Research Article Open Access
Modulation of ECG, Myocardial Oxidative Stress Markers and Connexion 43 Expression by Ascorbic Acid and Ferulic Acid in Isoproterenol-Induced Myocardial Infarction in Rats
Emile F Metias1, Nisreen M Aboelmaaty1, Abdelaziz M Hussein1*, Eman W Abdallah1 and Azza Abdelaziz21Department of Physiology, Faculty of Medicine, Mansoura University, Egypt
2Department of Pathology, Faculty of Medicine, Mansoura University, Egypt
- Corresponding Author:
- Abdelaziz M Hussein
Department of Physiology
Faculty of Medicine
Mansoura University, Egypt
Tel: 201002421140
E-mail: zizomenna28@yahoo.com
Received date: November 09, 2016; Accepted date: November 11, 2016; Published date: December 19, 2016
Citation: Metias EF, Aboelmaaty NM, Hussein AM, Abdallah EW, Abdelaziz A (2016) Modulation of ECG, Myocardial Oxidative Stress Markers and Connexion 43 Expression by Ascorbic Acid and Ferulic Acid in Isoproterenol-Induced Myocardial Infarction in Rats. Biochem Physiol 5:210. doi:10.4172/2168-9652.1000210
Copyright: © 2016, Metias EF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Objective: To assess the effects of ferulic acid (FeA) and ascorbic acid (AS) and combination of both on ECG variables, serum cardiac enzymes (AST, LDH, CK-MB), myocardial oxidative stress markers (MDA, SOD and GSH) and connexin 43 (Cx43) expressions in isoproterenol (ISO)-induced myocardial infarction. Methods: 40 male rats were equally allocated into 5 groups, 1) Control group, 2) ISO-induced MI group (rats received ISO 150 mg/Kg ip for 2 consecutive days at 24 h intervals), 3) FeA group (rats received ISO+FeA at 20 mg/kg/day po for 6 days), 4) AS group (rats received ISO+AS at 80 mg/kg/day po for 6 days) and 5) Combined group (rats received ISO+AS+FeA in the same previous doses). Results: The ISO group showed significant increase in serum cardiac enzymes (AST, CK-MB, and LDH), myocardial MDA and myocardial histopathological damage score with significant decrease in myocardial antioxidants (SOD and GSH) and Cx43expression compared to the control group (p<0.05). ECG traces of rats of ISO-induced MI, showed ST segment elevation, prolonged QT interval, shortened RR interval and increased heart rate. A combination of FeA and AS caused more significant improvement in the studied parameters than did each agent alone. ECG changes were improved significantly in the combined treatment group only. Conclusion: A combination of FeA and AS seems to offer a greater protective effect against ISO-induced myocardial infarction. This might be due to the synergism between their antioxidant properties as well as their effects on the density and location of Cx43 in myocardium.
Keywords
Isoproterenol; Myocardial infarction; Rats; Connexin 43; ECG
Abbreviations
AS: Ascorbic Acid; AST: Aspartate Transaminase; CK-MB: Creatine Phosphokinase; Cx43: Connexin-43; ECG: Electrocardiogram; FeA: Ferulic Acid; GSH: Reduced Glutathione; HR: Heart Rate; IHD: Ischemic Heart Disease; ISO: Isoproterenol; LDH: Lactate Dehydrogenase; MDA: Malondialdehyde; MI: Myocardial Infarction; SOD: Superoxide Dismutase; ZO-1: Zona Occludin-1
Introduction
Ischemic heart disease (IHD) is considered the chief reason of morbidity and mortality world-wide and, according to the world health organization, it will be the major cause of death in the world by the year 2020 [1]. Myocardial infarction (MI), as a type of IHD, results from the prolonged ischemia of the myocardium with necrosis of myocytes [2]. Oxidative stress plays an important role in pathophysiology of myocardial infarction and the use of antioxidants is considered an important counter measure against condition in which oxidative stress is implicated such as MI. They can retard the process of lipid peroxidation by blocking the generation of free radical chain reaction [3]. So, the use of antioxidants could improve the outcome of MI. Ferulic acid (FeA) is a natural antioxidant present particularly in fruits and vegetables such as tomatoes, sweet corn, rice bran, etc. [4]. Epidemiological studies have additionally shown that a high concentration of whole grain products rich in FeA decreases the risk of chronic cardiovascular diseases by an antioxidant mechanism [5]. Also, ascorbic acid (AS) is an important water soluble antioxidant that may affect the risk of atherosclerosis and ischemic heart disease [6]. Humans cannot synthesize AS and its ingestion from exogenous supplement or diet is necessary. AS promotes endothelial prostacyclin that decreases vascular tone and represses platelet aggregation. Moreover, AS may prevent oxidized low-density lipoprotein induced increase in leukocyte–platelet aggregation [7]. It acts both directly by reaction with aqueous peroxyl radicals and indirectly by restoring the antioxidant properties of vitamin E [8].
The pathophysiological mechanisms underlying cardiac arrhythmias in an injured myocardium involve several components, including altered ion channels in cell membranes and abnormal gap junction channels [9]. The chief cardiac gap junction subtype connexin43 (Cx43) is regarded as a promising target in the treatment of cardiovascular disease, and could prompt novel therapies in the future. Cx43 is the main Cx in the ventricular myocardium. Cx43 has four transmembrane, two extracellular and three cytosolic domains including the amino and carboxy terminus [10]. Connexins interact with other proteins in the cell, such as the peripheral membrane protein ZO-1 in rat cardiomyocytes [11]. ZO-1 binds to α-spectrin protein that is highly expressed at the intercalated disk [12]. We hypothesized that upregulation of Cx43 might be possible mechanisms for the cardioprotective effects of antioxidants. Moreover, a previous study demonstrated synergistic effects for AS and FeA against ISOmyocardial infarction [13], however, the possible underlying mechanisms for this synergistic effect were not investigated. So, the present study was designed to investigate the effects of AS, FeA and a combination of both on biochemical, electrocardiographic and histopathological changes and the expression of connexin 43 in a rat model of ISO-MI.
Materials and Methods
Chemicals
Ferulic acid, L-ascorbic acid, and isoproterenol hydrochloride were procured from Sigma Chemical Co., St. Louis, MO, USA.
Animals
Forty adult male Sprague Dawley rats weighing between 200-250 g, aged 4-6 months were enrolled in this study. Animals were bred and housed in the animal house of the Medical Experimental Research centre (MERC), Mansoura University, at controlled environmental conditions (temperature of 24°C and 12 h light/dark cycles) fed a standard laboratory chow and had a free access to tap water. All experimental protocols were approved by our local committee of animal care and ethics.
