Opinion Article Open Access
Molecular Mechanisms Involved in the Immune Control of the Malignant Disease Process
Arlen M*, Arlen P, Coppa G, Tsang K, Conte C, Crawford J, Molmenti E, Saric O and Doubokovskiy A
Department of Surgery and Pathology, Division of Surgical Oncology Northwell Health System, Hofstra College Medicine and Precision Biologics, USA
- *Corresponding Author:
- Myron Arlen
Co ordinator Surgical Oncology
North Shore/LIJ Medical Center
Surgical Oncology, 450 Lakeville Rd
Lake Success, NY 11042, USA
Tel: 1-516-482-1200
E-mail: marlenmd@msn.com
Received Date: August 12, 2017; Accepted Date: August 25, 2017; Published Date: September 1, 2017
Citation: Arlen M, Arlen P, Coppa G, Tsang K, Conte C, et al. (2017) Molecular Mechanisms Involved in the Immune Control of the Malignant Disease Process. J Mol Immunol 2:111.
Copyright: © 2017 Arlen M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Molecular Immunology
Abstract
In order to control Cancer effectively, it is essential to define the role of the host immune system in responding to the presence of growing tumour. For the most part the immune system is virtually oblivious to the existence of malignant cells developing within an organ. Understanding how immune reactivity can be activated to recognize a malignant process and the mechanism of how growing tumour cells can be eliminated immunologically is of major importance to the clinician.
Keywords
Malignant disease; Growing tumour; IgG1; ADCC; Apoptosis
Introduction
In defining the potential action of the cytotoxic T cell component of the immune system, we have noted that such cells act mainly as bystanders in the presence of a cancerous lesion while actively attacking acquired bacterial and viral disease processes. We have been able to show that under the right circumstances, when host immunity is activated to target a tumour, it is via the expression of IgG1 derived from the B cell component of the immunocytes. Understanding the interplay that exists via the mechanism of IgG1 inducing antibody dependent cell cytotoxicity, (ADCC), and tumour destruction secondary to the presence of IgG1 is an important concept. This can only help in developing those immunotherapeutic agents essential for use by the clinician. Developing agents that enhance T cell activity such as checkpoint inhibitors are only partially effective in reaching the needed clinical responses for effective tumour control.
It is apparent from clinical observations that a competent immune system existing in the host has the capability of controlling for the most part, the presence of foreign invading cells. This includes organisms such as bacteria, fungi and viruses. In contrast to the host’s ability to destroy most foreign invaders, the onset of a malignant disease process continues to progress from its inception, unabated, and without immune host intervention, until metastasis eventually appears.
On evaluation of the protein composition of the malignant lesion, the same immunologic characterization found in the infectious invader is also present in the cancer cell. The immunologic difference that can be found in the cancer cell vs. the benign invaders relates to the existence of below threshold levels of the immunogenic that characterizes the tumour in contrast to other foreign cell types. The presence of tumour immunogen can be easily identified in a similar manner as one does for benign foreign invading cells at the time they enter the host. The question arising for the immunologist of course is, as to the origin of this malignant immunogenic protein, one that characterizes a known tumour system, how the immunogenic becomes effective in controlling tumour growth, and ways to incorporate its presence in designing newer therapeutic approaches for control of disease.
When invading infectious cells are challenged by the immune system a specific immunogen is noted to be present for each of these foreign cells. They are characterized at that threshold level necessary for the immunocytes to recognize these foreign invaders and allow for a destructive process to take place. As a result one can note the elimination of such invading cells.
A similar concept has prevailed in the analysis of the malignant lesion, one that appears to explain why tumour growth and progression invariably occurs and is rarely eliminated if ever from its developing field. It appears that for all lesions examined to date, that the necessary level of expression of tumour antigen in the form of an immunogenic protein that presents to the host is far below that level needed for recognition and subsequent destruction by the immune system. It explains why, with the failure of the immune system to recognize a transforming premalignant cell, that progression continues in the growth pattern of the tumour cell until invasion of surrounding tissue and eventual metastasis is seen.
The tumour immunogen, while suboptimal in its expression, arises in the earliest premalignant state of the tumour growth. It seems to arise and then be expressed in a field effect initiated thru viral or carcinogenic transformation of the underlying cells such as the colonocytes of bowel. The resulting immunogen found in the mature tumour cell is oncofetal in origin resulting from a post translational modification of the existing oncofetal protein [1]. One notes that in the presence of a metastatic lesion that does not appear to be affected by the hosts immune system, that one frequently sees infiltrating lymphocytes dispersed throughout the lesion (tumour infiltrating lymphocytes or TIL cells). It would be hard to conceive that their presence as many still believe is a result of the body’s defence system to destroy the lesion. Scale up of such immune cells from the tumor to date has failed to effectively control metastatic lesions when these immune cells are then delivered IV to patients. If such cells did represent a host attack on the existing metastatic lesion then this process should have occurred at the time of conversion from the insitu to the fully malignant cell. There is some suggestion that under electron microscopy that examination of such immune system cells reveals them to serve as transporters of tumour cells to the blood supply feeding the tumour and as such result in progression to further the metastatic process.
