Opinion Article Open Access
Molecular Diagnostics in Melanocytic Tumors: The Pathologists Perspective
Gerardo Ferrara1* and Giuseppina Improta2
1Department of Oncology, Anatomic Pathology Unit, Geatano Rummo General Hospital, Benevento, Italy
2Laboratory of Clinical Research and Advanced Diagnostics, IRCCS-CROB Centro di Riferimento Oncologico della Basilicata, Rionero in Vulture, Italy
- *Corresponding Author:
- Gerardo Ferrara
Department of Oncology
Anatomic Pathology Unit
Geatano Rummo General Hospital
Benevento, Italy
Tel: 39082457315
E-mail: gerardo.ferrara@libero.it
Received: January 27, 2016; Accepted: January 29, 2016; Published: February 01, 2016
Citation: Ferrara G, Improta G (2016) Molecular Diagnostics in Melanocytic Tumors: The Pathologist’s Perspective. Adv Mol Diag 1:102.
Copyright: © 2016 Ferrara G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Advances in Molecular Diagnostics
Abstract
During the last 15 years, molecular techniques have led to a fast gain in our knowledge on the development of melanocytic tumors. The potential implications of these advances for prognosis and therapy of melanoma patients are outstanding. There is, however, an even greater problem which has to be raised: since the histopathological diagnosis of melanoma is matter of considerable disagreement even among experts [1]. Pathologists have been increasingly asking for a molecular ‘key to the code’ in order to overcome the diagnostic limitations of conventional morphology. Table 1 summarizes the main molecular techniques and their expected results in melanoma [2], are these data meaningful also for the histopathological differential diagnosis between ‘nevus’ and ‘melanoma’?
Keywords
Melanoma; Spitzoid melanocytic tumors; Histopathology; Molecular diagnostics
During the last 15 years, molecular techniques have led to a fast gain in our knowledge on the development of melanocytic tumors. The potential implications of these advances for prognosis and therapy of melanoma patients are outstanding. There is, however, an even greater problem which has to be raised: since the histopathological diagnosis of melanoma is matter of considerable disagreement even among experts [ 1]. Pathologists have been increasingly asking for a molecular ‘key to the code’ in order to overcome the diagnostic limitations of conventional morphology. Table 1 summarizes the main molecular techniques and their expected results in melanoma [2], are these data meaningful also for the histopathological differential diagnosis between ‘nevus’ and ‘melanoma’.
| Technique | Expected results in melanoma |
|---|---|
| (Array) Comparative Genomic Hybridization | Gains at 1q, 6p, 7p, 7q, 8q, 17q, 20q, 4q, 8q, and 11q. Losses at 6q, 9p, 10p, 10q, 11q, and 21q |
| Fluorescence in situ hybridization | RREB1 gain>29%; RREB1 gain relative to Cep6>55%; CCND1 gain >38%; MYB loss relative to CEP6>31%; MYC gain >29%; CDKN2A biallelic loss relative to Cep9 >29%; kinase fusions of ROS1, NTRK1, ALK, BRAF, and RET in Spitzoid melanoma (39%) |
| Gene expression profiling | Compared with nevi, different expression of a set of genes including PRAME, S100A7, S100A8, S100A9, S100A12, PI3, CCL5, CD38, CXCL9, CXCL10, IRF1, LCP2, PTPRC, SELL |
| DNA sequencing | In familial melanoma: mutations of CDKN2A (40%), MITF (20%), CDK4, BAP1, TERT, POT1 In sporadic melanoma: mutations of BRAF (53-66%), NRAS (9-29%), NF1 (12-14%), KIT (36% of acral melanomas; 88% of oral melanomas), GNAQ/GNA11 (50% of uveal melanomas) |
| DNA methylation profiling | Methylation of promoters of CDKN2A, PTEN, RASSF-1A, RASSF10, RAR-beta2 |
| Micro-ribonucleic acid (MiRNA) profiling | Upregulation of miRNA192; down regulation of miRNA132 |
| Mass spectrometry | Actin, Vimentin, and three unknown peptidesdifferently expressed in Spitz nevi and Spitzoid melanoma |
Table 1: Molecular investigations for melanoma diagnosis.
Whole exome sequencing performed on metastatic tumor tissue has demonstrated that melanoma has the highest mutation rate among all human cancer types (16.8 mutations/Mb) [3] However, these data do not necessarily apply to primary cutaneous tumors. Of the latter, over 80% of cases harbor mutations involving the RAS-RAF-MEK-ERK pathway which is also affected in nevi [4]. In the field of Spitzoid melanocytic tumors, fluorescence in-situ hybridization with break-apart probes demonstrate kinase fusions of ROS1, NTRK1, ALK, BRAF, and RET; but these chromosomal rearrangements are present along the entire spectrum of Spitzoid tumors (55% of Spitz nevi; 56% of atypical Spitz tumors; 39% of Spitzoid melanomas) [5], a finding that clearly hampers the diagnostic usefulness of such a molecular signature. The same is also true for homozygous BAP1 (3p21) mutations, which can be found in a subset of syndromic and sporadic atypical epithelioid (Spitzoid) cell nevi but also in morphologically clear-cut melanomas [6]. Despite early claims about 11p gains or mutations of the HRAS exon 3 as a hallmark of benignity in Spitzoid neoplasms [7], cases of melanoma with HRAS mutations can be found as well [8]. One could therefore conclude, along with Dummer et al. [9] that the expectations on molecular biology in the differential diagnosis of melanocytic lesions have been overestimated.
