Molecular Imaging for Post-Operative Spinal Infection Diagnosis
Received: 06-Apr-2024 / Manuscript No. JIDT-24-131568 / Editor assigned: 08-Apr-2024 / PreQC No. JIDT-24-131568 (PQ) / Reviewed: 23-Apr-2024 / QC No. JIDT-24-131568 / Revised: 30-Apr-2024 / Manuscript No. JIDT-24-131568 (R) / Published Date: 07-May-2024 DOI: 10.4172/2332-0877.1000588
Abstract
Post-operative spinal infection is a serious and dreaded complication faced by spine surgeons all over the world. Statistically postoperative infections have been decreasing in the last decades. Although infrequent, post-operative spinal infections are still an important source of increased morbidity, mortality and health care cost. Early diagnosis can be challenging. Biopsy sample cultures may be negative due to technical difficulties and perioperative antibiotics. Post-operative biochemical markers may be abnormal, and MRI (Magnetic Resonance Imaging) images (the gold standard for imaging spondylodiscitis) are usually hard to interpret early after surgery and in the presence of implanted hardware. The optimal imaging tool for post-operative spinal infection remains to be established. Molecular metabolic imaging of infection has been the focus of intense interest in preclinical and clinical settings and now plays an important role in the diagnosis of musculoskeletal infections. Ubiquicidin, an antimicrobial 59-amino acid peptide, is part of our immune system. Its cationic fragment, (UBI 29-41) binds preferentially to the anionic membrane of microbial cells. Tc99 Pertechnate is a common technetium radiopharmaceutical used in molecular imaging. Technetium (99mTc) labelled UBI, (99mTec UBI 29-41) can be imaged by Single-Photon Emission Computed Tomography (SPECT), providing anatomic correlation for the metabolic image with clear delineation between structures, potentially differentiating between infectious site and sterile inflammation. The aim of this review is to evaluate the use of this molecular imaging modality in the setting of post-operative spinal infection.
Keywords: Post-operative; Spine infection; Molecular imaging; SPECT; 99mTec; UBI 29-41
Introduction
A post-surgical spine infection is a serious medical condition that requires multi-disciplinary care. Current infection reported rates vary between 1% and 4% [1-4]. Higher rates of infection have been reported in extensive procedures and when instrumentation hardware is used [5,6]. Although infrequent, a post-surgical spinal infection is a very serious complication that entails increased morbidity, mortality, prolonged hospital stays and substantial increase in health care costs [7].
When a post-surgical spinal infection is suspected, a clear diagnosis must be rapidly established, and appropriate antibiotic treatment immediately administered. Early and accurate diagnosis is crucial to avoid or diminish neurological sequela. One third of post-spinal infection patients suffer from residual spinal dysfunction [8].
Even if the offending microorganism is detected and appropriate antibiotic treatment administered, the response to treatment may remain uncertain. Since the discal space is avascular and hardware instrumentation may have been used, intravenous antibiotics may not adequately reach the surgical site. It is important to determine if additional debridement surgery and/or hardware removal are necessary to resolve the infectious process [9].
Ideally, a post-surgical spine infection is diagnosed early, based on clinical suspicion, neuroimaging, blood workup and the identification of the causal organism. The gold standard for diagnosis is a positive culture from the infected site that identifies the microorganism and the anti-biogram [10].
In the normal clinical setting, the diagnosis of an early post-surgical spine infection remains elusive. Positive cultures have low yield due to pre and post-surgery antibiotics, technical difficulties reaching the appropriate site and long incubation periods [10].
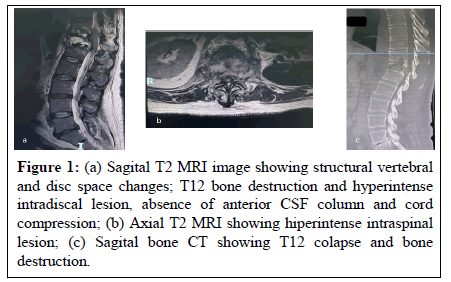
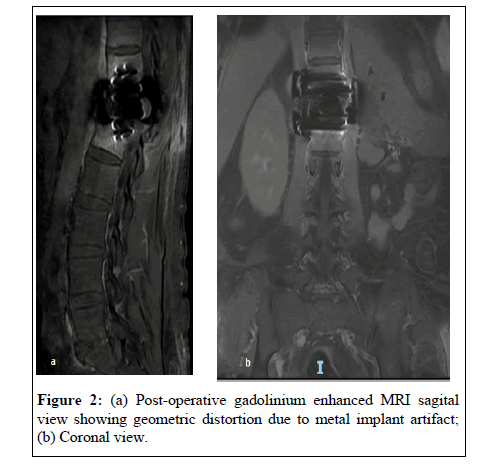
Biochemical parameters remain altered early after a surgical procedure. Imaging modalities (Contrast enhanced MRI, CT), the cornerstones of spondylodiscitis diagnosis, relay on detectable structural anatomical changes caused by the infectious process. These changes may take from 2 to 8 weeks to be detectable [11] (Figure 1). They may be absent or hard to interpret in the postoperative setting, more so, in the presence of instrumentation hardware known to cause geometric distortion (susceptibility artifact) on MRI images (Figure 2).
