Modification and Validation of Respiratory Health Questionnaire Derived from ST. George's Respiratory Questionnaire Abuja, Nigeria
Received: 26-May-2022 / Manuscript No. JCPHN-22-65037 / Editor assigned: 28-May-2022 / PreQC No. JCPHN-22-65037(PQ) / Reviewed: 06-Jun-2022 / QC No. JCPHN-22-65037 / Revised: 13-Jun-2022 / Manuscript No. JCPHN-22-65037(R) / Accepted Date: 13-Jun-2022 / Published Date: 20-Jun-2022 DOI: 10.4172/2471-9846.1000348
Abstract
Respiratory questionnaires, such as St George’s Respiratory Questionnaire (SGRQ), are used to assess the nonrespiratory effects of COPD and other respiratory illnesses.
The SGRQ has been validated in more than 60 languages and has been widely used for quality-of-life assessment. However, several studies have identified that the SGRQ is too lengthy for easy use within a clinical setting and is often challenging to complete in low-literacy environments, including in many developing countries.
Objectives: The objectives of this study were to extract questions from SGRQ and develop a simple questionnaire to be used in areas where SGRQ is relatively new. Modify the reporting period, and assess the validity, and reliability of this modified version in a population of COPD and asthma patients in Abuja Nigeria.
Method: The questions were extracted from SGRQ and reviewed by the supervisory team and the pulmonologists. Participants are registered COPD and asthma patients registered in the two hospitals used for the study who were from an existing cohort used to study the effect of O3, PM10, and NOx on respiratory health in Abuja, Nigeria. Focus groups and interviews were conducted to explore the perceptions of patients and clinicians on the original and modified questionnaires using qualitative methods (thematic analysis).
Result: The modified version showed good internal consistency, test-retest reliability, and responsiveness was shown by significant changes in responses to the questions. In the participants’ opinion, the modified questionnaire can be successfully used as a respiratory questionnaire for adult COPD and asthma patients in Nigeria.
Conclusion: This modified SGRQ is a reliable, valid, and responsive instrument for the evaluation of respiratory patients in countries where SGRQ is relatively new such as Nigeria. We observed that the modified questionnaire is equivalent to other versions and can be used in countries where health-related questionnaire such as SGRQ is relatively new and or low literacy/developing countries.
Keywords
SGRQ; Modification and validation; COPD; Asthma; Nigeria
Introduction
Respiratory questionnaires, such as St George’s Respiratory Questionnaire (SGRQ), are used to evaluate the health effects of respiratory diseases, especially COPD and asthma. SGRQ consists of three sections (symptoms, physical activities, and psychosocial impacts) that correlate with breathlessness, exercise, and depression or anxiety [1].
The SGRQ has been validated in more than 60 languages and has been widely used for quality-of-life assessment. However, several studies have identified that the SGRQ is too lengthy for easy use within a clinical setting [2] and are often challenging to complete in low-literacy environments, including in many developing countries [3]. According to Stahlberg (2009), 5% of the participants could not complete the SGRQ in their study. There is a need for a simpler tool to identify the influence of breathing difficulties on daily life. This study, therefore, aimed to develop a simple questionnaire suitable for use in Nigeria.
Literature Review: The SGRQ was developed to measure the effect of diseases of chronic airflow restrictions on health and well-being, to be sufficiently sensitive and respond to changes in disease activity. The St. George's respiratory questionnaire (SGRQ) is used to assess respiratory health diseases such as asthma and COPD, among other respiratory health-specific questionnaires. SGRQ was first designed in 1991 by Professor Paul Jones, from St. George’s University London Division of Cardiac and Vascular Science. SGRQ has been used extensively to evaluate the effects of respiratory illness on individuals. SGRQ has provided a consistent and validated approach used widely in many studies, including those of [4,5]. SGRQ was initially developed to measure the effect of chronic airflow restriction on patients, with both the language and the questionnaire's context being British. The SGRQ is a self-administrated questionnaire consisting of 50 items, divided into three subscales: symptoms, activities, and impact. The symptom subscale deals with the outcome of respiratory symptoms, severity, and frequency. The activity subscale details activities that limit or cause breathlessness. The impact subscale is about psychological disturbance and social functioning ensuing from airway diseases.
