed by the linear trend between concentrations of airborne particulate and contamination of pads on the face and anterior thigh on top of overalls. Hands were regularly contaminated by Buprofezin and hand concentrations contributed about 24% on average to real doses. Cotton gloves worn under latex gloves did not seem to indicate high protection or were not used correctly, since they showed average contamination levels about double those of hands, and in some cases there was more Buprofezin on the hands than on gloves. The coefficients of dermal transfer between hands and leaves and between cotton gloves and leaves were 0.94 cm2/h and 3.43 cm2/h (geometric mean), respectively. These values are much lower than that observed during stapling of Shindapsus treated with Chlorthalonil [13] and Imidacloprid [16]. In the former study on Chlorthalonil, for DFR levels similar to the present ones, hand contamination was much higher, whereas in the latter study with Imidacloprid, hand contamination was of the same order of magnitude as in the present study but DFR was lower. The differences in dermal transfer coefficients may be due to the protection afforded by latex gloves used constantly by the women during their work, and above all to daily replacement of cotton gloves. This was sustained by the fact that hand contamination contributed more than 50% and 80% to real doses of Chlorthalonil [13] and Imidacloprid [16], respectively, against 24% for Buprofezin in the present study.
For workers engaged in stapling, the data demonstrates that the average protection afforded by cotton overalls was about 87%. Another study conducted during re-entry (stapling) in tunnels of ornamental plants previously treated with Imidacloprid showed 13% penetration [16], confirming the mean penetration values of the present study.
For all workers, real and absorbed doses estimated for Buprofezin were below the AOEL and ADI of 40 and 10 μg/kg bw, respectively. The AOEL is defined as the daily exposure level that does not cause adverse effects in persons working regularly with the pesticide for days, weeks or months. The ADI is the quantity of pesticide that can be absorbed per day for a lifetime without manifestation of toxic effects. Although ADI is calculated for the general population exposed through residues in food, it is often used as a benchmark, below which occupational risk is presumably negligible even under conditions of chronic exposure.
The results of the present analysis suggest that risk level is acceptable. Nevertheless, the choice of spraying equipment and protective clothing should be such as to keep exposure as low as possible. For workers engaged in stapling, daily replacement of cotton gloves used in the present study seems to have reduced real exposure in a major way.
The variability of the data obtained under homogeneous exposure conditions in the present study indicates that correct use of protective clothing by workers is essential. It is therefore of fundamental importance to train workers in the use of protective clothing and equipment, and in pesticide handling in general.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- PPDB. Pesticide Properties DataBase. Buprofezin. Accessed April 28, 2015.
- US EPA (2014) Chemicals Evaluated for Carcinogenic Potential Science Information Management Branch Health Effects Division Office of Pesticide Programs U.S. Environmental Protection Agency
- EFSA (2010) Conclusion on the peer review of the pesticide risk assessment of the active substance buprofezin. EFSA Journal 8:1624.
- Borrás E, Sánchez P, Munoz A, Tortajada-Genaro LA (2011) Development of a gas chromatography–mass spectrometry method for the determination of pesticides in gaseous and particulate phases in the atmosphere. Analytica Chimica Acta 699: 57-65.
- Iwata Y, Knaak JB, Spear RC, Foster RJ (1977) Worker reentry into pesticide-treated crops. I. Procedure for the determination of dislodgable pesticide residues on foliage. Bull Environ Contam Toxicol 18: 649-655.
- Popendorf WJ, Leffingwell JT (1982) Regulating OP pesticide residues for farmworker protection. In: Residue Reviews, residue of pesticides and other contaminants in the total environment. Vol. 82 New York: Springer Verlag 125-201.
- Du Bois D, Du Bois E (1916) A formula to estimate the approximate surface if height and weight be known. Clinical calorimetry, tenth paper. Arch Intern Med 863-871.
- EN 143:2000 and A1:2006. Respiratory protective devices - Particle filters - Requirements, testing, marking.
- EN 149:2001 and A1:2009. Respiratory protective devices - Filtering half masks to protect against particles - Requirements, testing, marking.
- Aprea C, Sciarra G, Sartorelli P, Mancini R, Di Luca V (1998) Environmental and biological monitoring of exposure to mancozeb, ethylenethiourea, and dimethoate during industrial formulation. J Toxicol Environ Health 53: 263-281.
- Aprea C, Sciarra G, Sartorelli P, Ceccarelli F, Centi L (1999) Multiroute exposure assessment and urinary metabolites excretion of fenitrothion during manual operation on treated ornamental plants in greenhouses. Arch Environ Contam Toxicol 34: 490-497.
- Aprea C, Sciarra G, Lunghini L, Centi L, Ceccarelli F (2001) Evaluation of respiratory and cutaneous doses and urinary excretion of alkylphosphates by workers in greenhouses treated with omethoate, fenitrothion and tolclofos-methyl. Am Ind Hyg Assoc J 62: 87-95.
- Aprea C, Centi L, Lunghini L, Banchi B, Forti MA, et al. (2002) Evaluation of respiratory and cutaneous doses of chlorothalonil during re-entry in greenhouses. J Chromatogr B Analyt Technol Biomed Life Sci 778: 131-145.
- Aprea C, Centi L, Santini S, Lunghini L, Banchi B, et al (2005) Exposure to omethoate during stapling of ornamental plants in intensive cultivation tunnels: influence of environmental conditions on absorption of the pesticide. Arch Environ Contam Toxicol 49: 577-588.
- Aprea C, Terenzoni B, De Angelis V, Sciarra G, Lunghini L, et al. (2005) Evaluation of skin and respiratory doses and urinary excretion of alkylphosphates in workers exposed to dimethoate during treatment of olive trees. Arch Environ Contam Toxicol 48: 127-134.
- Aprea C, Lunghini L, Banchi B, Peruzzi A, Centi L, et al. (2009) Evaluation of inhaled and cutaneous doses of imidacloprid during stapling ornamental plants in tunnels or greenhouses. J Expo Sci Environ Epidemiol 9: 555-569.
- Kidd H, James DR Eds (1991) The agrochemicals handbook, third edition. Royal Society of Chemistry Information Services, Cambridge, UK.
- Schneider T, Vermeulen R, Brouwer DH, Cherrie JW, Kromhout H et al. (1999) Conceptual model for assessment of dermal exposure. Occup Environ Med 56:765-773.
Citation: Aprea MC, Centi L, Lunghini L, Banchi B, Bracalente G, et al. (2016) Monitoring and Control of Exposure to Buprofezin in Greenhouses. Toxicol Open Access 2: 117. DOI: 10.4172/2476-2067.1000117
Copyright: © 2016 Aprea MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12227
- [From(publication date): 10-2016 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 11346
- PDF downloads: 881