Research Article Open Access
Molecular Pharmacology of Antihistamines in Inhibition of Oxidative Burst of Professional Phagocytes
| NosáГ?Вѕ R*, Drábiková K, JanГ?ВЌinová V and PereГ?ВЌko T | |
| Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences, Slovakia | |
| *Corresponding Author : | NosáГ?Вѕ R Institute of Experimental Pharmacology and Toxicology Slovak Academy of Sciences, Dubravska 9 4104 Bratislava, Slovakia Tel: +421-2-59410442 E-mail: Radomir.Nosal@savba.sk |
| Received February 25, 2014; Accepted March 16, 2014; Published March 21, 2014 | |
| Citation: NosáГ?Вѕ R, Drábiková K, JanГ?ВЌinová V, PereГ?ВЌko T (2014) Molecular Pharmacology of Antihistamines in Inhibition of Oxidative Burst of Professional Phagocytes. Biochem Physiol 3:129 doi:10.4172/2168-9652.1000129 | |
| Copyright: © 2014 NosáГ?Вѕ R et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Antihistamines of the H1 and H3/H4 groups interfere with oxidative burst of human professional phagocytes. Of H1 antihistamines, the most effective was dithiaden suppressing oxidative burst of whole human blood and chemiluminescence of isolated neutrophils both at extra- and intracellular level. Generation of free oxygen radicals in isolated neutrophils resulted most probably from the inhibition of proteinkinase C activation, as demonstrated for dithiaden. The differences in H1 antihistamines to inhibit oxidative burst of professional phagocytes correlates with their physicochemical properties, particularly with partition coefficient. The potentiation of recombinant caspase-3 by dithiaden is supportive of the antiinflammatory effect of dithiaden similarly to H1 antihistamines, by increasing the apoptosis of professional phagocytes. Of the H3/H4 antihistamines, the most effective was JNJ 7777 120 followed by JNJ 1019 1584. Thioperamide was less effective and it slightly potetntiated generation of free oxygen radicals. Their results demonstrated H1 antihistamines to be much more potent in the inhibition of free oxygen generation in human professional phagocytes as compared with the H3/H4 antihistamines investigated. This finding should be taken into account therapeutically.
| Keywords |
| Antihistamines; Histamine receptors; Oxidative burst |
| Introduction |
| Antihistamines represent a wide range of medicinal drugs and experimentally used chemicals with defined affinity to four histamine receptors. In addition to their specific activity [1-4] in different tissues and organs, antihistamines possesses many pharmacological side effects resulting from their non-specific activities [5]. H1 antihistamines of the 1st and 2nd generation, DithiadenR and LoratadineR, inhibited platelet aggregation by interfering with platelet phospholipase A2, [6], decreased the release of GM-CSF and IL-8 in granulocyte-macrophage colony [7], and dose-dependently decrease polymorphonuclear leukocyte chemiluminescence and aggregation [8]. Moreover, H1 antihistamines were found to improve ischaemia/reperfusion injury in rat mesenteric microcirculation by suppressing oxidative burst of blood phagocytes, particularly neutrophils [9,10]. Differences in the effect of H1 antihistamines on oxidative burst and nitric oxide production of professional phagocytes depend on their physico-chemical properties and are related to the dissociation rate at acidic pH [11-13]. On the other hand, pheniramines differ in suppressing the generation of reactive oxygen species of human professional phagocytes as a result of halogenisation [14] corresponding the inclusion complexes of betacyclodextrin with pheniramine and its halogenated derivative [15]. |
| Histamine H3 receptors act as presynaptic autoreceptors that inhibit the synthesis and release of histamine in histaminergic neurones in the central nervous system (CNS). They also occur as heteroreceptors on nonhistaminergic neurones, modulating the release of other neurotransmitters such as 5-hydroxytryptamine, dopamine, acetylcholine, noradrenaline and GABA in the CNS and periphery. Ligands for the H3 receptor have been reviewed [16,17]. |