Discussion
The above observations do not rule out the concept that the host, under proper circumstances, can utilize its immune system to destroy tumour cells as it does when it effectively recognizes other forms of foreign invaders. That mechanism associated with immune destruction of the tumour cell, in contrast to other cells invading the host, is clearly defined. It can now be readily utilized in its approach to the elimination of tumour systems via immunotherapy alone and in combination with chemotherapy
The approach toward cancer management via the host’s immune system was first originated in the 1950’s by Prehn [2]. He utilized animal models on an experimental basis to confirm that when a threshold level of antigen is present in the host or delivered intradermally, that the immune system can be turned on effectively to destroy malignant transplants given to animal models. These experiments were used to prove that cancer cells do express such immunogens, but at a suboptimal level.
Hollingshead at George Washington Univ. was, at a later time, in the 70’s and 80’s, able to define the presence of such immunogenic proteins in tumours removed at the time of surgery, from an array of cancer specimens [3]. She employed pooled allogeneic protein derived from human operative specimens as the source of antigenic material. Membrane protein derived from such specimens was used to help define the threshold level of a specific tumour antigen necessary to effectively turn on the immune system of the host so that destruction of the malignancy could then be noted.
During the 1980’s several clinical trials were established to define and characterize that the immunogenic antigen comprising the array of membrane proteins found to be expressed in the tumour, could be employed therapeutically [4]. These proteins when fully isolated were then fractionated and eventually skin tested to see which group initiated a delayed hyper sensitivity response (DHR). In its partially purified form, the protein was tested in animal systems challenged with human cancer. The tumour associated antigen (TAA) so tested, was found capable of exciting the hosts immune system to recognize existing tumour antigen and for the first time, attack the cancer. This now indicated that there was the potential for the host’s immunocytes to be employed as a means of eliminating tumour from the host.
For clinical use, it was necessary to define antigen levels in their purified form. Employing the Hollingshead tumour protein that had been derived from the pooled allogeneic antigen samples, it was found that to reach an effective dose that one required the delivery of approximately 500-1000 gums of this immunologic protein. Patients with lung and colon cancer as well as malignant melanoma were tested for reactivity and then treated for the existing tumour. The individuals so treated by the intradermal injection of protein purified by Sepahadex gel followed by isoelectrophoresis showed an overall improvement in survival.
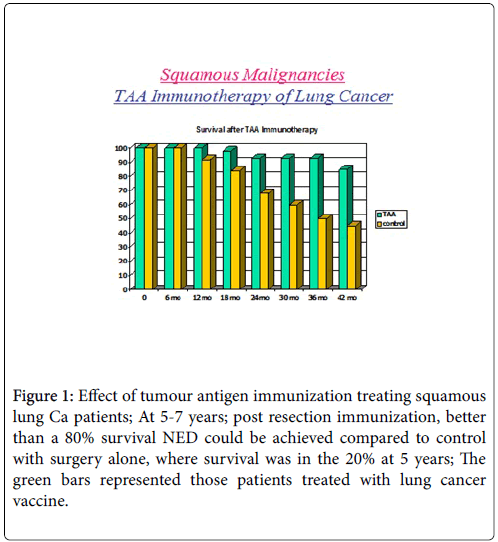
We were able to demonstrate that without chemotherapy or immunotherapy that the survival period, 5 years post-surgical resection, ranged from 20-30%. These patients did not receive any adjuvant immune or chemotherapy. If the proper therapeutic dose of vaccine was delivered one month post-surgery then survival approximated 70-90% over a 5-7 year period post resection Figure 1.
Figure 1: Effect of tumour antigen immunization treating squamous lung Ca patients; At 5-7 years; post resection immunization, better than a 80% survival NED could be achieved compared to control with surgery alone, where survival was in the 20% at 5 years; The green bars represented those patients treated with lung cancer vaccine.