Figure 1: (a) Sagital T2 MRI image showing structural vertebral and disc space changes; T12 bone destruction and hyperintense intradiscal lesion, absence of anterior CSF column and cord compression; (b) Axial T2 MRI showing hiperintense intraspinal lesion; (c) Sagital bone CT showing T12 colapse and bone destruction.
Molecular imaging is now an important tool in the diagnosis and management of infection. Current targeted probes seek to differentiate between post-op sterile inflammation and infection and identify the specific microbial species [10].
Currently, in immunocompetent patients, labelled leukocyte is the radio imaging study of choice for diagnosing skeletal infection. Unfortunately, technical difficulties limit its wide clinical application. The in vitro labelling is labour intensive, involves direct handling of blood products, and may not be widely available [11,12].
Radiolabelled Ubiquicidin, a naturally occurring antimicrobial peptide has gained interest for infection imaging. Ubiquicidin is an antimicrobial 59 amino acid peptide present in the human respiratory epithelium, activated macrophages and colonic epithelial cells [13-16]. The basis for its use as an imaging modality for infection is that its cationic fragment (UBI 29-41) binds preferentially to the anionic microbial membrane. UBI (29-41) has been successfully labelled with 68 Ga for PET, and 99mTec for SPECT imaging [13-18].
A study of molecular imaging diagnosis of musculoskeletal infection showed 99.0% sensitivity, 94.5% specificity, 92.5% positive predictive value, 98.5% negative predictive value and 99.4% accuracy for 99mTec (UBI 29-41) SPECT/CT in differentiating between soft tissue and bone infection [19-25].
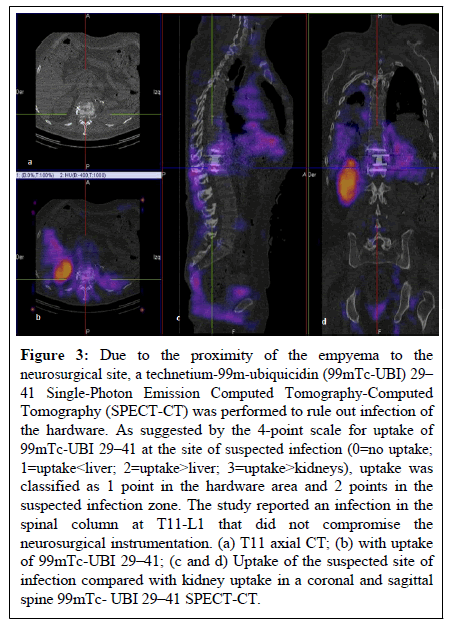
We previously reported a case of thoracic spondylodiscitis of hematogenous origin, managed initially with IV antibiotics, surgical debridement, minimally invasive T12 corpectomy and placement of a titanium expandable cage with supplemental bicortical vertebral screws [17]. 99mTec-UBI 29-41 SPECT-CT molecular imaging was used to assess response to treatment and rule out post-operative surgical re-infection in the presence of bilateral empyema (Figure 3).
Figure 3: Due to the proximity of the empyema to the neurosurgical site, a technetium-99m-ubiquicidin (99mTc-UBI) 29– 41 Single-Photon Emission Computed Tomography-Computed Tomography (SPECT-CT) was performed to rule out infection of the hardware. As suggested by the 4-point scale for uptake of 99mTc-UBI 29–41 at the site of suspected infection (0=no uptake; 1=uptake< liver; 2=uptake>liver; 3=uptake>kidneys), uptake was classified as 1 point in the hardware area and 2 points in the suspected infection zone. The study reported an infection in the spinal column at T11-L1 that did not compromise the neurosurgical instrumentation. (a) T11 axial CT; (b) with uptake of 99mTc-UBI 29–41; (c and d) Uptake of the suspected site of infection compared with kidney uptake in a coronal and sagittal spine 99mTc- UBI 29–41 SPECT-CT.
