Conference Proceeding Open Access
Molecular Docking to Test for Efficacy of Porphyrin Compounds to Cure Alzheimers Disease
Nadeem Kizilbash1* and Majed Alrowaili21Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, Northern Border University, P.O. Box 1321, Arar-91431, Saudi Arabia
2Department of Surgery, Faculty of Medicine, Northern Border University, P.O. Box 1321, Arar-91431, Saudi Arabia
- Corresponding Author:
- Nadeem Kizilbash
Faculty of Applied Medical Sciences
Department of Medical Lab Technology
Northern Border University, Arar-91431, Saudi Arabia
E-mail: fsd707@gmail.com
Received date: August 22, 2015; Accepted date: September 14, 2015; Published date: September 21, 2015
Citation: Kizilbash N, Alrowaili M (2015) Molecular Docking to Test for Efficacy of Porphyrin Compounds to Cure Alzheimer’s Disease. J Biotechnol Biomater 5:199. doi:10.4172/2155-952X.1000199
Copyright: © 2015 Kizilbash N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The cure for Alzheimer's disease involves searching for candidate compounds that can act as inhibitors for Acetylcholinesterase (AChE) enzyme. Regional cerebral blood flow can be increased in patients with Alzheimer’s disease by Acetylcholinesterase inhibitors. In this regard, Tetraphenylporphinesulfonate (TPPS), 5,10,15,20-Tetrakis (4-sulfonatophenyl) porphyrinato Iron(III) Chloride (FeTPPS) and 5,10,15,20-Tetrakis (4-sulfonatophenyl) porphyrinatoIron(III) nitrosyl Chloride (FeNOTPPS) were investigated as candidate compounds for inhibition of Acteylcholinesterase of Drosophila melanogaster (DmAChE) by use of Molecular Docking. FeNOTPPS was found to form the most stable complex with DmAChE.
Introduction
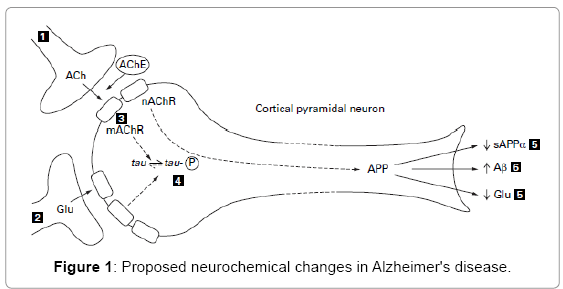
Alzheimer’s disease is a progressive neurodegenerative disorder. Brain regions that are associated with higher mental functions, such as the neocortex and hippocampus, are those affected by the disease [1]. The “Cholinergic Hypothesis” for Alzheimer’s disease proposes that degeneration of cholinergic neurons in the basal forebrain and the associated loss of cholinergic neurotransmission in the cerebral cortex and other areas contribute significantly to the deterioration in cognitive function seen in patients with Alzheimer’s disease [2] (Figure 1).
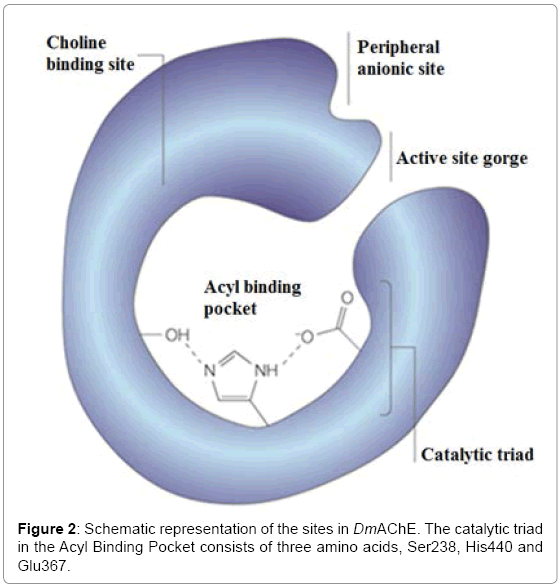
Acetylcholinesterase enzyme (AChE) is bound to cellular membranes of excitable tissues at cholinergic synaptic junctions. It catalyzes the hydrolysis of Acetylcholine neurotransmitter present in the brain [3]. The structure of AChE is a 12-stranded mixed β-sheet surrounded by 14 α- helices. There is a catalytic triad present in the active-site gorge of the Acteylcholinesterase enzyme of Drosophila melanogaster (DmAChE) which consists of three amino acids, namely Ser238, His440 and Glu367 (Figure 2).