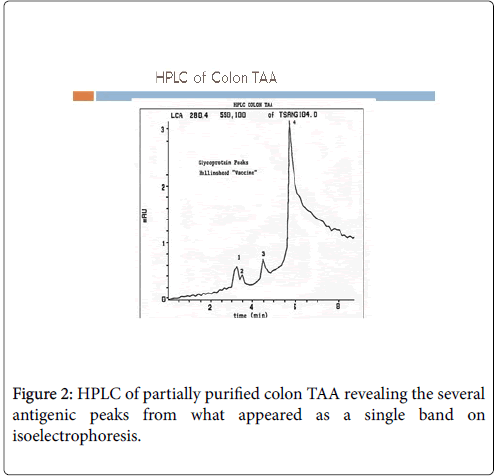
In order to produce such a therapeutic product (Antigen/Vaccine) for commercial purposes, the FDA was concerned that any potential host tumour specimen used in the pooled allogeneic preparation might possibly be contaminated with viruses such as HPV, HIV or Hepatoma To proceed with additional studies they now required that the necessary immunogenic molecule or molecules be sequenced and a recombinant antigen be produced for further vaccine studies. In order to further purify antigen as needed for sequencing, antibodies to the single band seen on electrophoretic separation were produced. As anticipated, at least 3 functional monoclonals were isolated and then developed for use in preparing a colon cancer vaccine. On HPLC, 3-4 peaks could be noted from the colon cancer partially purified antigen, explaining why several antibodies were derived from the antigen preparation Figure 2 [5].
At this point the question raised was why no higher than 80-90% long term survivals could be achieved by immunization in the original vaccine trials using pooled allogeneic antigen. Here after immunization 10-20% of the patients recurred, showing no evidence of response to the therapeutic protocol. Following immunization with the allogeneic protein, all patients responded with both a cellular and humoral response targeting the tumor. In roughly 10-20% of the patients, when recurrence was noted, there was almost complete failure to define a persistent humoral response. This humoral response appeared to be mediated through the production of an IgG1 antibody specific to tumour antigen. In the past, most believed that tumour destruction was cell mediated via CD8 cytotoxic T cells. We were now seeing evidence that even with an effective T cell response that recurrence could occur and be related to failure of the B cell system to initiate an effective IgG1 response.
Three distinct monoclonal antibodies derived from the partially purified colon antigen appeared to attack various combinations of the 3 immunogens expressed in colon cancer. They were now studied in detail. Hybridomas were produced in CHO cells with the human Fc attached to the hybridoma Fab’s. In each case the mechanism of tumour destruction was clearly demonstrated to occur via Antibody Dependent Cell Cytotoxicity (ADCC) [6]. This initial work was carried out using the initial group of monoclonal antibodies that we had developed. We essentially ruled out cell mediated destruction by the hosts CD8 cells. In this study it was demonstrated that ADCC was significant when employing the partially purified antigen we obtained from Hollinshead to produce the antibodies. In these studies it was also demonstrated for the first time that recombinant IL-6 enhanced the antitumor response of human peripheral blood mononuclear cells [7]. The assay utilized a 4 h. chromium release assay. It was recognized that the PMNC cells functioned by providing CD16 NK cells that allowed their attachment to the therapeutic monoclonal antibody at a receptor for the CD 16 cells on the Fc component of the effective monoclonal. Since the mechanism proved to be ADCC, it was apparent that to effectively bind to a sufficient number of NK cells that mAb delivery required a systemic intravenous approach rather than intra-arterial (intra lesion) delivery to a site where metastatic tumour was present.
In planning for clinical trials with the monoclonal antibodies, it was decided that those patients to be treated would be colon and pancreatic cancer patients having undergone previous treatment and among those having failed all standard forms of therapy that intravenous monoclonal antibody would be employed. We did not attempt to utilize partially purified protein in the form of a vaccine. If tumor antigen in the form of a genetically engineered peptide vaccine were to be utilized, it would take several months for the appearance of proper titers of mAb to appear and for an effective ADCC response to be noted.
Tumor Control via Antibody Dependent Cell Cytotoxicity
The term (ADCC), which is also referred to as antibody-dependent cellular cytotoxicity, has proven to be a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively, that is the NK cell, lyses the target cell, whose membranesurface antigens have been bound by specific antibodies such as those developed in our labs at Precision Biologics. NK cell lysis is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection. Of course should the monoclonal antibody recognize tumour immunogen, even at sub clinical levels, delivery of the NK cell to the tumor surface via the mAb can easily result in tumour destruction.
ADCC is independent of the immune complement system that also lyses targets but does not require any other cell. ADCC requires an effector cell which classically is known to be natural killer (NK) cells that typically interacts with IgG antibodies. However, macrophages, neutrophils and eosinophils can also mediate ADCC, such as the eosinophil killing of certain parasitic worms known as helminths via IgE antibodies.
The typical ADCC mechanism involves activation of NK cells by the antibodies. An NK cell expresses Fc receptors, mostly CD16. These receptors recognize, and bind to the Fc portion of an antibody, such as IgG, which has bound to the surface of a pathogen-infected target cell during the course of therapy. The most common Fc receptor on the surface of an NK cell is called CD16 or FcγRIII. Once the Fc receptor binds to the Fc region of IgG, the Natural Killer cell releases cytotoxic factors that cause the death of the target cell. Our studies have indicated that NO is one of the factors released by the NK cell to begin its process of cell tumour destruction. In several cases we have found recurrence of tumour so treated, appearing 10 years past the initial therapeutic approach to the tumour. Here we believe that NO (nitric oxide) synthase is released in the tumour field, initiating a process of dormancy.