Literature Review
Nuclear medicine has enhanced the diagnosis of infection because it is based on pathological tissue changes caused by the presence of viable microbes that start early in the infectious process and resolve quicker as the infection clears, compared to anatomical structural changes that take weeks to be detected by conventional imaging methods [26]. Blood-derived antimicrobial proteins and peptides form part of our innate immune system. They target microbial membranes leading to growth arrest and neutralization of surface polysaccharides [21].
Different inflammatory blood cells contain proteins and peptides that show affinity for surface lipids of microbial cells. The defensins are a family of peptides with cytotoxic activity against bacteria, fungi, parasites and host cells. The defensins calprotectin protein and ubiquicidin cationic peptide, are found in macrophages [27]. The antimicrobial activity of these peptides is based on the interaction of their cationic domains with the negatively charged microbial surface. The antimicrobial peptide ubiquicidin (UBI 29-41) was originally isolated from mouse macrophage cells [28]. Later an identical UBI was isolated from human airway epithelial cells and shown to be present in various murine and human tissues. The homology of the precursor protein and the fact that is probably widely expressed named this protein Ubiquicidin (Latin “ubique”, everywhere) [29].
The aim of radiolabeling techniques is to incorporate the radionuclide into the peptide without altering its biological functions allowing a reliable evaluation of its pharmacokinetics after intravenous administration. The labelling of antimicrobial peptides is rapid (within 10 minutes), effective (5% of total radioactivity fromimpurities), stable (minimal release of radioactivity from the 99mTc-peptide in diluted human serum), and safe (no adverse effect in mice and rabbits) [30].
99mTc labelled (UBI 29-41) selectively localized the infection sites of bacteria and fungi through binding with the cell membranes and revealed minimum to no uptake in inflammatory model by heat-killed microorganisms [31]. Similarly, no binding was found in inflammatory models of rabbits induced with formalin-killed Staphylococcus aureus [24].
Thigh muscle infection experiments in mice and rabbits with both gram-positive and gram-negative bacteria showed accumulation of 99mTc labelled (UBI 29-41) at the site of infection within 15 to 30 minutes after injection. No significant accumulation of labelled peptide was detected in thighs of mice and rabbits previously injected with LPS or heat-killed bacteria (i.e., sterile inflammation) [31].
A human study with 99mTc labelled (UBI 29-41) in patients with bone, soft tissue infection and prosthesis infection, showed overall sensitivity, specificity and accuracy of 100%, 80% and 94.4% respectively, with maximum tracer accumulation 30 minutes after injection [32].
A study comparing the efficacy of MRI, three-phase bone scan and 99mTc labelled (UBI 29-41) scan in vertebral osteomyelitis, showed antimicrobial peptide accuracy of 100% with maximum target to nontarget ratio at 15 minutes after tracer injection, while three-phase bone scan and MRI revealed 90% and 75% accuracy respectively. The study showed superiority of antimicrobial peptide scan over bone scan and MRI [33].
A study for monitoring infection evolution in orthopaedic patients showed better results on quantitative analysis of scan at 30, 60 and 120 minutes after tracer injection after 10-14, day interval with antibiotic treatment, compared with erythrocyte sedimentation rate, Creactive protein and radioisotope bone scan [34].
Radiolabelled peptides bind to bacterial and fungi cell membranes. However, there is non-uniform accumulation in different types of bacteria. Staphylococcus aureus showed more uptake than Escherichia coli which may be due to different mode of toxicity [35]. If bacteria are engulfed by the anti-inflammatory cells or become intracellular after invasion of the host immune cells, their detection may be difficult with scintigraphy.
In a prospective multicentre study comparing MRI, (18F) FDG PET/CT and 99mTc labelled (UBI 29-41) scan for post-op spondylodiscitis, the performance of 99mTc labelled (UBI 29-41) SPECT/CT was found to be suboptimal in the postoperative clinical setting, with 83% specificity and 44% sensitivity.
Discussion
Although our knowledge of microbial and antimicrobial behaviour has advanced significantly, infection continues to be a major source of patient morbidity and mortality. Spinal post-operative infection is a very serious complication that causes permanent neurological sequela in one third of affected patients [8]. To decrease the heavy burden of a post-operative spinal infection, prompt diagnosis and treatment must be initiated as soon as the infection is suspected. The suspicion however is ideally confirmed before antibiotic treatment starts. If the suspected diagnosis is discarded, there is no need to start antibiotics. It is equally important to know if the infection has subsided, so that treatment can be stopped.