Due to the toxic effects of pre-existing AChE inhibitors, current research is focused on developing new AChE inhibitors or modifying existing by computational methods to determine which ligand best fits the AChE binding site. In this study, molecular docking was used to predict the strength of binding of Porphyrin-derivatives: TPPS, FeTPPS and FeNOTPPS with DmAChE. The strength of binding was quantified by use of a Scoring Function that approximates the free energy of binding [4,5] obtained by Molecular Docking of TPPS, FeTPPS and FeNOTPPS with DmAChE.
Methodology
Ligands
Tetraphenyl porphinesulfonate (TPPS), 5, 10, 15, 20-Tetrakis (4-sulfonatophenyl) porphyrinato Iron (III) Chloride (FeTPPS) and 5,10,15,20-Tetrakis (4-sulfonatophenyl) porphyrinato Iron(III) nitrosyl Chloride (FeNOTPPS), were constructed on a Silicon Graphics Octane2 workstation using IRIX 6.5 operating system. The energies of all the molecules were minimized using the TRIPOS force field and Gasteiger- Hückel charges with a convergence gradient of 0.05 kcal/mol/Å. For FeTPPS, the coordinate bonds of Fe(III) and pyrrole nitrogen were defined first before energy minimization. For FeNOTPPS, the coordinate bonds of Fe(III) were first defined with pyrrole nitrogen and then with nitric oxide.
Molecular docking
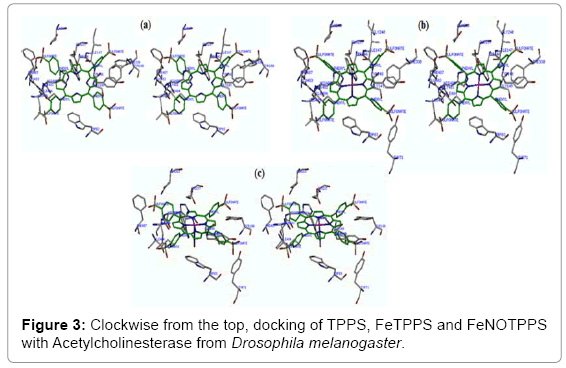
SYBL software was used for docking TPPS, FeTPPS and FeNOTPPS in the crystal structure of DmAChE (PDB code: 1QON) (Figure 3). These complexes were then subjected to molecular dynamics simulation for 10,000 fs then subjected to energy minimization using a TRIPOS force field and Gasteiger-Hückel charges with a convergence gradient of 0.05 kcal/mol/Å.
Analysis of binding
The strength of binding of TPPS, FeTPPS and FeNOTPPS to DmAChE (PDB code: 1QON) was determined by the use of Scoring Functions. Scoring Functions are expressed as a sum of separate terms that describe the various contributions to binding [6,7]. Scoring Functions include terms for van der Waals interactions, hydrogen bonding, de-solvation effects, metal ligand bonding, etc [8-11]. A high value of the Scoring Function represents “tight” binding between the protein and the ligand and vice versa.
Results and Discussion
The “Cholinergic hypothesis” states that the destruction of cholinergic neurons in the basal forebrain results in the deterioration of cognitive function in Alzheimer’s disease [12]. Biochemical investigations of biopsy tissue taken from patients show that presynaptic markers of the cholinergic system are reduced in number [13]. This is results in the reduction of AChE activity which leads to the degree of loss of cognition in patients with Alzheimer’s disease [13-16].