The typical ADCC mechanism involves activation of NK cells by the antibodies. An NK cell expresses Fc receptors, mostly CD16. These receptors recognize, and bind to the Fc portion of an antibody, such as IgG, which has bound to the surface of a pathogen-infected target cell during the course of therapy. The most common Fc receptor on the surface of an NK cell is called CD16 or FcγRIII. Once the Fc receptor binds to the Fc region of IgG, the Natural Killer cell releases cytotoxic factors that cause the death of the target cell. Our studies have indicated that NO is one of the factors released by the NK cell to begin its process of cell tumour destruction. In several cases we have found recurrence of tumour so treated, appearing 10 years past the initial therapeutic approach to the tumour. Here we believe that NO (nitric oxide) synthase is released in the tumour field, initiating a process of dormancy.
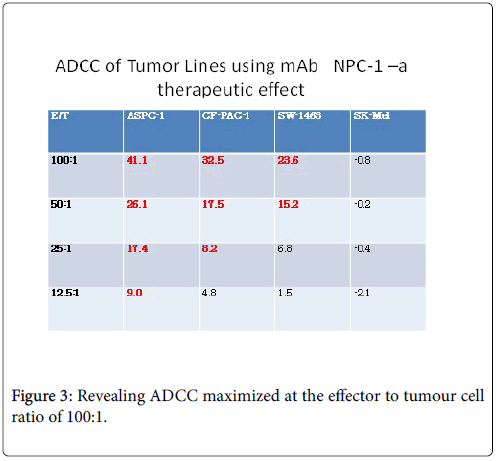
The effects against solid tumours of trastuzumab and rituximab monoclonal antibodies have been shown in experiments with mice to involve ADCC as an important mechanism of therapeutic action. In the clinic, the FcgRIII 158V/F polymorphism interferes with the ability to generate ADCC responses in vitro during trastuzumab treatment [8]. To be effective one must be able to demonstrate at least a 30-40% ADCC response Figure 3.
Usually, a target cell line expressing a certain surface-exposed antigen is incubated with antibody specific for that antigen. After washing, effector cells expressing Fc receptor CD16 are co-incubated with the antibody-labelled target cells. Effector cells are typically PBMCs (peripheral blood mononuclear cell), of which a small percentage are NK cells (Natural Killer cell); less often they are purified NK cells themselves. Over the course of a few hours a complex forms between the antibody, target cell, and effector cell which lead to lysis of the cell membrane of the target. If the target cell was pre-loaded with a label of some sort, that label is released in proportion to the amount of cell lysis. Cytotoxicity can be quantified by measuring the amount of label in solution compared to the amount of label that remains within healthy, intact cells.
The classical method of detecting this is the Chromium-51 [51Cr] release assay; the Sulfur-35 [35S] release assay is a little used radioisotope-based alternative. Target cell lysis is determined by measuring the amount of radiolabel released into the cell culture medium by means of a gamma counter or scintillation counter. A variety of non-radioactive methods are now in widespread use. Fluorescence-based methods include such things as direct labelling with a fluorescent dye like calcein or labeling with europium that becomes fluorescent when released Eu3+ binds to a chelator. Fluorescence can be measured by means of multi-well fluorometers or by flow cytometry methods. There are also enzymatic-based assays in which the contents of the lysed cells includes cellular enzymes like GAPDH that remain active; supplying a substrate for that enzyme can catalyze a reaction whose product can be detected by luminescence or by absorbance.
In carefully examining the total picture of tumour destruction by monoclonal antibody, a number of additional mechanisms associated with ADCC have been noted by us. The ability to apply each of these mechanisms alone or with ADCC appears to be a possible effective approach for tumour control. The advantage of the use of monoclonal antibody infusion in the patient with advanced and recurrent tumour, especially those having failed standard chemotherapy, is in the rapidity of attack on the tumour, this response could be seen in approximately 6-8 h. after delivery of the specific mAb whereas a comparable vaccine from which the mAb was derived needed months to become effective in the level of antibody production needed to reach proper serum titers. Those titers have been demonstrated to remain at therapeutic levels in the serum for many years.
Antibodies bound to malignant target cells along with Fc receptors expressed by cytotoxic cells are the major factors of ADCC. IgG and IgA can trigger ADCC by binding specifically to FcγR and FcαR, respectively. Clinically we have determined that the IgG1’s play the major role in controlling the malignancy following immunization with a specific immunogenic protein. ADCC mechanisms that lead to target cell death vary depending on effector cells that are recruited by antibodies.