Diagnosis confirmation is difficult in the post-surgical period. Patients may present with post-op pain and increased inflammatory markers. Contrast MRI may be inconclusive, biopsy culture, the gold standard of infection diagnosis, may be negative due to previous antibiotic treatment or technical difficulties in tissue sampling.
The question of a new or ongoing post-op spinal infection is a pressing one. Misdiagnosis and consequently, mistreatment, either giving unnecessary antibiotics for weeks or withholding necessary treatment and perhaps additional revision surgery, is likely to have costly and possibly catastrophic consequences.
Molecular imaging of infection is a very promising tool that can potentially help solve the question of an ongoing active infection vs. post-surgery sterile inflammatory changes and monitor the response to treatment [10]. Its usefulness rests on the detection of physio pathological changes that occur early, and resolve quicker when infection has been resolved, than the anatomical structural changes detected by neuroimaging [22].
Blood derived antimicrobial peptides are part of our innate immune system. They target the microbial membranes, leading to growth arrest [20]. The defensins peptides, calprotectin protein and Ubiquicidin cationic peptides are found in macrophages, respiratory and colonic epithelium [16,21].
99mTc-labeled (UBI 29-41) was found to target bacterial cells but not sterile inflammation in experimental animals [23]. In later studies, it also showed to accumulate with high accuracy in fungal infections [22].
99mTc-labelled (UBI 29-41) has shown encouraging results differentiating infection from sterile inflammation in multiple animal studies. The tracer accumulation has been shown to correlate directly with the number of viable bacteria so serial imaging can be used to monitor response to treatment [24]. Use of radiolabelled antimicrobial peptides is only recommended when faced with the dilemma of postop infection vs. sterile inflammation. Nuclear medicine uses radiation and must only be used when other monoisotopic diagnostic methods fail to achieve the desired aim [21].
Phase I clinical trials using 99mTc-labeled (UBI 29-41) in patients with soft tissue infection and osteomyelitis have yielded overall sensitivity of 100%, specificity of 80%, accuracy of 94% with 30´after IV administration of the radiotracer as the optimal time for diagnosis [25].
Molecular imaging with radiolabelled Ubiquicidin seems to show marked advantages over conventional biochemical and imaging diagnostic modalities in the setting of a suspected post op spinal infection. It has the potential to safely distinguish between postoperative infection and sterile inflammation, and to monitor response to treatment.
It should be considered a useful additional tool in the management of these very complicated and vexing clinical scenario.
Conclusion
Physicians face the problem of a possible new or ongoing infectious process after spine surgery. Discriminating between sterile inflammation or infection in the post-operative setting is difficult.
Radiolabelled antimicrobial peptide tracers constitute a promising diagnostic tool since they bind specifically to bacterial cell membranes. 99mTc labelled (UBI 29-41) SPECT/CT has shown encouraging results in human clinical trials. This diagnostic method can also be used to track efficacy and duration of antibiotic treatment. There are limitations with this diagnostic tool. High specificity at the expense of low sensitivity is one of them. The possibility of peptide bacterial resistance is another. 99mTc labelled (UBI 29-41) SPECT/CT seems to be a useful diagnostic modality to discriminate between active infection and sterile inflammation in the complicated setting of post-operative spine infection.
References
- Pham MH, Mehta VA, Patel NN, Jakoi AM, Hsieh PC, et al. (2016) Complications associated with the dynesys dynamic stabilization system: A comprehensive review of the literature. Neurosurg Focus 40:E2.
[Crossref] [Google Scholar] [PubMed]
- Boden SD, Davis DO, Dina TS, Sunner JL, Wiesel SW (1992) Postoperative diskitis: Distinguishing early MR imaging findings from normal postoperative disk space changes. Radiol 184:765-771.
[Crossref] [Google Scholar] [PubMed]
- Yang C, Zheng Z, Liu H, Wang J, Kim YJ, et al. (2016) Posterior vertebral column resection in spinal deformity: A systematic review. Eur Spine J 25:2368-2375.
[Google Scholar] [PubMed]
- Shillingford JN, Laratta JL, Reddy H, Ha A, Lehman RA Jr, et al. (2018) Postoperative surgical site infection after spine surgery: An update from the Scoliosis Research Society (SRS) morbidity and mortality database. Spine Deform 6:634-643.