Induction of ISO-induced myocardial infarction rat model
ISO is a β-adrenergic agonist that causes severe stress in myocardium and necrotic injuries in the heart muscles. Higher levels of ISO deplete the energy reserve of cardiac muscle cells, leading to complex biochemical and structural changes that cause irreversible cellular damage and necrosis [14]. For induction of MI, rats were receiving ISO (150 mg/kg b.w) intraperitoneally for 2 days [15]. Histopathological examination of cardiac specimens proved the occurrence of MI.
Experimental groups
The rats were divided into five groups (n=8 in each group). Group 1: control, Group 2: ISO-induced MI, Group 3: ISO+FeA in which rats received FeA at a dose of 20 mg/kg b.w for 6 days orally via gastric gavage after induction of MI, Group 4: ISO+AS in which rats received AS at a dose of 20 mg/kg bw for 6 days orally via gastric gavage after induction of MI and Group 5: ISO+FeA+AS, in which rats received both FA and AS for 6 days orally via gastric gavage in the same previous doses. These doses were used because it was previously demonstrated that they exhibited the maximum cardioprotective effect against ISO-induced MI [13,16].
ECG Recording
In all groups, ECG recording was performed at day 0 and then repeated at 2, 5 and 8 days after induction of MI in groups 2, 3, 4 and 5. ECG recording was done under light ether anaesthesia using biopac student lab system (software BSL 3.7.5), data acquisition unit MP45, biopac electrode lead set x2 and disposable vinyl electrodes, 3 electrodes per rat.
Collection of blood samples and harvesting heart
The blood samples were collected from the ophthalmic venous plexus by using fine walled Pasteur pipettes under halothane light anaesthesia before induction of MI (basal) and at 2,5 and 8 days after induction of MI. The blood was collected in test tubes and centrifuged to obtain serum for estimation of cardiac enzymes (CK–MB, AST and LDH). At the end of the experimental protocol, rats were sacrificed using a large dose of Na+ thiopental (120 mg/Kg) and the thorax was opened and the heart was rapidly excised. Then the heart was washed in ice-cold isotonic saline and dissected into 2 parts; the small part was used for determination of markers of oxidative stress, while the large portion of the heart was soaked in 10% neutral formalin to be used for histopathological and immunostaining studies.
Biochemical measurements
Assay of cardiac enzymes (CK-MB, AST ALT and LDH)
Cardiac enzymes (CK-MB, AST, ALT and LDH) were measured using commercially available kits according to the manufacturer’s instructions. Kits for AST, ALT and CK-MB were purchased from bioMérieux Diagnostics, Milan, Italy, for LDH were purchased from Bayer Diagnostics Ltd., Baroda, India.
Estimation of oxidative stress markers
The small portion of cardiac tissue was homogenized in cold phosphate buffer saline (pH 7.4, 50 mm) and aliquoted into different ependorffs. Malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) were measured by colorimetric kits (Bio- Diagnostics, Giza, Egypt) according to the manufacturer’s instructions.
Histopathological examination
The heart tissues obtained from all experimental groups were washed immediately with saline and then fixed in 10% buffered neutral formalin solution. After fixation, the heart tissue was embedded in paraffin. Then, the heart tissue was sectioned and stained with haematoxylin and eosin (H&E). Briefly, the findings were graded as no changes; +mild (focal myocyte damage or small multifocal degeneration with slight degree of inflammatory process); ++moderate (extensive myofibrillary degeneration and/or diffuse inflammatory process); +++marked (necrosis with diffuse inflammatory process). Accordingly these histopathological changes in myocardial tissue, all rats were divided into three groups: group A (no histopathological change), group B (mild histopathological change) and group C (moderate and/or severe histopathological changes).
Immunohistochemical examination for connexin 43
The tissue section was deparaffinised, rehydrated, washed, immersed in 3% hydrogen peroxide, and then digested with pepsin for antigen retrieval [17]. After the blocking of unspecific binding by serum, the section was incubated with rabbit polyclonal anti-rat Cx43/ GJA1 antibodies (1:100, ab11370, Abcam, USA) at 4°C overnight. Diaminobenzidine/peroxidase substrate was used to produce a browncolored signal. The section was counterstained, dehydrated, cleared and cover slipped. PBS was used to replace primary antibody and adjacent sections were used as negative control. Cx43 was quantified as the percent of myocardial area occupied by positive staining (calculated by averaging the values from ten fields at 10x magnification) for each left ventricular area.
Statistical analysis
The data were expressed as Mean ± SD. Comparison for parametric data were carried out by analysis of variance (ANOVA) followed by turkey’s post hoc analysis for intergroup multiple comparisons. P<0.05 was considered significant.
Results
Results of animal survival in different groups
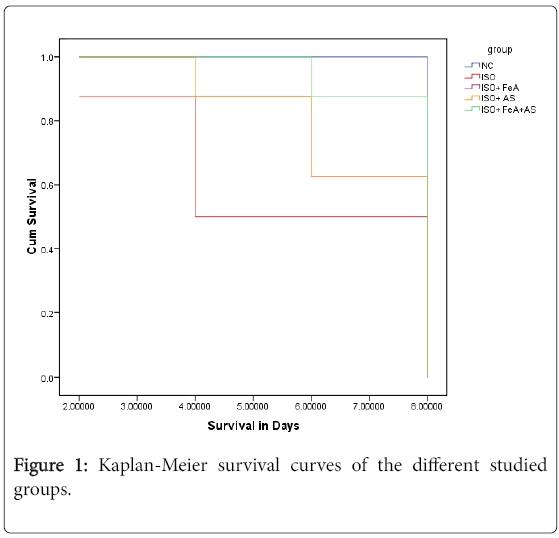
Kaplan curve showed that survival rate was greater in AS and combined groups compared to ISO and ISO+FeA groups. By the end of experiment 8 rats survived in the NC group, 5 rats in ISO group, 5 rats in the ISO+FeA group, 7 rats in the ISO+AS group and 8 rats in ISO+FeA+AS group (Figure 1).