In most cases, human‐activating Fcγ receptors comprise an IgG binding α chain associated with a transducing subunit, a γ chain (γγ) homodimer. In NK cells, the presence of a γ/ζ heterodimer or of a ζ/ζ homodimer has also been reported (the ζ chain is also part of the T cell receptor (TcR) complex). The associated γ or ζ chain contains an ITAM (immuno‐receptor tyrosine‐based activating motif) that is phosphorylated upon crosslinking of FcγR. Human FcγRIIa is the only activating receptor where the IgG‐binding α chain intracellular domain contains an ITAM. NK cells express activating FcγRIIIa, although the expression of activating FcγRIIc and of inhibitory FcγRIIb by small NK cell subsets has also been reported. FcγRIIIa is also expressed by some T cells. FcγRIIIb, a GPI‐linked surface receptor expressed by neutrophils is not presented. In mouse, there is no FcγRIIa and inhibitory FcγRIIb is referred only as FcγRII. In contrast, there is another activating FcγR, termed FcγRIV, also associated with a γ chain homodimer.
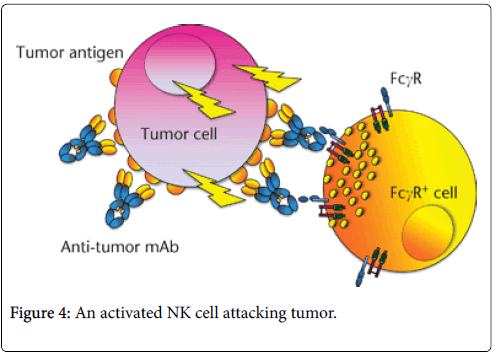
Schematic view of ADCC is noted above in Figure 4. The binding of an IgG antitumor mAb to the target cell (here a tumor cell) allows the recruitment and cross linking of activating FcγR expressed by effector cells (NK cells, neutrophils, etc.). In humans, activating receptors include FcγRI (CD64), FcγRIIa (CD32) and FcγRIIIa (CD16). Effector cells are then activated and release molecules that will lead to the death of tumor cells.
The diagram in Figure 4 represents an NK cell containing granules that have polarized towards the contact zone with tumor cell once FcγRIIIa (CD16) has been engaged and cross linked. The granule content (perforin and granzyme B) is then released in the close vicinity of the tumor cell (NK cell ‘synapse’), initiating an apoptotic process that will lead to cell death. In all of the cases examined the major component of tumor destruction has been the NK cell and not the cytotoxic CD8 cell.
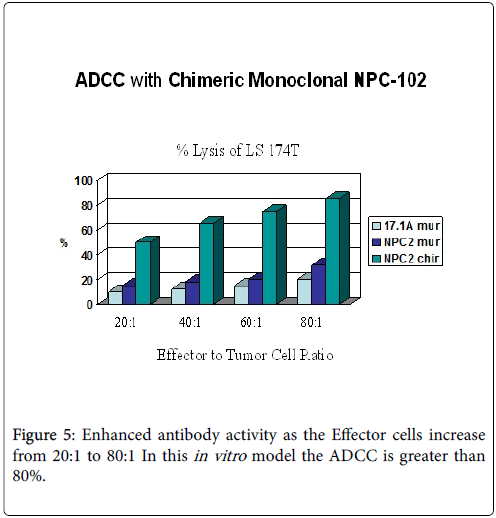
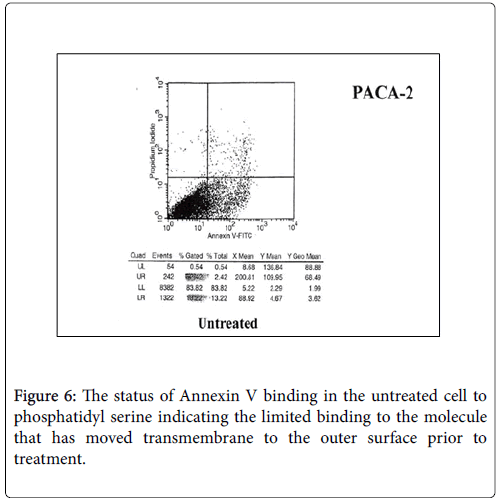
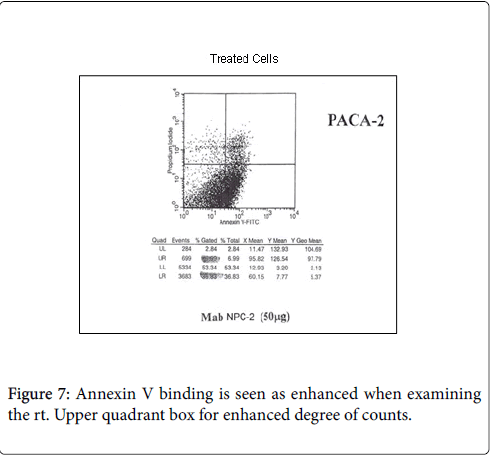
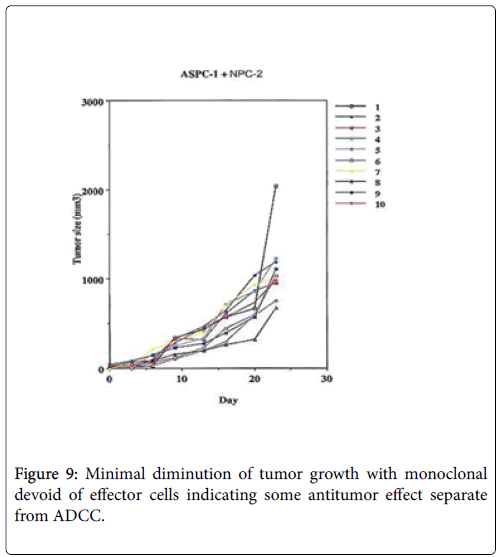
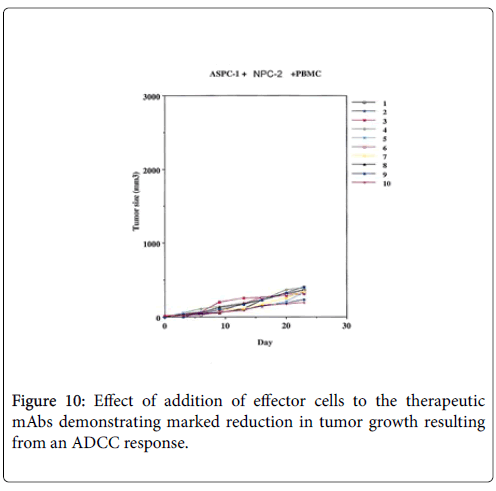
While we had noted that a major effect in patients receiving therapeutic vaccines was the initiation of ADCC, Figures 5-7. We also found that in many instances that a certain degree of Annexin V binding representing an Apoptotic effect (9) had taken place via infusion of the therapeutic mAb. Apoptosis in general refers to programmed cell death which inadvertently was identified while evaluating for the mechanism for the ADCC response resulting from our infusion with our monoclonals. We were looking to see if there was any relationaship of an existing apoptotic effect associated with the delivery of our antibodies and did come across the fact that such did occur but to a much lesser extent. The effect appeared to arise from Trail ligation by the monoclonal antibody and could be measured by Annexin V binding.