Each item and scope in the SGRQ have a weight and score attributed to it. The SGRQ is scored on a hierarchy ranging from 0 to 100, with zero representing no impairment on the patient's quality of life and 100 indicating the highest impairment. Conducted a meta-analysis on the validation of the US, Swedish, Spanish, Chinese, English, and French versions of the SGRQ. Analysing all the six studies' outcomes shows that the SGRQ is adequately reliable, with the symptoms subscale having the lowest reliability of the three subscales. Moreover, a study presented that the SGRQ could be shortened from 50 to 31 items without any considerable effect on the reliability. Given that both health professionals and patients prefer shorter to longer questionnaires, the researchers recommended removing the unnecessary questions, thus making the questionnaire easier to complete.
Additionally, evaluated the SGRQ and removed 19 questions due to their poor psychometric properties. The questions removed include six out of eight questions from part1 on symptoms, three questions on medication, questions on employment, the question on walking up the hill from section 2 part 2, and the question on recall period. The study by Paap also found no effect on the removal of 19 questions on the validity of the questionnaire, as a 31-question questionnaire was developed from the study. Also, some questions in the SGRQ are challenging to complete. For instance, in the Dutch version of the SGRQ, reporting periods differ from “the last four weeks” for the scope of the symptoms to "usually" or "these days" for the other domains. As a result, the patients have to carefully read the instructions [6] suggest that the fifth and the eighth items of the symptom subscale be removed because they did not apply to patients with COPD.
Consequently, these questions' answers were often missing [7]. A Swedish study found that 7 out of 131 patients could not complete the whole SGRQ. Stalberg and colleagues highlighted that the seven patients could not complete some questions of the SGRQ because they were challenging to complete.
Although the questionnaire has been translated and validated for use in other countries, some of which are in developing nations including and Nepal use SGRQ. The problems associated with the use of SGRQ in Nigeria have also been noted by other researchers. The problems may be due to the diverse range of languages spoken in the same state and the applicability of the questions to Nigerian patients' lifestyle. Also, several authors have expressed concerns about the use of SGRQ in the developing world due to misunderstanding of COPD however; COPD is a global issue affecting 384 million people.
Methods
This study adopted a qualitative approach for the modification and validation of the questions [8, 9] from the SGRQ among COPD and asthma patients in two government-owned hospitals in Abuja, Nigeria. The qualitative analysis was exploratory and adopted an inductive technique using thematic analysis to explore how the participants perceived the new questionnaires and the validation by providing rich descriptions of their opinion about the questionnaire and giving voice to those views that were not heard.
Modification of the questionnaire: Modification of the SGRQ began with the examination of the questionnaire, including the language, suitability in the Nigeria setting, and the number of questions. The questions in the SGRQ were checked for their relevance to the Nigerian setting and consideration (such recreation including light gardening, shoveling snow, playing golf) of the issues participants have reported in previous studies using the SGRQ. Although the SGRQ has been extensively used, validated questionnaires can be modified [10] because a questionnaire that asks too many irrelevant questions reduces participants’ responses to those questions and decreases study power. This is vital when researching in a hospital setting because they usually have a lower response rate than other populations. Questionnaires used in clinical settings need to be simple and straightforward. This was considered carefully in relation to the data that are needed. The researcher, respiratory clinicians in the respiratory clinic and project team checked that each question was straightforward, concise, and without bias. The language used targeted those at the lowest educational level in the cohort. The modification of the questionnaire took place in five stages.
Decision making: Based on existing literature and feedback from both hospitals, some questions were excluded. Examples of the questions examined in SGRQ include “Have you ever had paid employment?" “My chest trouble interferes with my work or made me change my work”. "My chest trouble is a nuisance to my family, friends, or neighbours”. Patients felt these questions as irrelevant because work and their respiratory condition are two different things [11].