Effect FeA, AS and combination of both on cardiac enzymes in ISO-MI
At day 0, there was no statistically significant difference in serum levels of cardiac enzymes (CK-MB, LDH, ALT and AST) among all groups (NC, ISO, ISO+FeA, ISO+AS and ISO+comb groups). At days 2, 5 and 8, the level of these markers showed significant increase in all studied groups (ISO, ISO+FeA, ISO+AS and ISO+comb) compared to the NC group (p ≤ 0.01). Serum levels of CK-MB and AST showed significant decrease in all treated groups (ISO+AS, ISO+FeA, ISO +comb) at days 5 and 8 compared to ISO-induced MI group (p<0.05). Compared to the ISO group, ISO+AS and ISO+FeA groups showed significant decrease in serum LDH at day 8 while the ISO+comb group showed a significant decrease at days 5 and 8 (p ≤ 0.03). Moreover, the ISO+comb group showed significant decrease in serum LDH and AST compared to ISO+FeA and ISO+AS groups at day 5 only (p ≤ 0.01). Also, the level of ALT showed no statistically significant difference among all studied groups at different times (Table 1).
| Group | N.C | ISO | ISO+FeA | ISO+AS | ISO+ comb | |
|---|---|---|---|---|---|---|
| Days | ||||||
| CK–MB (U/L) | Basal | 23.35 ± 2.25 | 22.71 ± 2.19 | 21.62 ± 1.97 | 22.12 ± 3.29 | 22.3 ± 2.7 |
| 2 days | 21.95 ± 2.6 | 175.1 ± 60.5†a | 216.7 ± 63.4†a | 186.8 ± 95.5†a | 183.2 ± 34.6†a | |
| 5 days | 21.8 ± 2.84 | 159.2 ± 56.79a | 54.2 ± 18.79*b | 55.4 ± 11.88*b | 18.87 ± 4.67*b | |
| 8 Days | 21.78 ± 2.93 | 103.87 ± 8.79a | 29.5 ± 4.63b | 28.76 ± 6.04b | 20.75 ± 2.71b | |
| AST (U/L) | Basal | 21.82 ± 1.40 | 21.92 ± 1.58 | 22.17 ± 1.78 | 21.65 ± 1.44 | 22.15 ± 1.01 |
| 2 days | 21.82 ± 1.39 | 225.37 ± 56.96†a | 197.37 ± 30.88†a | 252.62 ± 45.59†ac | 236 ± 29.33†ac | |
| 5 days | 22 ± 1.85 | 196.62 ± 29.44a | 72.5 ± 13.16*ab | 83.62 ± 18.87*ab | 22.12 ± 1.8*bcd | |
| 8 days | 22.4 ± 2.35 | 84.75 ± 11.73#a | 35 ± 7.96ab | 38.87 ± 6.42#b | 23.25 ± 2.37b | |
| ALT (U/L) | Basal | 21.68 ± 1.1 | 21.97 ± 1.4 | 22.32 ± 2.88 | 21.81 ± 2.6 | 20.8 ± 1.94 |
| Day 2 | 22.16 ± 3.5 | 22.75 ± 3.69 | 27.25 ± 11.11 | 26.68 ± 8.4 | 22 ± 4 | |
| Day 5 | 21.97 ± 1.780 | 21.75 ± 3.37 | 22 ± 7.01 | 22.75 ± 3.195 | 21.875 ± 6.46 | |
| Day 8 | 22.02 ± 3.09 | 23.93 ± 7.21 | 19.625 ± 3.42 | 21.35 ± 2.847 | 23.5 ± 3.207 | |
| LDH (U/L) | Basal | 223.8 ± 31.56 | 245.53 ± 33.28 | 234.3 ± 56.3 | 224.57 ± 48.11 | 240.7 ± 36.51 |
| Day 2 | 216.4 ± 54.9† | 704.5 ± 91.5†a | 672.2 ± 95.2†a | 746.25 ± 64.2†a | 623.87 ± 85.19†a | |
| Day 5 | 242.41 ± 46.25 | 600.12 ± 100.7a | 430.5 ± 64.58*a | 495.13 ± 88.8*a | 199.5 ± 16.56*bcd | |
| Day 8 | 246.27 ± 63.17 | 480.5 ± 58.31a | 270.75 ± 53.08b | 291.38 ± 23.83#b | 188.87 ± 25.20b | |
| All results are expressed as mean ± SD, One way ANOVA with Turkey’s post hoc test (significance at p ≤ 0.05). NC: Normal Control; ISO: Isoprenaline; FeA: Ferulic Acid; AS: Ascorbic Acid; Comb: Combination; †: Significant vs. Basal group; *: Significant vs. 2 days group; #: Significant vs. 5 days group; a: Significant vs. NC; b: Significant vs. Iso Group; c: Significant vs. Ferulic acid; d: Significant vs. Ascorbic acid | ||||||
Table 1: Markers of myocardial damage (CK-MB, AST, ALT and LDH) in different groups.
Effect FeA, AS and combination of both on ECG (PR, QRS, RR, QT intervals, ST segment and HR) in ISO-MI
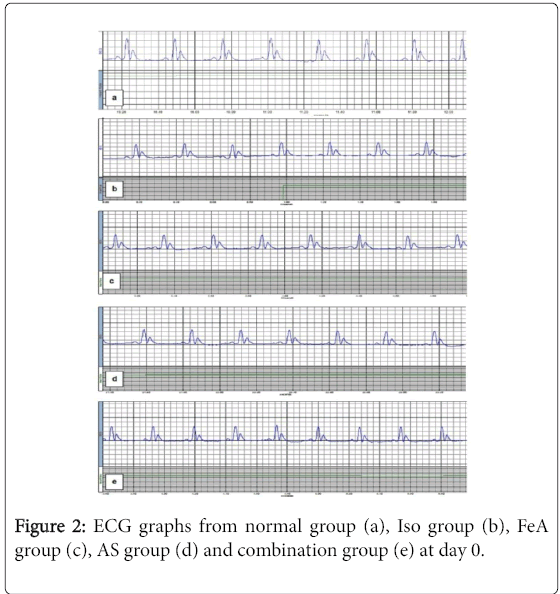
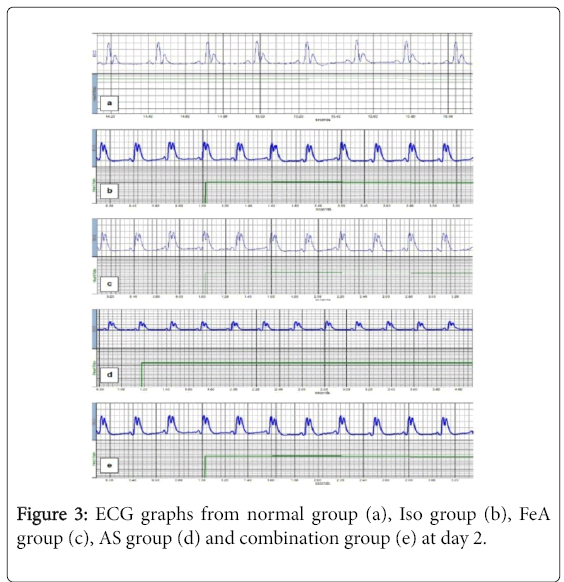
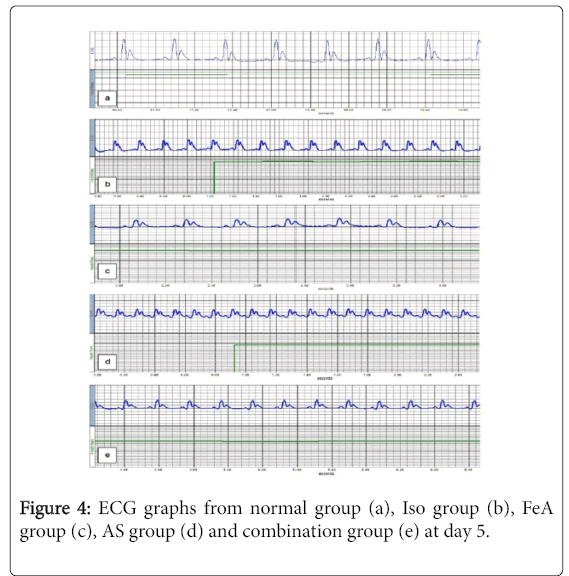
For PR interval, there was no statistically significant difference among all groups. Compared to the NC group, in all studied groups (ISO, ISO+FeA, ISO+AA and ISO+comb), the duration of each of QRS complex and QT interval was significantly decreased while there was significant increase in HR and S-T segment (p<0.01). The QRS complex duration showed significant improvement in all treated compared to ISO group at day 8 (p<0.01). Also, HR, QT interval and S-T segment showed significant improvement in all treated compared to ISO group at days 5 and 8 (p<0.01). Moreover, HR, QT interval and S-T segment showed more significant improvement in ISO+Combined treatment group compared to ISO+FeA and ISO+AS groups at day 5 (p<0.05) (Table 2). Figures 2-5 are representative samples of ECG recordings from different groups at days 0, 2, 5 and day 8, respectively.