Annexin V binding
As noted above, Annexin V binding [9,10] has been found to accompany many situations where ADCC is the primary mechanisn of a therapeutic antibody response. Annexin A5 (or annexin V) is a cellular protein in the Annexin group. In flow cytometry, Annexin V is commonly used to detect apoptotic cells by its ability to bind to phosphatidylserine, a marker of apoptosis. Normally the phosphatidylserine is located on the inner surface of the tumor membrane. With apoptosis the membrane becomes permeable allowing the serine molecule to migrate to the surface of the cell where it is then detected by Annexin V binding. When the degree of ADCC was measured, Annexin V binding was secondarily examined for tumor destruction.
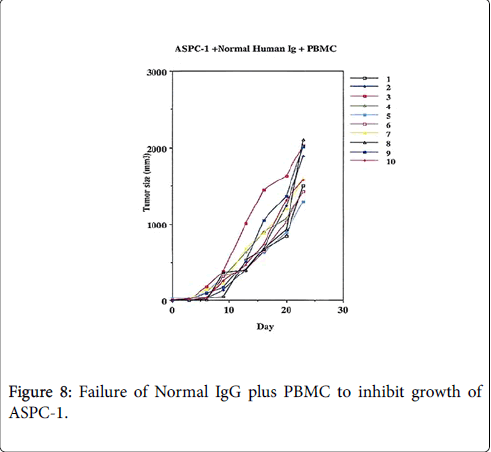
An experimental model demonstrating the degree of tumor destruction defined when normal human IgG is employed in 10 animals, then when therapeutic mAb is employed without effector cells and then when the effector antibody is given with PBMC Figures 8-10.
The function of the membrane protein that migrates to the cell surface during apoptosis is unknown; however, Annexin A5 has been proposed to play a role in the inhibition of blood coagulation by competing for phosphatidylserine binding sites with prothrombin and also to inhibit the activity of phospholipase A1. These properties have been found during in vitro experiments. Annexin A5 is used as a nonquantitative probe to detect cells that have expressed phosphatidylserine (PS) on the cell surface, an event found in apoptosis as well as other forms of cell death. Platelets also expose PS and PE on their surface when activated, which serves as binding site for various coagulation factors. The Annexin A5 affinity assay typically uses a conjugate of Annexin V and a fluorescent or enzymatic label, biotin or other tags, or a radioelement, in a suitable buffer (Annexin V binding to aminophospholipids is Ca2+ dependent). The assay combines Annexin V staining of PS and PE membrane events with the staining of DNA in the cell nucleus with propidium iodide (PI) or 7- Aminoactinomycin D (AAD-7), distinguishing viable cells from apoptotic cells and necrotic cells. Detection occurs by flow cytometry or a fluorescence microscope. When destruction occurs as a reflection of what we detect with the Annexin V assay the mechanism is reflected in the development of the apoptosome.