[Crossref] [Google Scholar] [PubMed]
- Bachy M, Bouyer B, Vialle R (2012) Infections after spinal correction and fusion for spinal deformities in childhood and adolescence. Int Orthop 36:465-469.
[Crossref] [Google Scholar] [PubMed]
- Lee MJ, Hacquebord J, Varshney A, Cizik AM, Bransford RJ, et al. (2011) Risk factors for medical complication after lumbar spine surgery: a multivariate analysis of 767 patients. Spine 36:1801-1806. [Crossref] [Google Scholar]
[PubMed]
- Blumberg TJ, Woelber E, Bellabarba C, Bransford R, Spina N (2018) Predictors of increased cost and length of stay in the treatment of postoperative spine surgical site infection. Spine J 18:300-306.
[Crossref] [Google Scholar] [PubMed]
- Smids C, Kouijzer IJ, Vos FJ, Sprong T, Hosman AJ, et al. (2017) A comparison of the diagnostic value of MRI and 18F-FDG-PET/CT in suspected spondylodiscitis. Infect 45:41-49.
[Crossref] [Google Scholar] [PubMed]
- Longo M, Granata F, Ricciardi K, Gaeta M, Blandino A (2003) Contrast enhanced MR imaging with fat suppression in adult-onset septic spondylodiscitis. Eur Radiol 13:626-637.
[Crossref] [Google Scholar] [PubMed]
- Li YD, Wong CB, Tsai TT, Lai PL, Niu CC, et al. (2018) Appropriate duration of post-surgical intravenous antibiotic therapy for pyogenic spondylodiscitis. BMC Infect Dis 18:468.
[Crossref] [Google Scholar] [PubMed]
- Lawal I, Zeevaart J, Ebenhan T, Ankrah A, Vorster M, et al. (2017) Metabolic imaging of infection. J Nucl Med 58:1727-1732.
[Crossref] [Google Scholar] [PubMed]
- Stradiotti P, Curti A, Castellazzi G, Zerbi A (2009) Metal-related artifacts in instrumented spine. Techniques for reducing artifacts in CT and MRI: state of the art. Eur Spine J 18:102-108.
[Crossref] [Google Scholar] [PubMed]
- Love C, Palestro CJ (2004) Radionuclide imaging of infection. J Nucl Med Technol 32:47-57.
[Google Scholar] [PubMed]
- Welling MM, de Korne CM, Spa SJ, van Willigen DM, Hensbergen AW, et al. (2019) Multimodal tracking of controlled Staphylococcus aureus infections in mice. ACS Infect Dis 5:1160-1168.
[Crossref] [Google Scholar] [PubMed]
- Kim HO, Na SJ, Oh SJ, Jung BS, Lee SH, et al. (2014) Usefulness of adding SPECT/CT to 99mTchexamethylpropylene amine oxime (HMPAO)-labeled leukocyte imaging for diagnosing prosthetic joint infections. J Comput Assist Tomogr 38:313-319.
[Crossref] [Google Scholar] [PubMed]
- Lazzeri E, Bozzao A, Cataldo MA, Petrosillo N, Manfrè L, et al. (2019) Joint EANM/ESNR and ESCMID-endorsed consensus document for the diagnosis of spine infection (spondylodiscitis) in adults. Eur J Nucl Med Mol Imaging 46:2464-2487.
[Crossref] [Google Scholar] [PubMed]
- Govaert GA, IJpma FF, McNally M, McNally E, Reininga IH, et al. (2017) Accuracy of diagnostic imaging modalities for peripheral post-traumatic osteomyelitis: a systematic review of recent literature. Eur J Nucl Med Mol Imaging 44:1393-1407.
[Crossref] [Google Scholar] [PubMed]
- Ostovar A, Assadi M, Vahdat K, Nabipour I, Javadi H, et al. (2017) A pooled analysis of diagnostic value of 99mTc-ubiquicidin (UBI) scintigraphy in detection of an infectious process. Clin Nucl Med 38:413-416.
- Flores-Vázquez JG, Rodriguez-Hernandez LA, Becerril-Vega G, Rodríguez-Hernández IA, Eguiluz-Melendez AG, et al. (2024) Technetium-99m-ubiquicidin 29-41 SPECT-CT to detect postsurgical spinal infection: A case report. Surg Neurol Int 26;15:24.
[Crossref] [Google Scholar] [PubMed]
- Sathekge M, Garcia-Perez O, Paez D, El-Haj N, Kain-Godoy T, et al. (2018) Molecular imaging in musculoskeletal infections with 99mTc-UBI 29-41 SPECT/CT. Ann Nucl Med 3:54-59.