| Group | N.C | ISO | ISO+FeA | ISO+AS | ISO+ comb | |
|---|---|---|---|---|---|---|
| Days | ||||||
| PR interval (ms) | Basal | 51.1 ± 2 | 50.1 ± 2.1 | 50.5 ± 1.9 | 50.3 ± 1.7 | 50.6 ± 1.9 |
| 2 days | 52.1 ± 1.7 | 49.7 ± 1.6 | 50 ± 1.6 | 50.6 ± 2.5 | 50.3 ± 2.32 | |
| 5 days | 51 ± 2.8 | 50.2 ± 1.4 | 50.8 ± 1.7 | 50.6 ± 1.5 | 50.1 ± 1.4 | |
| 8 Days | 50.5 ± 1.9 | 48.3 ± 1.4 | 50.1 ± 2.1 | 50.3 ± 1.4 | 50.1 ± 1.2 | |
| QRS complex (ms) | Basal | 50.5 ± 1.9 | 48.3 ± 1.4 | 50.1 ± 2.1 | 50.3 ± 1.4 | 50.1 ± 1.2 |
| 2 days | 35.6 ± 1.8 | 37.2 ± 1.4 | 36.7 ± 1 | 36.2 ± 1.4 | 36. ± 1.6 | |
| 5 days | 35.7 ± 1.6 | 23.7 ± 1†a | 24.2 ± 1.28†a | 23.5 ± 1.19†a | 24.2 ± 1.7†a | |
| 8 days | 36 ± 1.36 | 25.5 ± 1.3a | 29 ± 1*ab | 29.3 ± 1.14*ab | 30.5 ± 1.1*ab | |
| HR (bpm) | Basal | 195 ± 5 | 194 ± 3 | 197 ± 29 | 195 ± 4 | 187 ± 22 |
| Day 2 | 192 ± 3 | 458 ± 10†a | 454 ± 15†a | 444 ± 21†a | 449 ± 17†a | |
| Day 5 | 194 ± 3 | 338 ± 25*a | 281 ± 8*ab | 290 ± 5*ab | 201 ± 12*bcd | |
| Day 8 | 196 ± 3 | 252 ± 1.8#a | 219 ± 2#b | 225 ± 4#ab | 188 ± 6bc | |
| QT interval (ms) | Basal | 65.13 ± 3.68 | 62 ± .1.6 | 63.75 ± .1.48 | 63.75 ± .1.38 | 64.5 ± 1.6 |
| Day 2 | 62 ± .1.6 | 136.75 ± 1.9†a | 137.63 ± .2.5†a | 137.38 ± .2.67†a | 137.1 ± .2.8†a | |
| Day 5 | 62.63 ± 1.99 | 129.13 ± 2.416*a | 93.12 ± .5.72*ab | 95.87 ± .3.9*ab | 75.25 ± .2.8*abcd | |
| Day 8 | 61.75 ± .1.58 | 105.88 ± .4.3#a | 81.38 ± .3.068#ab | 78.75 ± .1.669#ab | 64.88 ± 2.99#bcd | |
| ST segment (uV) | Basal | 6.70 ± 1.30 | 6.3 ± 1.9 | 6.34 ± 2.2 | 7.2 ± 1.8 | 5.95 ± 2 |
| Day 2 | 7.7 ± 3.2 | 125 ± 1.6†a | 130 ± 18.5†a | 132.5 ± 16.6†a | 141 ± 34.4†a | |
| Day 5 | 7.1 ± 01.6 | 10.36 ± 10a | 62.5 ± 0.026*ab | 66 ± 15.9*ab | 31.2 ± 18.8*bcd | |
| Day 8 | 6.8 ± 2.8 | 57 ± 12.6#a | 28.7 ± 14#b | 25.5 ± 8.8#b | 5.9 ± 2.3b | |
| All results are expressed as mean ± SD, One way ANOVA with Turkey’s post hoc test (significance at p ≤ 0.05). NC: Normal Control; ISO: Isoprenaline; FeA: Ferulic Acid; AS: Ascorbic Acid; Comb: Combination; †: Significant vs. Basal group; *: Significant vs. 2 days group; #: Significant vs. 5 days group; a: Significant vs. NC; b: Significant vs. Iso Group; c: Significant vs. Ferulic acid; d: Significant vs. Ascorbic acid |
||||||
Table 2: ECG variables (PR, QT intervals, ST segment, QRS complex and HR) in different groups.
Effect FeA, AS and combination of both on myocardial oxidative stress markers in ISO-MI
As shown in Table 3, there was a significant increase in MDA and a significant decrease in (GSH and SOD activity) in ISO-induced MI group compared to NC group (p<0.05). On the other hand, in all treated groups (ISO, ISO+FeA, ISO+AS and ISO+comb) there was significant improvement in these markers compared to the ISOinduced MI group (p<0.05). Moreover, there was more improvement in these markers in the ISO+Combined treatment group compared to ISO+FeA or ISO+AS groups (p<0.05).
| NC | ISO | ISO+FeA | ISO+AS | ISO+comb | |
|---|---|---|---|---|---|
| SOD (µ/g tissue) | 860.01 ± 9.118 | 212.53 ± 5.888a | 417.67 ± 11.671ab | 422.16 ± 11.366abc | 594.96 ± 15.419abcd |
| GSH (mmol/g tissue) | 7690.75 ± 122.89 | 3124.87 ± 41.865a | 4180.25 ± 46.40ab | 4191.75 ± 22.5ab | 6357.125 ± 199.536abcd |
| MDA (mmol/g tissue) | 1.9725 ± 0.302 | 15.8125 ± 1.05a | 6.6838 ± 1.504ab | 8.250 ± 1.227abc | 3.7225 ± 0.35abcd |
| All results are expressed as mean ± SD, One way ANOVA with Turkey’s post hoc test (significance at p ≤ 0.05). NC: Normal Control; ISO: Isoprenaline; FeA: Ferulic Acid; AS: Ascorbic Acid; Comb: Combination, a: Significant vs. NC , b: Significant vs. Iso group, c: Significant vs. Ferulic acid, d: Significant vs. Ascorbic acid | |||||
Table 3: Markers of oxidative stress in the myocardium (SOD, GSH and MDA) in different groups.