| The H4 receptor is preferentially expressed in various cells of the immune system and mast cells and induces the chemotaxis of, for example, eosinophils and mast cells. It has also been identified on lymphocyte T cells, dendritic cells and basophils. It is suggested that the H4 receptor is involved, along with the H2 receptor, in the control of IL-16 release from human lymphocytes, and it has been speculated that an H4-selective antagonist might be useful in helping to treat asthma. Antagonists, such as JNJ 7777120 have also been reported to be effective in various models of inflammation [18,19]. |
| In this study the effect of dithiaden, loratadine, pheniramine, brompheniramine and chlorpheniramine was compared with that of JNJ 7777 120, JNJ 1019 1584 and thioperamide on oxidative burst of human whole blood and isolated neutrophils in vitro. |
| Materials and Methods |
| Chemicals |
| Luminol, isoluminol, PMA (phorbol-4β-12β-myristate-13α- acetate), Ca2+-ionophore A23187, superoxide dismutase, dextran (average MW 464,000), zymosan A (from Saccharomyces cerevisiae), luciferase (from firefly Photinus pyralis) and D-luciferin sodium salt from Sigma-Aldrich Chemie (Deisenhofen, Germany). HRP (horseradish peroxidase), catalase and Folin–Ciocalteu’s phenol reagent were purchased from Merck (Darmstadt, Germany) and lymphoprep (density 1.077 g/ml) from Nycomed Pharma AS (Oslo Norway), human purified caspase-3 was from Enzo Life Sciences, Lausen, Switzerland. All other chemicals used were of analytical grade and obtained from commercial sources. |
| The phosphate buffered saline solution (PBS) used in this study contained 136.9 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 1.8 mM CaCl2 and 0.5 mM MgCl2× 6H20 and had a pH of 7.4. Tyrode’s solution used in this study consisted of 136.9 mM NaCl, 2.7 mM KCl, 11.9 mM NaH2CO3, 0.4 mM NaH2PO4 × 2H20, 1 mM MgCl2 × 6H20 and 5.6 mM glucose, pH of 7.4. |
| Antihistamines |
| Dithiaden: 4-[3-(dimethylamino)propylidene]-4,9- dihydrothieno[2,3-b]benzo[e]thiepin, Zentiva, Czech Republic, |
| Loratadine: ethyl 4-(8-chloro-5, 6-dihydro-11H-benzo[5,6] cyclohepta [1,2-b]pyridin-11-ylidene)-1-piperidinecarboxylate, Zentiva, Slovak Republic; |
| Brompheniramine: 3-(4-bromophenyl)-N,N-dimethyl-3-pyridin- 2-yl-propan-1-amine, Wyeth, Austria; 4.2.4 Chlorpheniramine: 3-(4-chlorophenyl)-N, N-dimethyl-3-pyridin-2-yl-propan-1-amine, GLAXO Smithkline, Great Britain, |
| Pheniramine: N,N-dimethyl-3-phenyl-3-pyridin-2-yl-propan-1- amine, Hoechst, Germany, |
| JNJ-7777120: 5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]- 1H-indole and JNJ 10191584: 1-[(5-Chloro-1H-benzimidazol-2-yl) carbonyl]-4-methylpiperazine maleate, Sigma-Aldrich; |
| Thioperamide: N-Cyclohexyl-4-(imidazol-4-yl)-1- piperidinecarbothioamide maleate, abcam Biochemicals. |
| Blood collection and neutrophil separation |
| Fresh human blood was obtained at the blood bank by venepuncture from healthy male volunteers (20–50 years) who had not received any medication for at least 7 days. It was anticoagulated with 3.8% trisodium citrate (blood : citrate ratio = 9:1). The Ethical Committee license for blood sampling at the National Transfusion Service NTS-KRA/2012/ SVI was registered. Human neutrophils were isolated from whole blood, as described previously [20,21]. The blood was gently mixed and erythrocytes were allowed to sediment in 3% dextran solution by centrifugation (10×g, 25 min, 22°C). The neutrophil suspension (3 mL) was layered on Lymphoprep (3 mL) and centrifuged (500×g, 30 min, 22°C). After hypotonic lysis and centrifugation (500×g, 10 min, 22°C), the neutrophils were resupended in Ca2-Mg2-free PBS. After counting, they were adjusted to a final concentration of 105 cells/μL (Coulter Counter, Coulter Electronics, England) and kept on ice. The final suspension of neutrophils contained more than 96% of viable cells, as evaluated by trypan blue exclusion and was used within 2 h, as long as the control chemiluminescence remained constant. |