Removal of questions: Several questions were removed in response to the feedback from clinicians, patients and literature search (Table 1). Clinicians and participants felt some questions were irrelevant and/or time-consuming. This is consistent with other studies. The questions rephrased included 1 to 5 in Part 1 (Symptoms), and question 7 was removed. In Part 2 (Activities), questions 1, 3, and 4 were rephrased and questions 2, 4, 6 and 7 removed to make the questions simple and straightforward [12].
| SGRQ questions | Retained | Rephrased | Deleted |
|---|---|---|---|
| Part1 Questions about how much chest trouble you have had over the past 3 months. Over the past 3 months, in an average week, how many good days (with little chest trouble) have you had? |
Rephrased to how often do have symptoms | ||
| Part 2 If you have ever had paid employment. Section 1 How would you describe your chest condition? Questions about what activities usually make you feel breathless these days. Questions about your medication We would like to know how your chest usually affects your daily life. |
Rephrased options Rephrased | Deleted Deleted | |
| Now would you tick in the box (one only) which you think best describes how your chest affects you: How would you describe your chest condition? Questions on cough, wheezing and sputum |
Retained Retained | Rephrased |
Table 1: Questions that were rephrased, deleted, or retained.
Different studies highlighted that question 5 “During the past 3 months how many severe or very bad unpleasant attack of chest trouble have you had” is only for patients with asthma, so the wording of the question was rephrased due to feedback. The rephrasing of question 5 was similar to a study conducted in Nepal conducted a psychometric evaluation of the SGRQ where 19 questions were removed due to poor psychometric properties. Some sections, such as section four (“Questions about other effects that your chest trouble may have on you these days”) and six (“Questions on how your activities might affect your breathing”), were not included in the modified questionnaire, and others rephrased to fit into the system. From the feedback, some sections were seen as negative and inconsistent to their condition as the participants felt that individual status and health condition are two different things. This was consistent with validation studies conducted in Hong Kong, China and the United States where some questions were removed or rephrased due to ambiguity or cultural beliefs [13-15].
Focus groups: Three focus groups in each hospital were used from an existing cohort of the effects of photochemical smog (ozone, oxides of nitrogen and particulate matter 10) on COPD and asthma in Abuja, Nigeria from the respective hospitals to evaluate the SGRQ and the modified version.
Interviews: Four health professionals were interviewed to identify any problematic words or questions and suggest an alternative for application to Nigerian patients. These health professionals were encouraged to read through the questionnaires before the interview.
Review: The project team reviewed the rephrased questions to ensure the language was appropriate and that the meaning was retained. Respiratory clinic consultants also reviewed the revised copy.
Evaluation of the instrument: Modified SGRQ: The modified SGRQ is self-administered with 35 structured questions which include the following areas: (a) demographics, (b) respiratory disease symptoms, (c) duration and (d) severity of the symptoms. The first version of the modified SGRQ was presented and proofread by the research team, who are fluent in English. This ensured the clarity of terms and appropriateness of the questionnaire to the population of interest. The respiratory clinic consultants in the two government-owned hospitals used for this research suggest dividing the questionnaire into sections similar to SGRQ (Table 2).
| Domain | Questions |
|---|---|
| Sociodemographic | Age, weight, height, BMI and gender |
| Respiratory symptoms | How long have you had the respiratory symptoms? |
| What respiratory symptoms do you have? | |
| If you have a cough, how many attacks have you had recently? | |
| How often do you notice respiratory symptoms? Once a day, Twice a day, 3 times a day and Other specify | |
| Severity | On a scale of 1 to 10, how do you rate the severity of your symptoms? |
| How would you describe your chest condition? Very severe/Severe/ Moderate/Mild |
Table 2: Three sections of the modified questions.
Sampling: The general aim of qualitative studies is to test how the conditions, relationships, perceptions, attitudes, or problems are in a population. The sample representativeness is crucial. In choosing a sample, the objective of the study and the characteristics of the population are considered. Purposive sampling was used in selecting participants that were involved in the existing cohort used to study the effect of O3, PM10 and NOx on respiratory health in Abuja [16].
Procedure: The researcher recruited participants from an existing cohort used to study the effect of O3, PM10 and NOx on respiratory health in Abuja. The inclusion criteria were:
• Residents of Abuja registered as respiratory patients in either of the hospitals used for the research.
• At least 18 years of age.
• Gave verbal consent or signed the consent form.
• Can speak and understand English
• Patients that saw SGRQ when it was introduced and rejected. This ensured that the participants gave accurate information on their experience when they used SGRQ and the modified questionnaire [17- 19].