Effect FeA, AS and combination of both on myocardial morphology in ISO-MI
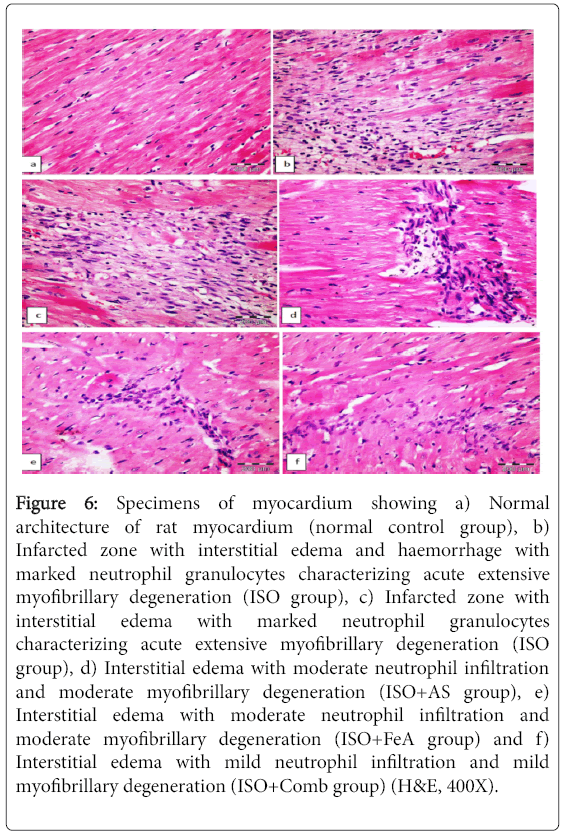
Table 4 shows the number of rats and their scores in different groups. The myocardial histopathological damage score from all rats from the NC group was A. In the ISO-induced MI group, most rats (3 of 5) became score C. The treated groups showed significant decrease in the damage score. Figure 6a shows the normal architecture of the rat myocardium, while Figures 6b and 6c showed an infarcted zone with interstitial edema with neutrophil granulocytes characterizing acute extensive myofibrillary degeneration in the ISO group. Treatment with AS, FeA and combination of both in ISO-induced myocardial infarction showed mild degenerative changes of myocardial tissue (Figures 6d-6f, respectively).
| Group A | Group B | Group C | |
|---|---|---|---|
| Normal Control | 8 | - | - |
| ISO | - | 2 | 3 |
| ISO+FeA | 2 | 3 | - |
| ISO+AS | 2 | 5 | - |
| ISO+Combi | 4 | 4 | - |
| ISO: Isoproterenol; FeA: Ferulic Acid; AS: Ascorbic Acid; Group A: No histopathological changes; Group B: Mild histopathological changes; Group C: Moderate and/or severe histopathological changes | |||
Table 4: Number of rats and histopathological damage score of myocardium in different groups.
Figure 6: Specimens of myocardium showing a) Normal architecture of rat myocardium (normal control group), b) Infarcted zone with interstitial edema and haemorrhage with marked neutrophil granulocytes characterizing acute extensive myofibrillary degeneration (ISO group), c) Infarcted zone with interstitial edema with marked neutrophil granulocytes characterizing acute extensive myofibrillary degeneration (ISO group), d) Interstitial edema with moderate neutrophil infiltration and moderate myofibrillary degeneration (ISO+AS group), e) Interstitial edema with moderate neutrophil infiltration and moderate myofibrillary degeneration (ISO+FeA group) and f) Interstitial edema with mild neutrophil infiltration and mild myofibrillary degeneration (ISO+Comb group) (H&E, 400X).
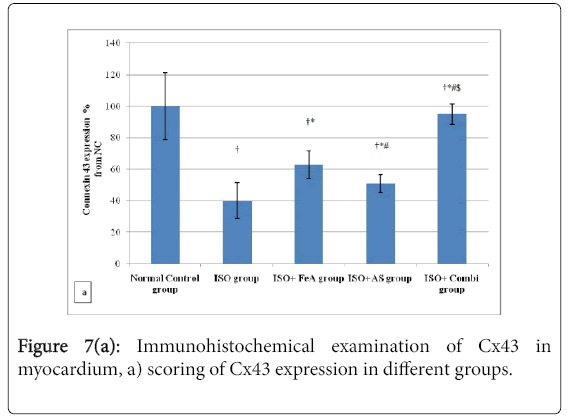
Effect FeA, AS and combination of both on immunohistochemistry of myocardial connexin 43 in ISOMI
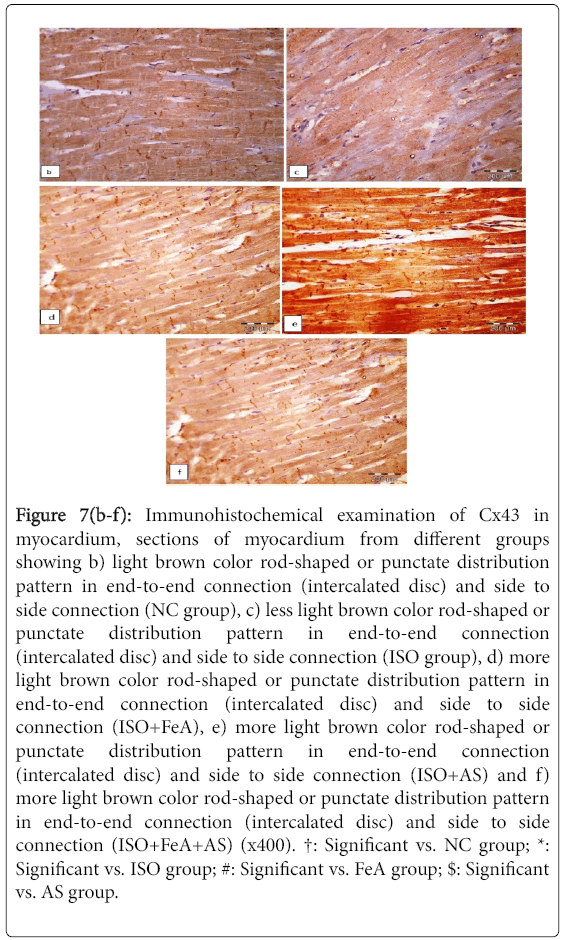
As shown in Figure 7a, Immunohistochemical detection of Cx43 protein in the cardiomyocytes of the control group revealed light brown color with the cardiomyocytes exhibiting a rod-shaped or punctate distribution pattern in end-to-end connection (intercalated disc) and side to side connection. On the other hand, in the ISOinduced MI group there was less rod shaped or punctate Cx43 in the cardiomyocytes (Figure 7b). In all treated groups, there was more rodshaped or punctate Cx43 staining in the cardiomyocytes with more Cx43 in intercalated disc than in side to side connection of cardiomyocytes (Figures 7c-7e).