| Chemiluminescence (CL) assay of whole blood and isolated neutrophils |
| The oxidative burst in whole blood was stimulated with phorbol myristate acetate (PMA 0.05 μM). CL was measured in 250 μL samples consisting of 50 μL aliquots that contained blood (50× diluted), luminol (200 μM), antihistamines (AH; 0.01–100 μM) and phosphate buffer [20]. Horseradish peroxidase (HRP 8 U/mL) was added to the system and maintained a sufficient extracellular peroxidase concentration. The effect of AH on extra- and intracellular ROS production was measured in unstimulated and PMA- (0.05 μM) stimulated neutrophils (5×105 per sample) by isoluminol/luminol-enhanced CL. Extracellular CL was determined in the system containing isoluminol (5 μM) and HRP (8 U/ml). Intracellular CL was measured with luminol in the presence of extracellular scavengers – superoxide dismutase (100 U/ml) and catalase (2000 U/ml) [22]. The CL of both whole blood and isolated neutrophils was evaluated in a microplate luminometer Immunotech LM-01T (Czech Republic) at 37°C. The data were based on integral values of CL over 3600 s (whole blood) or 1800 s (isolated neutrophils) (RLU×s, RLU = relative light units). |
| Recombinant caspase-3 activity |
| To determine the caspase-3-activity, a modified method was applied [23]. Briefly, the cleavage of the Z-DEVD-amino-luciferin substrate by caspase releases amino-luciferin. The following reaction with luciferase was detected by CL. The light production was measured in the Luminometer Immunotech LM-01T. According to the manufacturer’s instructions, 10 μL of 0.1 IU caspase was added to 20 μL aliquots of different DIT concentrations and buffered solution. Finally, 50 μL of Caspase-Glo 3/7 Reagent was added and the mixture was measured for 60 min to determine caspase-3 activity. |
| Protein kinase C activation |
| Phosphorylation of protein kinase (PKC) isoenzymes α and βII was detected [20]. Isolated human neutrophils (5×106) were incubated at 37°C with DIT for 1 min, stimulated with PMA (0.15 μM, 1 min) and lysed by the addition of solubilisation buffer. After sonication on ice, samples were centrifuged to remove unbroken cells, the supernatant was boiled for 5 min with sample buffer and samples were loaded on 9.8% SDS polyacrylamide gels. Proteins were separated by electrophoresis, transferred to Immobilon-P Transfer Membrane (Millipore Corp., USA). From the two strips taken, one was detected for PKC and the second for β-actin, which represented the internal control. Membrane strips were blocked for 60 min with 1% bovine serum albumin in Tris buffered saline. This was followed by 60 min incubation in the presence of the Phospho-PKC α and βII (Thr638/641) antibody (rabbit antihuman, 1:8000, Cell Signaling Technology) or β-actin Antibody (rabbit anti-human, 1:4000, Cell Signaling Technology, Danvers, MA, USA). The membranes were subsequently washed six times with TBS and incubated 60 min with the secondary antibody conjugated to horseradish peroxidase (anti-rabbit from donkey, 1:10,000, Amersham, UK). The activity of horseradish peroxidase was visualised using Enhanced Chemiluminiscence Western Blotting Detection Reagents (Amersham, UK), followed by autoradiography. The optical density of each PKC band was corrected by the optical density of the corresponding β-actin band. |
| Statistical analysis |
| Data represent the mean ± SEM, unless stated otherwise. Statistical analysis was performed using the ANOVA paired test to examine differences between the treatments and control. Differences were considered to be statistically significant when p < 0.05 (*) or p < 0.01 (**). |
| Results |