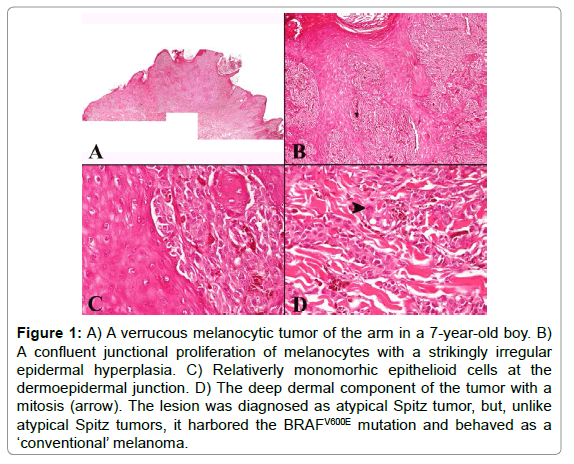
In our opinion, a completely sceptic approach about the diagnostic impact of molecular techniques in this field is probably NOT justified. First of all, molecular techniques may help recognize as melanoma an undifferentiated malignancy with a ‘null’ immunophenotype [ 10]. Furthermore, and even more important, some subgroups of melanocytic tumors, irrespective of the degree of histopathological atypia, can be identified on a molecular basis. As underlined above, Spitzoid neoplasms can be typified by kinase fusions [5], BAP1 biallelic inactivating mutations [6], or HRAS gains/mutations [7]; along with 83% of uveal melanomas [11] activating mutations of GNAQ (9p21) and GNA11 (19p13) are a hallmark for dermal dendritic melanocytic tumors (blue nevus and related lesions) [12]. Both in Spitzoid and in dendritic cell melanocytic tumors, BRAF or NRAS mutation are very rare [12,13]; thus, if a BRAF or NRAS mutation is detected in a seemingly ‘Spitzoid’ or ‘dendritic cell’ morphologic context, a careful histopathological re-evaluation is warranted in order to exclude a conventional melanocytic malignancy. Figure 1 illustrates a case of melanocytic tumor of the arm in a 7-year-old boy. The lesion was initially diagnosed as an atypical Spitz tumor, mainly because of the age of the patient, the epidermal hyperplasia, and the epithelioid cell morphology. Unfortunately however, four years after wide excision of the primary tumor, the patient developed a cutaneous satellitosis, along with nodal and distant metastases. Both the primary and the metastatic tumor tissue were found to harbor the BRAFV600E mutation, a finding which was obviously much more in keeping with a conventional melanoma rather than with an atypical Spitz tumor.
Figure 1: A) A verrucous melanocytic tumor of the arm in a 7-year-old boy. B) A confluent junctional proliferation of melanocytes with a strikingly irregular epidermal hyperplasia. C) Relativerly monomorhic epithelioid cells at the dermoepidermal junction. D) The deep dermal component of the tumor with a mitosis (arrow). The lesion was diagnosed as atypical Spitz tumor, but, unlike atypical Spitz tumors, it harbored the BRAFV600E mutation and behaved as a ‘conventional’ melanoma.
In conclusion, the main goal which can be achieved with molecular techniques in the diagnosis of melanocytic skin lesions is the identification of a given “subgroup” of tumors (“conventional” vs “Spitzoid” vs “dendritic cell”). A ‘red flag’ must be raised for a melanocytic tumor in which molecular data are conflicting with the apparent clinicopathological context.
References
- Ferrara G, Argenziano G, Soyer HP, Corona R, Sera F, et al. (2002) Dermoscopic and histopathologic diagnosis of equivocal melanocytic skin lesions. An interdisciplinary study on 107 cases. Cancer 95: 1094-1100.
- Ferrara G. The histopathological diagnosis and reporting of melanoma. In: Argenziano G (ed). Cutaneous melanoma: a pocket guide for diagnosis and management. In press.
- (2015) The Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 161: 1681-1696.
- Zalaudek I, Gülly C, Pellacani G, Hofmann-Wellenhof R, Trajanoski S, et al. (2011) The dermoscopic and histopathologic pattern of nevi correlate with the frequency of BRAF mutations. J Invest Dermatol 131: 542-545.
- Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, et al. (2014) Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Comm 5: 3116.
- Piris A, Mihm MC, Hoang MP (2015) BAP1 and BRAFV600E expression in benign and malignant melanocytic proliferations. Hum Pathol 46: 239-245
- Bastian BC, LeBoit PE, Pinkel D (2000) Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathologicalfeatures. Am J Pathol 157: 967-972.
- Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, et al. (2015) Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet 47: 996-1002.
- Dummer R, Kerl K, Mihic D, Kamarachev J (2011) Differential diagnosis of melanocytic lesions and molecular biology. Arch Dermatol 147: 232-233.
- Kiuru M, McDermott G, Berger M, Halpern AC, Busam KJ (2014) Desmoplastic melanoma with sarcomatoid dedifferentiation. Am J Surg Pathol 38: 864-870.
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, et al. (2010) Mutations in GNA11 in uveal melanoma. N Engl J Med 363: 2191-2199.
- Zembowicz A, Phadke PA (2011) Blue nevi and variants: an update. Arch Pathol Lab Med 135: 327-336.
- Indsto JO, Kumar S, Wang L, Crotty KA, Arbuckle SM, et al. (2007) Low prevalence of RAS-RAF mutations in Spitz melanocytic nevi compared with other mlanocytic lesions. J Cutan Pathol 34: 448-55.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 10427
- [From(publication date):
April-2016 - Jul 09, 2025] - Breakdown by view type
- HTML page views : 9512
- PDF downloads : 915