Figure 7(b-f): Immunohistochemical examination of Cx43 in myocardium, sections of myocardium from different groups showing b) light brown color rod-shaped or punctate distribution pattern in end-to-end connection (intercalated disc) and side to side connection (NC group), c) less light brown color rod-shaped or punctate distribution pattern in end-to-end connection (intercalated disc) and side to side connection (ISO group), d) more light brown color rod-shaped or punctate distribution pattern in end-to-end connection (intercalated disc) and side to side connection (ISO+FeA), e) more light brown color rod-shaped or punctate distribution pattern in end-to-end connection (intercalated disc) and side to side connection (ISO+AS) and f) more light brown color rod-shaped or punctate distribution pattern in end-to-end connection (intercalated disc) and side to side connection (ISO+FeA+AS) (x400). †: Significant vs. NC group; *: Significant vs. ISO group; #: Significant vs. FeA group; $: Significant vs. AS group.
Discussion
In the present study, a documented rat model of ISO-induced myocardial infarction was used to study whether a combination of FeA and AS is exerting a greater protective effect on different parameters known to be disturbed following AMI. Isoproterenol, at such a dose (150 mg/Kg), induces severe stress in the cardiac muscle, membrane permeability alterations, which bring about the loss of function and integrity of myocardial membrane and finally causing myocardial necrosis [18]. Determination of cardiac enzymes is considered as a definite and sensitive early marker of MI. Undoubtedly, the significant rise in serum levels of CK-MB, LDH & AST was expected as a consequence of ISO-induced cardio toxic effect and destruction of cell membranes of myocardial cells. The role of these enzymes as diagnostic markers of myocardial tissue damage is well documented [19] and their increased level in ISO-induced MI infarction was also reported before [20,21]. However, serum ALT levels did not show any significant increase in the ISO group compared to the NC group, this might due to its insensitivity as a marker of myocardial injury. Treatment with FeA, AS or a combination of both caused significant improvement in the serum levels of cardiac enzymes (LDH, AST and CKMB) compared to the ISO group, findings suggesting therapeutic effects for AS and FeA against ISO-MI especially at days 5 and 8, suggesting delayed effects for AS and FeA in protection of the myocardium from ISO-MI. These findings are in agreement with previous studies [15,16]. These effects of FeA and AS on cardiac enzymes might be explained by their antioxidant effects or their stimulation of metabolically active organs such as liver and kidney and degradation of the released cardiac enzymes.
In line with abnormalities in cardiac enzymes, histological examination of heart tissues from the ISO-induced MI group showed focal lesions of necrosis, interstitial edema with marked neutrophil granulocytes characterizing acute extensive myofibrillary degeneration. These changes may be attributed to oxidative stress and free radical produced by ISO which are thought to be involved in catecholamine related toxicity [22]. At the tissue level, this was supported by the improved scoring in heart specimens from the AS +FeA treated group. AS being an efficient free radical scavenger might have protected nitric oxide from its inactivation by free radicals and therefore increased the availability of nitric oxide, which improves the cardiac performance during coronary ischemia [23]. On the other hand, FeA is known to protect by inhibiting lipid peroxidation and thus would increase the myocardial antioxidant activity [24]. Thus, a combination of FeA with AS can cooperate in preserving the physiological integrity of cells exposed to free radicals [5].
The oxidative stress products represent an important link through which many of the detrimental effects induced by ISO are explained. In the present study, the elevated MDA with concomitant reduction in the anti-oxidant activity of SOD and GSH markers are in line with such an explanation. Activated lipid peroxidation (LPO) is an important pathogenic event in myocardial infarction [25]. As the oxidation of polyunsaturated fatty acids in biological membranes may cause impairment of membrane function, decrease in membrane fluidity, and disruption of membrane structure [14], this may explain the association between increased MDA production and the detrimental effect noticed on myocardial cells as evidenced by the increased leakage of cardiac enzymes. Meanwhile, the reduction in the antioxidants, GSH & SOD, could be explained by their excessive utilization during the burst of reactive oxygen species production, in protecting SH group containing proteins from LPO [26]. Antioxidants constitute the defence system that limit the toxicity associated with free radicals, and thus are expected to be consumed by enhanced radical reactions [27]. The present results show that co-treatment with FeA and AS restored the levels of antioxidant markers and that of MDA to near normalcy when compared to individual treatment groups. This effect may be due to the free radical scavenging properties of FeA [5] and AS [28]. AS improves the variety of established cardiovascular risk factors, including markers of oxidative stress [29] and increasing NO bioavailability [30] resulting from increased NO production via up regulation of endothelial NO synthase gene expression and/or increased tetrahydrobiopterin stabilization [31]. Normally, phenolic compounds act by scavenging free radicals [32] and quenching the lipid peroxidative chain. Being a phenolic compound, FeA has strong efficiency to act as a scavenger of different free radicals, including anion superoxide (O2−), hydroxyl radicals (OH), NO−, and hydroxyl and peroxyl radicals, thus inhibiting lipid peroxidation [3]. The improvement observed in the present experiments might be explained on this basis; FeA being a phenolic compound might have inhibited LPO.
Also, in the present study the ISO-MI model showed disturbed ECG parameters in the form of reductions in the duration of QRS complex, R-R intervals, and prolongation of QT interval but there was no change in PR interval. There was also significant elevation in the ST segment and increase in heart rate. These ECG findings indicate development of myocardial infarction. These ECG findings of ISO-MI are in agreement with previous studies [33-35]. Such abnormalities in the ISO-induced MI model might be due to ISO-induced generation of free radicals which further produce oxidative stress. Increased oxidative stress causes loss of cell membrane function leading to elevation of ST segment, conduction disturbances and tachycardia [36]. Further, it has been demonstrated that an increase in heart rate is responsible for increased oxygen consumption leading to accelerated myocardial necrosis [13]. Also, the QT interval represents the period of electrical systole of the heart which is determined by inward Na+ and Ca+2 current and outward K+ and Cl- currents [37] and is a method of determining the functional integrity of the myocardium [34]. The QT interval prolongation and non-significant change in PR interval may be related to cardiac vagal dysfunction and represents cardiac toxic potential such as indication of arrhythmias, cardiac dysfunction and sudden cardiac collapse [38]. Interestingly, in the present study neither FA nor AA caused significant improvement in ECG abnormalities, however, only the combination group showed significant improvement in ECG variables compared to the ISO group. These findings suggest synergistic effects for FeA and AS. In the present experiments, treatment with FeA and AS improved ECG changes, showing a greater improvement with combination treatment rather than single treatments. These findings are in line with those of Peacock et al. [39] and Punithavathi et al. [40].