Exclusion Criteria
• Respiratory patients that are not registered in either hospital used for the research
• Patients less than 18 years
• Patients that did not give consent
• Patients that cannot speak English
Six focus group discussions, three from each hospital, were held. The 24 Participants’ ages ranged from 34 to 65 years old. Participants were asked to read through and complete the questionnaires before the focus group discussions. The common room in each hospital was used as the venue for focus group discussions. Participants in each hospital were informed that participation was voluntary, and they were free to withdraw at any point and without penalty. Participants were told that the reason for the focus group was to evaluate and finalise the adapted questionnaire and there are no right or wrong answers to the questions. A topic guide was also developed to structure the focus group discussions. Responses were encouraged with prompts used to explore in-depth opinions. Discussions were between 45 and 60 minutes [20-23].
In addition to the above, clinicians from the study hospitals were interviewed to help interpret participants' opinions. Four respiratory clinicians from the respiratory clinic volunteered for the face-to-face interviews, two from each hospital [24]. The interviews were held at each hospital during working hours in a private consulting room. Interviews took between 30 and 45 minutes. They were asked to read through and complete the questionnaires before the interviews. An interview guide was used to structure the interview and responses were encouraged with prompts whilst reminding participants that there is no wrong or right answer [25].
Ethical approval: Ethical approval for this study was obtained from the research ethics committee of the lead author’s university from both hospitals.
Piloting: Prior to the validation exercises and dissemination of the modified questionnaire, the modified questionnaire was piloted using 15 participants a group of Nigerian students to evaluate the suitability and feasibility of implementing the questionnaire to Nigerian respiratory patients. The pilot study examined the following:
• perceptions of participants to the questions related to sensitive issues such as occupation, respiratory symptoms, duration and how the condition affects them.
• appropriateness of selected instrument and study type.
• appropriateness of the wording of questionnaires [26-28].
During the questionnaire piloting, most participants did not want to give their names and while signing the consent form. Thus, this may be a likely problem to participation in the main study. Other comments on some of the questions include how long they have had the condition, the severity and frequency. Thus, in the main study, participants after reading through the information sheet and having any question addressed, were informed that by signing or completing the questionnaire, they are giving informed consent to participate in the survey if they do not want to sign and the comments were also addressed and included in the questionnaire [29-30].
Data Collection and measures: A face-to-face administration method was used both in the pilot and in main study. Although the questionnaires were designed to be self-administered and anonymity is important, some participants were not literate enough to read and answer the questions themselves [31]. The researcher, therefore, assisted in administering the questionnaire to such participants by reading out each question and response option (when available) and recording their response to the questionnaire. Prior to the start of the survey in each hospital, a senior nurse or information officer made an announcement in the outpatients’ waiting area, introducing the researchers and the study to the patients, emphasising the voluntary nature of the study.
Those that indicated interest were approached by the researcher who gave them the participant information sheet and explained more about the research. In addition to the information sheet given to them, it was required for participants to give their consent and assured anonymity and confidentiality of their responses and identities.
Data analysis and Result
A qualitative method was adopted for this study using thematic analysis to analyse data obtained from the focus groups and interviews. This allowed a link to be established between the participants' feedback and the outcome after examining the feedback and responses from the interview and focus group discussion thus forming themes (Table 3). This was compared with the available literature.
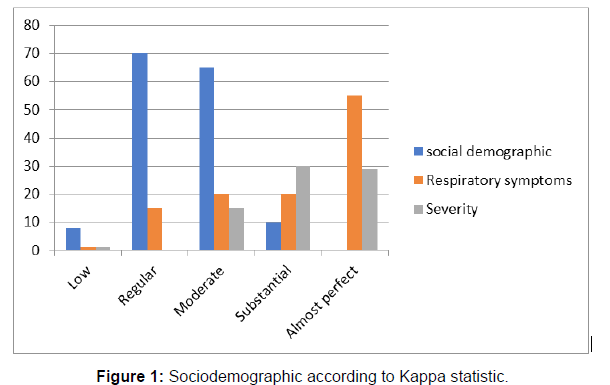
Reliability test: The modified questionnaire was tested on two different occasions within a one-month period to reduce the likelihood that the participants' respiratory condition had changed. In the two stages, eight participants were involved. Sixteen questionnaires (questionnaires from the same eight patients) were compared among the participants due to the number of present participants in both data collection phases. This was conducted to determine the reliability of the questionnaire in providing the same questionnaire to the same participants at different times (Bryman, 2015), under the same conditions (Patel & Joseph, 2016). Participant responses were compared for correlation using cross-tabulation. A 96% similarity was achieved from the test-retest exercise (Figure 1).