| Figure 1 shows the dose-response effect of histamine (HIS), dithiaden (DIT), loratadine (LOR), phenyramine (PE), brompheniramine (BPA) and chlorpheniramine (CPA) on whole blood chemiluminescence stimulated with PMA. In the concentration of 1 μM, dithiaden decreased the CL by 19 percent. In the concentration of 10 μM dithiaden, phenyramine, bromphenyramine and clorphenyramine decreased the CL by 60, 12, 38 and 20 percent, respectively. Increase of the concentration of H1 antihistamines to 100 μM resulted in inhibition of CL with dithiaden, loratadine, phenyramine, bromphenyramine and chlorphenyramine by 96, 98, 13, 97 and 61 percent, respectively. Histamine was ineffective in any concentration used. |
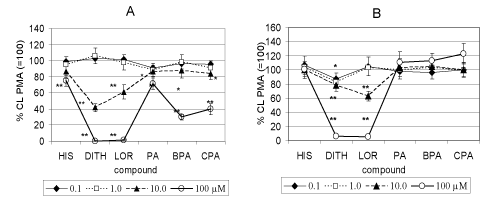
| The effect of the H1 antihistamines studied on extra- and intracellular CL of isolated human neutrophils stimulated with PMA is demonstrated in Figure 2. In concentrations of 0.1 and 1 μM any of the compounds tested decreased extracellular CL (Figure 2A). In 10 μM concentration DIT, LOR PA, BPA and CPA decreased stimulated CL by 58, 40, 17, 15 and 18 percent respectively. DIT and LOR completely inhibited extracellular CL, PA, BPA and CPA decreased CL by 30, 70 and 60 percent, respectively. Histamine decreased CL by 22 percent. |
| Figure 2B demonstrates that HIS, PA, BPA and CPA did not affect stimulated intracellular CL by any concentration used. Dithiaden decreased CL in the concentration of 0.1 to 100 μM by 9, 18, 38 and 96 per cent, respectively. LOR in concentrations of 10 and 100 μM was effective by 39 and 97 percent, respectively. |
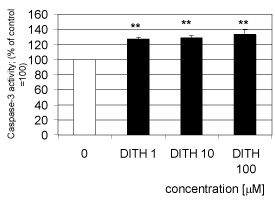
| Dithiaden increased significantly the activity of human recombinant caspase 3 starting at the concentration of 1 μM (Figure 3). The caspase-3 activity increased with dithiaden in concentrations of 1, 10 and 100 μM by 25.2, 29.8 and 32.3 per cent of the control value, respectively. |
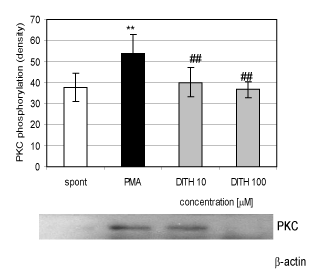
| Stimulation of isolated human neutrophils with PMA (0.15 μM) resulted in the increase of proteinkinase αβII by 34 percent (Figure 4). Dithiaden in concentrations of 10 and 100 μM decreased stimulated phosphorylation by 21 and 22 per cent, respectively. |
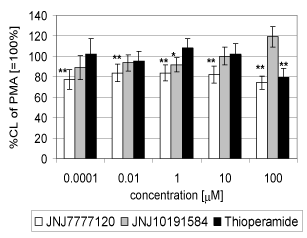
| Figure 5 demonstrates the effect of H3/H4 antihistamines JNJ 7777120, JNJ 10191584 and thioperamide on whole human blood chemiluminescence stimulated with PMA (0.05 μM). Compound JNJ 7777 decreased the CL with all concentration used by 23 to 17.5 percent of the control value without any concentration-dependent relationship. Compound JNJ 1019 and thioperamide were ineffective. |
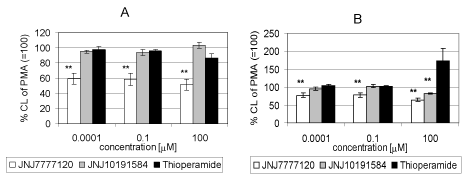
| Compound JNJ 7777 120 decreased significantly extracellular CL in the concentration range of 1 nm and 100 μM by 39.8 to 48.4 percent of the control value, respectively. Compound JNJ 10191584 and thioperamide had no effect on stimulated chemiluminescence. (Figure 6a). Concerning the effect of the compounds tested on intracellular CL, JNJ 7777 decreased CL in the concentration range of 1 nM , 0.1 and 100 μM to 76.4, 77.0 and 68.3 percent of the control value, respectively Thioperamide strongly potentiated stimulated intracellular CL to 170.8 percent of the control value (=100%) (Figure 6b). |
| Discussion |
| The tested H1 antihistamines of the 1st generation DIT and pheniramines and of the 2nd generation LOR decreased CL of human whole blood. The most effective was DIT starting to decrease CL at 1 μM concentration and significant differences were found between the drugs tested. Histamine did not affect stimulated whole blood CL. The difference between particular drugs resulted rather from physicochemical properties of particular drugs than from their specificity to affect histamine receptors [12]. This was also demonstrated for H1 antihistamines in inhibiting platelet aggregation and inhibition of ischaemia/reperfusion damage of vessels [9,10]. In pharmacological