Disturbance in gap junction proteins might be a potential underlying mechanism for myocardial arrhythmias in ISO-MI, so in the present study we investigated the expression of Cx43 (principal gap junction protein in the heart) in ISO-MI. Immunohistochemical examination for Cx43 in the present study revealed reduced expression of Cx43 and its loss from regions of intercalated discs in heart tissues obtained from ISO-induced MI group. Administration of either FeA or AS improved Cx43 expression and its retention in the regions of intercalated discs, but the combination of both enhanced the presence of Cx43 to a greater extent. Whether the anti-oxidant properties of FeA and AS are the main underlying mechanism in such improvement or whether there is another possible pathway that can explain it is to be explored by further work. It is established now that the balance between phosphorylation and dephosphorylation of Cx43 contributes to the regulation of gap junction conductance and permeability, and Cx43 is phosphorylated by several protein kinases such as the mitogenactivated protein, kinases, extracellular signal regulated kinase [41]. Several reports linked the dephosphorylation of Cx43 with ischemia in tissues such as the brain [42], heart [43], pig myocardium [44] and cardiomyocytes [45]. In the heart, ischemia causes Cx43 redistribution along the lateral plasma membrane away from the intercalated disk region [43]. Lateralized Cx43 is dephosphorylated, whereas phosphorylated forms remain at the intercalated disk region [46]. These acute ischemic changes in Cx43 phosphorylation are followed by Cx43 loss due to degradation [47], coinciding with the development of arrhythmias. It is of interest that functional recovery of isolated perfused hearts from ischemia and reperfusion is closely linked to the ability of reperfusion to re-establish Cx43 phosphorylation [43]. Although, we found loss of Cx43 in the region of intercalated disc of hearts obtained from ISO-MI rats by immunohistochemistry, one of the important limitations of the present study was the assessment of phosphorylated and dephosphorylated Cx43 by western blot and 2D gel electrophoresis.
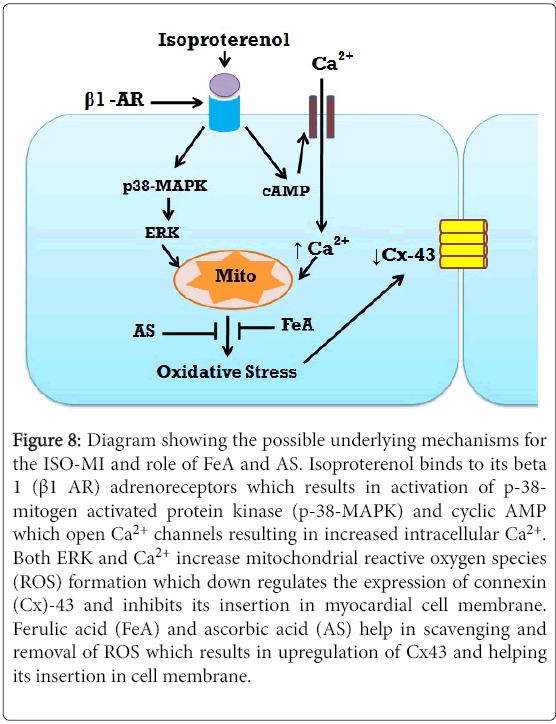
In conclusion, the combination of FeA and AS offered more protection against myocardial damage induced by ISO in rats. The mechanism of such protection seems to be due their antioxidant properties as shown by the enhancement of activity of anti-oxidant enzymes and lowering of the level of the oxidative marker MDA. Further, the present results suggest that they exerted a protective effect over the density and location of membrane Cx43, which may explain the improvement in some of the ECG parameters recorded. Further studies are needed to elucidate the underlying mechanism between the improvement in Cx43 density and these antioxidant agents (Figure 8).
Figure 8: Diagram showing the possible underlying mechanisms for the ISO-MI and role of FeA and AS. Isoproterenol binds to its beta 1 (β1 AR) adrenoreceptors which results in activation of p-38- mitogen activated protein kinase (p-38-MAPK) and cyclic AMP which open Ca2+ channels resulting in increased intracellular Ca2+. Both ERK and Ca2+ increase mitochondrial reactive oxygen species (ROS) formation which down regulates the expression of connexin (Cx)-43 and inhibits its insertion in myocardial cell membrane. Ferulic acid (FeA) and ascorbic acid (AS) help in scavenging and removal of ROS which results in upregulation of Cx43 and helping its insertion in cell membrane.
Conclusion
References
- Lopez AD, Murrau CC (1998) The global burden disease, 1990-2020. Nat Med 4: 124-123.
- Whellan DJ (2005) Heart failure disease management: Implementation and outcomes. Cardiol Rev 13: 231-239.
- Chidambaramurthy KN, Jayaprakasha GK, Singh RP (2002) Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem 50: 4791-4795.
- Paganga G, Miller N, Rice-Evans CA (1999) The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Radic Res 30: 153-162.
- Trombino S, Serinin S, Nicuolo FD, Cellono L, Ando S, et al. (2004) Antioxidant effect of ferulic acid in isolated membranes and intact cells: Synergistic interactions with a-tocopherol, ß-carotene and ascorbic acid. J Agric Food Chem 52: 2411–2420.
- Frei B, Stocker R, England L, Ames BN (1990) Ascorbate: The most effective antioxidant in human blood plasma. Adv Exp Med Biol 264: 155-163.
- Lefer AM (1990) Prostacyclin, high density lipoproteins and myocardial ischemia. Circulation 81: 2013-2015.
- Bendich A, Machlin LJ, Scandurra O (1986) The antioxidant role of vitamin C. Adv Free Radic Biol Med 2: 419–444.
- Xia HJ, Dai DZ, Dai Y (2006) Up-regulated inflammatory factors endothelin, NFkB, TNF[alpha] and iNOS involved in exaggerated cardiac arrhythmias in L-thyroxine-induced cardiomyopathy are suppressed by darusentan in rats. Life Sci 79: 1812-1819.
- Yancey SB, John SA, Lal R, Austin BJ, Revel JP (1989) The 43-kD polypeptide of heart gap junctions: Immunolocalization, topology and functional domains. J Cell Biol 108: 2241-2254.