Also, to test for reliability Landis and Koch’s approach was used to measure the consistency of the answers in the first and second administration of the questionnaire. The proposed criteria by Landis and Koch (1977) to measure agreement between items were used to classify reliability in the questionnaire two-phase administration. Questions were grouped into three sections following SGRQ categorisation for the reliability analysis: (1) sociodemographic (age, gender, weight, height and occupation), (2) respiratory symptoms (respiratory symptom duration, severity, description of cough), and (3) chest condition (cause of respiratory symptoms, what affects the symptoms).
One of the main concerns from the table above was the time taken for questionnaire completion and the number of questions. This modification process was similar to other studies where some phrases such as "If you have ever had paid employment” in part two, section 1 were perceived to be confusing and were therefore rephrased or removed to fit with the participants opinion.
The reporting of the symptoms was modified from a three-monthly period in the SGRQ to daily. Words like "phlegm" and questions on paid employment were removed. The question on "other effects that your chest trouble may have on you these days" was modified to better suit the culture and the beliefs of Nigerian participants, in a similar process to a study in America. A study conducted by Meguro modified the SGRQ and removed eight questions from the original version because the psychometric properties were poor. Also, in their study, the question on recalling period was not answered as participants found recalling their events of about three months challenging to remember due to the timeframe, which was similar to the findings in this study [32].
However, even when the recall period questions were removed in part one (eg ‘During the past 3 months how many severe or very unpleasant attacks of chest trouble have you had?’, ‘Over the past 3 months, I have brought up phlegm (sputum)’), participants still skipped questions as some are not applicable to COPD patients. The participants' great concern in this study was the time taken for completion. Some of the highlighted concerns on the SGRQ are shown in Table 3.
| Themes | Feedback | |
|---|---|---|
| Clinicians | Patients | |
| Time | "It will take time to complete, and this does not fit into the system we are operating." | "The modified version should be because you cannot pay for our time and time is important.” |
| “You cannot pay for the patients' time. It is important you consider time in this research and the type of questionnaire you are using" | "We cannot use all our time in this hospital to complete one questionnaire. " | |
| "Time is very important in everything, and this research is not an exception, the St George's Questionnaire will take too much time to complete, it will consume our time and that of the patients for free." | “There is something I want you to understand in this hospital everybody is busy, and there is limited time for everything” | |
| Setting | “Questions on snow, recreation and swimming need to be removed, because we do not experience snow here." | “The questionnaire is asking us about recreation and swimming when there is no close by a river or lake to swim [in]” |
| "When you use a questionnaire with sections, subsections or parts, it makes it difficult to handle” | "The system operating here will make it difficult from being used as we may miss our appointment because we are filling a questionnaire” | |
| "Mindset and level of exposure regarding the questionnaire are different" | "A questionnaire with too many questions or pages should be reduced to make it easy for everyone" | |
| "Nobody has the time to go through the questionnaire with many children" | ||
| Resource | “We do not have designated time or staff to attend to patients in this kind of work” | "Nurses are busy and may not attend to us while filling out the form during consultation" |
| Perceptions | “SGRQ has too many questions with many pages; what we need is a simple questionnaire." | |
Table 3: Highlighted concerns during interview and focus group discussion.
Discussion
The modification, validity and reliability of the modified version of the SGRQ among respiratory patients in two government-owned hospitals in Abuja Nigeria, who were aged between 35 and 55, were examined using qualitative methods. The data sources offered high levels of reliability among the results, allowing for a more substantial explanation and understanding.
Unlike studies conducted in other parts of the world, such as the United Kingdom, the application of the SGRQ in Nigeria had several challenges. From the participants' reports and interviews, some of the issues were due to inconsistencies in understanding questions, particularly because some questions (such as those related to snow or swimming) were not relevant to the Nigerian context and would potentially lead to misinterpretations.
Questions relating to weight, height and age incorporated into the modified version were widely understood by both patients and clinicians. During the modification process, five themes were developed from participant feedback: time, setting, resources, patients and perception. Participants highlighted the amount of time the questionnaire took to complete which affected the time they had available for treatment, and also led to concerns about potentially missing appointments. This is in line with work that found participants felt the original SGRQ to be overly long and time-consuming to complete. However, Berkanovic (1980) suggests that while a modified or translated questionnaire may save time in comparison to creating a new one, adaptations made in a simple language may not retain the same meaning as the original version due to language and cultural influence on issues linked with health.