non-toxic concentrations, H1-antihistamines significantly inhibited nitrite accumulation that was not caused by the scavenging ability of drugs against nitric oxide, measured amperometrically. The degree of inhibition of nitrite accumulation positively correlated with the degree of tested lipophilicity, measured by reversed-phase thin layer chromatography. Furthermore, H1-antihistamines differentially modulated the iNOS protein expression [11,12]. This was confirmed also for the ability of the H1 antihistamines tested to interfere with free oxygen generation measured extracellularly. All the compounds tested dose-dependently decreased the CL of stimulated human neutrophils extracellularly in the rank order of potency DIT=LOR >BPA>CPA> PA=HIS. |
| The difference between pheniramines migh t also result from their halogenisation as demonstrated by inhibition of oxidative burst of blood phagocytes during ischaemia/ reperfusion [9,10,14]. Inhibition of free oxygen radical generation by H1 antihistamines intracellularly (Figure 2B) could result from their ability to interfere with regulatory pathways responsible for oxidative burst in neutrophils. DIT decreased proteinkinase C activation in PMA-stimulated neutrophils, indicating its interference with oxidative burst in neutrophils. This finding might contribute also to the mechanism of antioxidative effect of DIT in whole blood phagocytes and isolated neutrophils. Similar results were demonstrated in human gastric adenocarcinoma and Caski cells [24,25] and for the polyphenolic compound N-feruloylserotonin and resveratrol in human neutrophils [21,26]. |
| Thus DIT may interfere with modulation of intracellular signalling pathways involved in down-regulation of COX-2 and iNOS expression and NF-κB activation, as demonstrated for resveratrol [27,28]. |
| DIT in all concentrations tested (1 to 100 μM) increased the activity of recombinant caspase in vitro. Caspase-3 belongs to the effector group of caspases, which are responsible for the executive phase of apoptosis [29]. Caspase activation from their pro-caspase form has been widely described in cells undergoing apoptosis, including neutrophils. Programmed cell death – apoptosis – is an important process for successful removal of recruited neutrophils. Neutrophils express high levels of the pro-apoptotic proteins [30]. Activation of recombinant caspase-3 by DIT might thus contribute to the inhibition of oxidative burst in human neutrophils resulting from suppression of protein kinase C activation and to the antiinflammatory effect of DIT. A similar effect was described for natural stilbene derivatives on human neutrophils [31]. |
| In comparison with the H1 antihistamines tested, only compound JNJ 777120 expressed suppression of oxidative burst in whole blood and in isolated neutrophils both at extra- and intracellular sites. Interestingly, this effect was not concentration dependent. Compound JNJ 10191584 in 100 μM concentration potentiated CL in whole blood, yet on the other hand it inhibited CL intracellularly. Thioperamide was effective in the same concentration in whole blood but potentiated CL intracellularly. |
| The antihistamines tested showed marked differences in the inhibition of oxidative burst in human blood and isolated neutrophils. It is evident from our results that H1 antihistamines are much more potent in inhibiting stimulated chemiluminescence as compared with H3/H4 antihistamines. |
| Acknowledgements |
| This work was supported in part by the project APVV-0052-10, APVV-0315-07 of the Slovak Research and Development Agency and by the grant VEGA 2/0010/13. The authors wish to thank Professor Magda Kouřilova for correcting the English of the manuscript. |
References
|
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 16013
- [From(publication date):
June-2014 - Jul 16, 2025] - Breakdown by view type
- HTML page views : 11367
- PDF downloads : 4646