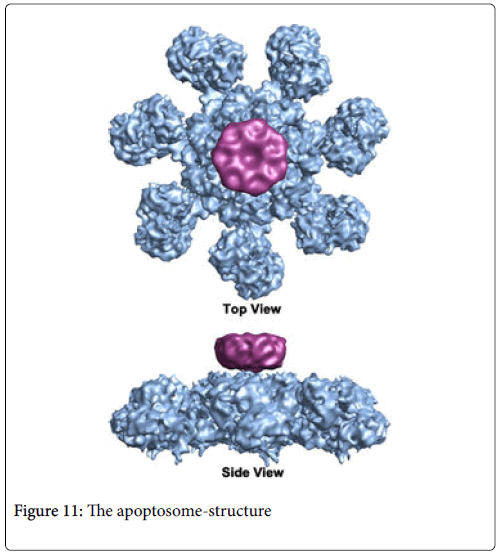
The apoptosome is a large quaternary protein structure formed in the process of apoptosis. Its formation is triggered by the release of cytochrome c from the mitochondria in response to an internal (intrinsic) or external (extrinsic) cell death stimulus. In leading to the process of apoptosis in contrast to that of ADCC the Caspases are one of the main molecules initiated in this apoptotic process which occurs as a secondary immunologic mechanism at least for the destruction of the cancer cell [11]. Caspases are a family of endoproteases that provide critical links in cell regulatory networks controlling inflammation and cell death [12]. The activation of these enzymes is tightly controlled by their production as inactive zymogens that gain catalytic activity following signaling events promoting their aggregation into dimers or macromolecular complexes. Activation of apoptotic caspases results in inactivation or activation of substrates, and the generation of a cascade of signaling events permitting the controlled demolition of cellular components. Dysregulation of caspase sequences underlies human diseases including cancer. It probably arises secondarily to the monoclonal responses that not only binds to the NK cell but secondarily ligates to TRAIL as has been suggested when we were examining the ADCC response to our therapeutic monoclonals as well as the effect of Annexin V binding [13]. These enzymes are represented by an array of genes important for maintaining homeostasis through regulating cell death and inflammation.
These endoproteases have been found to hydrolyze peptide bonds in a reaction that depends on catalytic cysteine residues in the caspase active site and occurs only after certain aspartic acid residues in the substrate. Although caspase-mediated processing can result in substrate inactivation, it may also generate active signaling molecules that participate in ordered processes such as the apoptotic event that occurs with malignancy.
The Caspase enzymes are the central components of the apoptotic response. The apoptotic caspases are generally divided into two classes: the initiator caspases, which include caspase-2, -8, -9 and -10 and the effector caspases, which include caspases-3, -6 and -7. Caspase 8 represents the apical caspase in the TNF death receptor pathway [14]. Caspase 9 serves as the apical caspase of the mitochondrial pathway. All caspases are produced in cells as catalytically inactive zymogenes and must undergo proteolytic activation during apoptosis. Activation of effector caspases is carried out by an initiator caspases . Once activated, the effector caspases are responsible for the proteolytic cleavage of a broad spectrum of cellular targets, which ultimately leads to cell death as seen within the cancer cell system.
The intrinsic pathway is mediated by mitochondria. In response to apoptotic stimuli several proteins, such as cytochrome c, are released from the intermembrane space of mitochondria into the cytoplasm. Cytochrome c binds to and activates the protein APAF1 in the cytoplasm that allows APAF1 to bind to ATP/dATP and to form the apoptosome, which mediates activation of caspase-9, thereby triggering a cascade of caspase activation. The extrinsic pathway is initiated by binding of an extracellular death ligand, such as FasL and TNF-alpha, to its cell-surface death receptor, such as FasR and TNFR. Adaptor protein FADD, TRADD, RIDD transmits an activating signal to effector caspases caspase-2, -8, and -10. Caspase-10 can function independently of caspase-8 in initiating Fas- and tumor necrosis factor-related apoptosis-inducing ligand-receptor-mediated apoptosis [15].
The apoptosome as noted in Figure 11. is a large quaternary protein molecule formed in the process of apoptosis. Its formation is triggered by the release of cytochrome c from the mitochondria in response to an internal (intrinsic) or external (extrinsic) cell death stimulus. Stimuli can vary from DNA damage and viral infection. In mammalian cells, once cytochrome c is released, it binds to the cytosolic protein Apaf-1 to facilitate the formation of an apoptosome. An early biochemical study suggests a one -to-one ratio of cytochrome c to apaf-1 for apoptosome formation. Once formed, the apoptosome can then lead to the destruction of the malignant lesion. This results initially in membrane permeability and shifting of components of the inner surface of the membrane i.e. phosphatidylserine to its outer surface where it can then be detected by Annexin V binding [16].