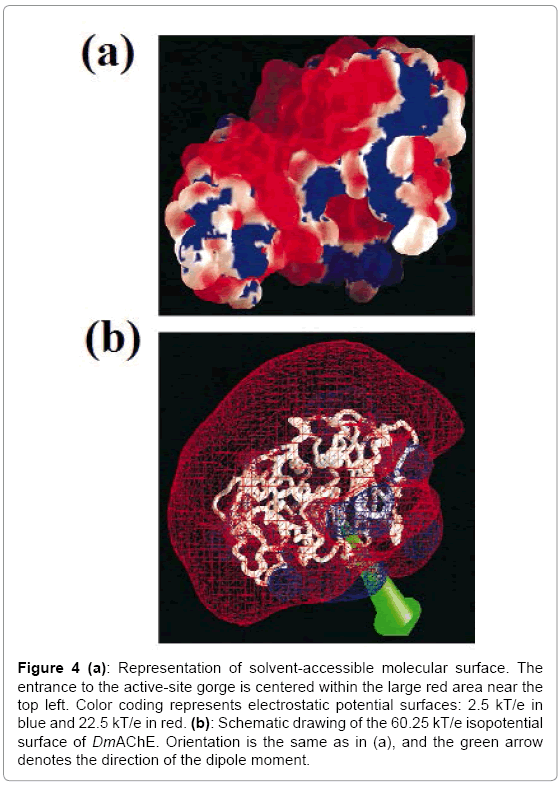
The tertiary structure of DmAChE is similar to that of other vertebrate AChEs. The differences are in some of the surface loops which deviate by up to 8 Å, and the C-terminal helix is also shifted substantially. The potential surface of DmAChE is also similar to that of other AChE molecules which includes the vertebrate AChE [17,18]. It shows the presence of negative charges near the opening of the active-site gorge and positive charges on the opposite side of the molecule (Figure. 4a). The direction of the molecular dipole moment is approximately along the axis of the active-site gorge (Figure. 4b).
Figure 4: (a): Representation of solvent-accessible molecular surface. The entrance to the active-site gorge is centered within the large red area near the top left. Color coding represents electrostatic potential surfaces: 2.5 kT/e in blue and 22.5 kT/e in red. (b): Schematic drawing of the 60.25 kT/e isopotential surface of DmAChE. Orientation is the same as in (a), and the green arrow denotes the direction of the dipole moment.
An important feature of the active site of DmAChE is a 20 Å long, deep and narrow gorge that is coated with aromatic residues (Figure 2). Their side chains can interact with various inhibitors via noncovalent interactions by assuming different conformations [19]. The experimental results show that the active-site gorge of DmAChE can allow the porphyrin inhibitors to enter which can result in blockage of the further entry of the of acetylcholine substrate. The experimental data (Table 1) also demonstrates that FeNOTPPS is energetically the most stable in DmAChE. This can be due to the greater hydrophobicity of FeNOTPPS as compared to TPPS and FeTPPS. The larger size of FeNOTPPS makes it less soluble in water and more stable in the activesite gorge of DmAChE. FeNOTPPS is energetically more stable than TPPS and FeTPPS when bound to DmAChE.
| Molecule | *Valueof Scoring Function |
|---|---|
| TPPS | 1955738102 |
| FeTPPS | 1604890320 |
| FeNOTPPS | 21918620930 |
Table 1: *Scoring Functions include terms for van der Waals interactions, hydrogen bonding, desolvation effects and metal-ligand bonding. They predict the strength of the non-covalent interactions between two molecules.
The breakdown of cholinergic neurons observed in Alzheimer’s disease cause atherosclerosis, arteriosclerosis, arterial stiffness, and endothelial dysfunction which result in damage to the blood-brain barrier and brain function. These factors can reduce perfusion of the brain by arterial blood, resulting in ischemia/hypoxia and neuronal and glial injury [20]. Inestrosa et al. [21] have reported that AChE enzyme promotes amyloid plaque formation from amyloid-β peptide. The formation of amyloid plaques can be inhibited by ligands such as TPPS, FeTPPS and FeNOTPPS.