[Crossref] [Google Scholar] [PubMed]
- Akhtar MS, Imran MB, Nadeem MA, Shahid A (2012) Antimicrobial peptides as infection imaging agents: better than radiolabeled antibiotics. Int J Pept 2012:965238.
[Crossref] [Google Scholar] [PubMed]
- Das SS, Hall AV, Wareham DW, Britten KE (2002) Infection imaging with radiopharmaceuticals in the 21st century. Brazil Arch Biol Technol 45:25-37.
- Welling MM, Mongera S, Lupetti A, Belter HS, Bonetto V, et al. (2002) Radiochemical and biological characteristics of 99mTc-UBI 29-41 for imaging of bacterial infections. Nucl Med Biol 29:413-422.
[Crossref] [Google Scholar] [PubMed]
- Akhtar MS, Iqbal J, Khan MA, Irfanullah J, Jehangir M, et al. (2004) “99mTc-labeled antimicrobial peptide ubiquicidin (29-41) accumulates less in Escherichia coli infection than in Staphlococcus aureus infection.” J Nucl Med 45:849-856.
[Google Scholar] [PubMed]
- Akhtar MS, Qaisar A, Irfanullah J, Iqbal J, Khan B, et al. Antimicrobial peptide 99mTc-Ubiquicidin 29-41 as human infection-imaging agent: Clinical trial. J Nucl Med 46:567–573.
- Levy O (2000) Antimicrobial proteins and peptides of blood: Templates for novel antimicrobial agents. Blood 96:2664-2672.
- Hiemstra PS, van Den Barselaar MT, Roest M, Nibbering PH, van Furth R (1999) Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J Leukoc Biol 66:423-428.
[Crossref] [Google Scholar] [PubMed]
- Pauwels EKJ, Welling MM, Feitsma RIJ, Atsma DE, Nieuwenhuizen W (1993) The labeling of proteins and LDL with 99mTc: a new direct method employing KBH4 and stannous chloride. Nucl Med Biol 20:825-833.
- Welling MM, Lupetti A, Balter HS, Lanzzeri S, Souto B, et al. (2001) 99mTc-labeled antimicrobial peptides for detection of bacterial and Candida albicans infections. J Nucl Med 42:788-794.
- Welling MM, Paulusma-Annema A, Balter HS, Pauwels EK, Nibbering PH (2000) Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med 27:292-301.
- Akhtar MS, Khan ME, Khan B, Irfanullah J, Afzal MS, et al. (2008) An imaging analysis of 99mTc-UBI (29-41) uptake in S. aureus infected thighs of rabbits on ciprofloxacin treatment. Eur J Nucl Med Mol Imaging 35:1056-1064.
[Crossref] [Google Scholar] [PubMed]
- Assadi M, Vahdat K, Nabipour I, Sehhat MR, Hadanvand F, et al. (2011) Diagnostic value of 99mTc-ubiquicidin scintigraphy for osteomyelitis and comparison with 99mTc-methylene diphosphonate scintigraphy and magnetic resonance imaging. Nucl Med Commu 32:716-723.
[Crossref] [Google Scholar] [PubMed]
- Nazari B, Azizmohammadi Z, Rajaei M (2011) Role of 99mTc ubiquicidin 29-41 scintigraphy to monitor antibiotic therapy in patients with orthopaedic infection: a preliminary study. Nucl Med Commun 32:745-751.
[Crossref] [Google Scholar] [PubMed]
- Singh AK, Verma J, Bhatnagar A, Sen S, Bose M (2003) Tc- 99m isoniazid: a specific agent for diagnosis of tuberculosis. World J Nucl Med 2:292-305.
- Paez D, Sathekge MM, Douis H, Giammarile F, Fatima S, et al. (2021) Comparison of MRI, [18F] FDG PET/CT, and 99mTc-UBI 29-41 scintigraphy for postoperative spondylodiscitis-a prospective multicenter study. Eur J Nucl Med Mol Imaging 48:1864-1875.
[Crossref] [Google Scholar] [PubMed]
Citation: Suarez-Rivera O (2024) Molecular Imaging for Post-Operative Spinal Infection Diagnosis. J Infect Dis Ther 12:588. DOI: 10.4172/2332-0877.1000588
Copyright: © 2024 Suarez-Rivera O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1725
- [From(publication date): 0-2024 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 1410
- PDF downloads: 315