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, et al. (1998) Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273: 12725-12731.
- Barker RJ, Price RL, Gourdie RG (2002) Increased association of ZO-1 with connexin-43 during remodeling of cardiac gap junctions. Circ Res 90: 317-324.
- Yogeeta SK, Gnanapragasam A, Senthil Kumar S, Subashini R, Devaki T (2006) Synergistic interactions of ferulic acid with ascorbic acid: its cardioprotective role during isoproterenol induced myocardial infarction in rats. Mol Cell Biochem 283: 139–146.
- Rona G (1985) Catecholamine cardiotoxicity. J Mol Cell Cardiol 17: 291-306.
- Sathish V, Ebenezar KK, Devaki T (2003) Synergistic effect of nicorandil and amlodipine on tissue defence system during experimental myocardial infarction in rats. Mol Cell Biochem 243: 133–138.
- Yogeeta SK, Hanumantra RB, Gnanapragasam A, Subramanian S, Rajakannu S, et al. (2006b) Attenuation of abnormalities in the lipid metabolism during experimental myocardial infarction induced by isoproterenol in rats: Beneficial effects of ferulic acid and ascorbic acid. Basic Clin Pharmacol Toxicol 98: 467–472.
- Wang K, Xu BC, Duan HY, Zhang H, Hu FS (2015) Late cardioprotection of exercise preconditioning against exhaustive exercise-induced myocardial injury by up-regulation of connexin 43 expression in rat hearts. Asian Pac J Trop Med 8: 658–663.
- Asdaq SM, Inamdar MN (2009) Pharmacodynamic interaction of garlic with hydrochlorothiazide in rats. Indian J Physiol Pharmacol 53: 127-136.
- Gürgün C, Ildizli M, Yavuzgil O, Sin A, Apaydin A, et al. (2008) The effects of short term statin treatment on left ventricular function and inflammatory markers in patients with chronic heart failure. Int J Cardiol 123: 102-107.
- Sabeena, Farvin KH, Anandan R, Kumar SH, Shiny KS, et al. (2004) Effect of squalene on tissue defence system in isoproterenol induced myocardial infarction in rats. Pharmacol Res 50: 231-236.
- Rajadurai M, Prince SMP (2006) Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in wistar rats: Biochemical and histopathological evidences. Toxicology 228: 259-268.
- Dhalla N, Yates J, Naimark B (1992) Cardio toxicity of catecholamines and related agents. Cardiovasc Toxicol 2: 239-282.
- Tousoulis D, Davies G, Toutouzas P (1999) Vitamin C increases nitric oxide availability in coronary atherosclerosis. Ann Intern Med 131: 156-157.
- Perfilova VN, D'iakova AV, Tiurenkov IN (2005) Cardioprotective action of ferulic acid upon heart under stressor damage conditions. Eksp Klin Farmakol 68: 19-22.
- Rajadurai M, Prince PSM (2005) Comparative effects of Aegle marmelos extract and alpha-tocopherol on serum lipids, lipid peroxides and cardiac enzyme levels in rats with isoproterenol-induced myocardial infarction. Singapore Med J 46: 78–81.
- Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186: 1-85.
- Sun F, Hamagawa E, Tsutsui C, Sakaguchi N, Kakuta Y, et al. (2003) Evaluation of oxidative stress during apoptosis and necrosis caused by D-galactosamine in rat liver. Biochem Pharmacol 65: 101–107.
- Shite J, Qin F, MaO W, Kawai H, Suzzanne YS, et al. (2001) Antioxidant vitamins attenuates oxidative stress and cardiac dysfunction in Tachycardia-induced cardiomyopathy. J Am Coll Cardiol 38: 1734– 1740.
- Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, et al. (2002) Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: Role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106: 3073–3078.
- MacKenzie A, Martin W (1998) Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: Restoration by superoxide dismutase mimetics. Br J Pharmacol 124: 719–728.
- Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, et al. (2001) L-Ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetra hydrobiopterin. J Biol Chem 276: 40–47.
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA (2000) Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 48: 4581-4589.
- Rajadurai M, Stanely MPP (2007) Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology 230: 178-188.
- Thippeswamy BS, Thakker SP, Tubachi S, Kalyani GA, Netra MK, et al. (2009) Cardioprotective effect of Cucumis trigonus roxb on isoproterenol-induced myocardial infarction in rat. Amer J Pharmacol Toxicol 4: 29-37.
- Yadav CH, Akhtar M, Khanam R (2014) Isoproterenol toxicity induced ECG alterations in Wistar rats: Role of histamine H3 receptor agonist imetit. Int J Pharm Pharm Sci 6: 654-658.
- Prince SMP, Rajkumar S, Dhanasekar K (2011) Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur J Pharmacol 668: 233-240.
- Kela AK, Reddy LP, Thombre DP (1980) ECG findings in normal rats and after administration of isoproterenol. Indian J Physiol Pharmacol 24: 84-90.
- Mazzoleni A, Cutin ME, Wolff R, Reiner L, Somes G (1975) On the relationship between heart weights, fibrosis and QRS duration. J Electrocardiol 8: 233-236.
- Peacock WE, Hollander JE, Smalling RW, Bresler MJ (2007) Reperfusion strategies in the emergency treatment of ST-segment elevation myocardial infarction. Am J Emerg Med 25: 353-366.
- Punithavathi VR, Stanely MPP (2011) The cardioprotective effects of a combination of quercetin and α-tocopherol on isoproterenol-induced myocardial infarcted rats. J Biochem Mol Toxicol 25: 28-40.
- Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM (2004) Structural bases for the chemical regulation of connexin-43 channels. Cardiovasc Res 62: 268-275.
- Li WEI, Ochalski PA, Hertzberg EL, Nagy JI (1998) Immunorecognition, ultrastructure and phosphorylation status of astrocytic gap junctions and connexin-43 in rat brain after cerebral focal ischemia. Eur J Neurosci 10: 2444–2463.
- Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, et al. (2000) Dephosphorylation and intracellular redistribution of ventricular connexin-43 during electrical uncoupling induced by ischemia. Circ Res 87: 656-662.
- Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, et al. (2003) Ischemic preconditioning preserves connexin-43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J 17: 1355–1357.
- Jeyaraman M, Tanguy S, Fandrich RR, Lukas A, Kardami E (2003) Ischemia-induced dephosphorylation of cardiomyocyte connexin-43 is reduced by okadaic acid and calyculin-A but not fostriecin. Mol Cell Biochem 242: 129–134.
- Severs NJ, Bruce AF, Dupont E, Rothery S (2008) Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 80: 9–19.
- Huang XD, Sandusky GE, Zipes DP (1999) Heterogeneous loss of connexin-43 protein in ischemic dog hearts. J Cardiovasc Electrophysiol 10: 79-91.
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 5713
- [From(publication date):
December-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 4880
- PDF downloads : 833