Conclusion
The results show that Tetraphenylporphinesulfonate (TPPS), 5,10,15,20-Tetrakis (4-sulfonatophenyl) porphyrinato Iron(III) Chloride (FeTPPS) and 5,10,15,20-Tetrakis (4-sulfonatophenyl) porphyrinatoIron(III) nitrosyl Chloride (FeNOTPPS) can serve as inhibitors of acteylcholinesterase of Drosophila melanogaster (DmAChE). This is significant in light of the fact that this model can be transposed to humans and these inhibitors can be employed to increase regional cerebral blood flow in patients with Alzheimer’s disease.
References
- Burns A, Robert P (2009) The National Dementia strategy in England. BMJ 338: b931.
- Bartus RT, Dean RL 3rd, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408-414.
- Taylor P, Radić Z (1994) Thecholinesterases: from genes to proteins. Annu Rev PharmacolToxicol 34: 281-320.
- Kuntz ID (1992) Structure-based strategies for drug design and discovery. Science 257: 1078-1082.
- Leach AR, Shoichet BK, Peishoff CE (2006) Prediction of protein-ligand interactions. Docking and scoring: successes and gaps. J Med Chem 49: 5851-5855.
- Blanco, Mario (1991) Molecular silverware. I. General solutions to excluded volume constrained problems. Journal of computational chemistry 12: 237-247.
- Wendy D. Cornell, PiotrCieplak, Christopher I. Bayly, Ian R. Gould, Kenneth M. Merz, et al. (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. Journal of the American Chemical Society 117: 5179-5197.
- Mittal RR, Harris L, McKinnon RA, Sorich MJ (2009) Partial charge calculation method affects CoMFA QSAR prediction accuracy. J ChemInf Model 49: 704-709.
- Kuntz ID (1992) Structure-based strategies for drug design and discovery. Science 257: 1078-1082.
- Ballester PJ, Mitchell JB (2010) A machine learning approach to predicting protein-ligand binding affinity with applications to molecular docking. Bioinformatics 26: 1169-1175.
- Kitchen DB, Decornez H, Furr JR, Bajorath J (2004) Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov 3: 935-949.
- Bartus RT, Dean RL 3rd, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408-414.
- Francis PT, Sims NR, Procter AW (1993) Cortical pyramidal neuron loss may cause glutamatergichypoactivity and cognitive impairment in Alzheimer’s disease: investigative and therapeutic perspectives. J Neurochem60:1589–1604.
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, et al. (1978) Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J 2: 1457-1459.
- Wilcock GK, Esiri MM, Bowen DM, Smith CC (1982) Alzheimer's disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J NeurolSci 57: 407-417.
- Sims NR, Bowen DM, Allen SJ, Smith CC, Neary D, et al. (1983) Presynaptic cholinergic dysfunction in patients with dementia. J Neurochem 40: 503-509.
- Felder CE, Botti SA, Lifson S, Silman I, Sussman JL (1997) External and internal electrostatic potentials of cholinesterase models. J Mol Graph Model 15: 318-327, 335-7.
- Botti SA, Felder CE, Sussman JL, Silman I (1998) Electrotactins: a class of adhesion proteins with conserved electrostatic and structural motifs. Protein Eng 11: 415-420.
- Harel M, Kryger G, Rosenberry TL, Mallender WD, Lewis, et al. (2000) Three-dimensional structures of Drosophila melanogaster acetylcholinesterase and of its complexes with two potent inhibitors. Protein Science 9: 1063-1072.
- Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, et al. (2012) Cerebral blood flow in Alzheimer's disease. Vasc Health Risk Manag 8: 599-611.
- Inestrosa NC, Alvarez A, Pérez CA, Moreno RD, Vicente M, et al. (1996) Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron 16: 881-891.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15078
- [From(publication date):
September-2015 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 10422
- PDF downloads : 4656