Conclusion
Under normal circumstances, the host immune response to a developing malignant lesion is rarely effective as a result of sub thresh hold levels of tumour immunogen. When the threshold level of tumour antigen is delivered in the form of a vaccine, the major response targeting the tumour is via antibody dependent cell cytotoxicity. We have also seen evidence of TRAIL (TNF-related apoptosis-inducing ligand) membrane receptors interacting with our mAb, resulting in an apoptotic process functioning in concert with the initial ADCC mechanism. Here, when apoptosis does develop, Annexin V binding can now be measured as a reflection of membrane permeability secondary to the development of the apoptosome, a molecule designed to initiate apoptosis within the cell. In combination we believe that the proper antibody given with an NK cell stimulant such as IL- 15 will result in the responses expected. This process can be further enhanced when low dose chemotherapy is then administered to minimize release of inhibitory molecules from the tumour that interferes with immune reactivity targeting the tumour for final destruction.
References
- Arlen M, Arlen P, Coppa G, Crawford J, Deutsch G, et al. (2017) Rapid Immunohistochemistry of resected bowel margins at the time of Colectomy: Defining the presence of pre-malignant cells undetected by H&E. J Vaccine 1: 5-9.
- Prehn R (2007) Methylcholanthrene Induced Metastasis. Mol Oncology 1: 263-264.
- Hollinshead A, Elias G, Arlen M, Mosley MS, Scherrer J (1985) Specific Active Immunotherapy in Patients with Adenocarcinoma of the colon using TAA. Cancer 56: 480-489.
- Arlen M, Tsang K, Wang T (1998) Immunotherapy of Colon Cancer Using Chimeric mAb 31.1 Crit. Rev in Immunol 18: 133-138.
- Arlen M, Arlen P, Tsang K, Wang XP (2010) The Therapeutic Value of Monoclonal Antibodies Directed against Immunogenic Tumour Glycoproteins J Cancer 1: 209-222.
- Pearson GR, Arber W, Henle W, Hofschneider P, Klein J, et.al. (1978) In Vitro- and In Vivo investigations on Antibody Dependent Cellular Cytotoxicity. Contempt Top Microbiol and Immunol 80: 65-96.
- Lida S, Kuni-Kamochi R , Katsuhiro M (2009) Two Mechanisms of the enhanced Antibody-Dependent Cellular Cytotoxicity (ADCC) efficacy of non-fucosylated therapeutic antibodies in human blood. BMC Cancer 58: 1471-2407.
- Collins DM, O’Donovan N, McGowan PM (2012) Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann Oncol 23: 1788-1795.
- Reed JC (2000) Mechanisms of Apoptosis. Am J Pathology 157:1415-1430.
- Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. (2016) Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harbor Protoc.
- Ried SJ, Shi Y (1998) Molecular mechanisms of caspase regulation during apoptosis. Nature reviews. Mol cell biol 5: 897-907.
- Thornberry N , Lazebnik Y (1998) Caspases: Enemies within, Science 281: 1312-1316.
- Genderen H (2008) Annexin A5: Functions of phosphatidylserine-binding and two-dimensional crystallization: Biochimica et Biophysica Acta (BBA) – Molecular Cell Research 1783: 953-963.
- Muzio M, Stockwell BR, Stennnicke HR (1998) An induced proximity model for caspase 8 activation. J Biol Chem 273: 2926-2930.
- Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ (2001) Caspase-10 is an initiator caspase in death receptor signaling. Proceedings of the National Academy of Sciences of the United States of America 98: 13884-13888.
- Feng Y, O'Connell PJ (1998) Apoptosis detection by Annexin V binding: a novel method for the quantitation of cell-mediated cytotoxicity. J Immunol Methods 217: 61-70.
Relevant Topics
- Bacteriostatic antibiotics
- Cell signaling and activation
- Chemokines
- Class I MHC molecules
- Class II MHC molecule
- Colitis Antibiotics
- Immune response
- Immunochemistry
- Immunogenicity of biopharmaceuticals
- Immunogenomics
- Immunoglobulins
- Immunoglycomics
- Immunomodulatory xenobiotics
- Immunopharmacology
- Immunoproteomics
- Immunosenescence
- Immunotolerance
- Molecular Immunology
- Non classical MHC class I molecules
Recommended Journals
Article Tools
Article Usage
- Total views: 13899
- [From(publication date):
October-2017 - Jul 16, 2025] - Breakdown by view type
- HTML page views : 12833
- PDF downloads : 1066