Considering the difference in the environment, participants pointed out that the structure of the questionnaire is different. One participant pointed out that there is a queuing system in a Nigerian clinic that causes much congestion, which in turn brings about rigidity when it comes to patients being attended to. Thus, if a patient missed their place in the queue, the likelihood of them being treated was slim. In the global initiative for chronic obstructive lung disease (GOLD) report, the reliability of any questionnaire is determined by the setting. Thus, as observed in this study questionnaire that fits into other countries may not work in Nigeria. Barr stated when using a questionnaire, it must be first modified to the patients’ understanding and way of life. Studies have shown that when using extensive questionnaires such as the SGRQ, there is a need for trained staff to explain vital information or difficult questions.
Other research on the SGRQ shows that some of the questions are problematic. For instance, the SGRQ time of reporting differs between "last three months", "last four weeks", these days", "usually" and "few days". Participants may not remember or notice the difference when answering the questionnaire questions (Rutten-van, Roos and Van Noord, 1999). Other studies also used a modified version of the SGRQ known as St George's Respiratory Questionnaire-COPD specific version (SGRQ-C).
Limitations: One limitation of this study was that clinicians discouraged the use of the SGRQ for studies in a clinical setting after seeing the number of pages, even before the patients saw the SGRQ. The data drawn from the modified SGRQ were based solely on the information collected from respiratory patients from the two hospitals in Abuja and the clinicians.
Conclusion
In conclusion, the modified SGRQ questionnaire showed validity and reliability among the participants of patients with asthma and COPD. The modified questionnaire can be successfully used as a respiratory questionnaire for obstructed adults in developing countries where questionnaires such as SGRQ are relatively new. Thus, the modified version is a valuable questionnaire to use in low literacy countries.
Acknowledgments
We acknowledge and thank all the consultants, staff from the Respiratory unit/clinic involved in data collection, and information officers from Gwagwalada Teaching hospital and National hospital in this study. We appreciate the effort and support of the project team. My deepest appreciation to Yvonne Forde for giving us permission and access to St George’s Respiratory questionnaire.
References
- Adeloye D, Chua S, Lee C, Basquill C (2015) Global Health Epidemiology Reference Group (GHERG). Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health 5(2): 56-57
Google Scholar, Indexed at, Crossref
- Paap MCS, Brouwer D, Glas CAW (2015) The St George’s Respiratory Questionnaire revisited: A psychometric evaluation. Qual Life Res24(1):67-79.
Google Scholar, Indexed at, Crossref
- Obaseki D, Adeniyi B, Erhabor G (2016) Biomass exposure and its association with respiratory symptoms, quality of life, and lung function: A population study in Ife, Nigeria. Eur Respir J. 23: 45-48.
Google Scholar, Indexed at, Crossref
- Barr T, Schumacher G, Freeman S (2000) American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther 22(9): 56-59
Google Scholar, Indexed at, Crossref
- Meguro M, Barley EA (2007) Development and validation of an improved, COPD-specific version of the St. George Respiratory Questionnaire. Ches132(2): 456-463.
Google Scholar, Indexed at, Crossref
- Rutten-van Molken M, Roos B (1999) An empirical comparison of the St George’s Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Disease Questionnaire (CRQ) in a clinical trial setting. Thorax 54: 45-46.
Google Scholar, Indexed at, Crossref
- Jones P, Brusselle G, Dal Negro R (2010) Health-related quality of life in patients by COPD severity within primary care European. Respir Med 105(1) doi: 10.1016/j.rmed.2010.09.004.
Google Scholar, Indexed at, Crossref
- Monninkhof E, Pieterse M (2004) A qualitative evaluation of a comprehensive self-management programme for COPD patients: effectiveness from the patients' perspective. Patient Educ Couns 55(2): 177-184.
Google Scholar, Indexed at, Crossref
Google Scholar, Indexed at, Crossref
- Alderman AK, Salem B (2010) Survey research. Plast Reconstr Surg 126:1381-1389.
- Bryman A (2015) Social research methods. Oxford university press.
- Creswell JW (2013) Research design: Qualitative, quantitative, and mixed methods approach. 4th Ed. Sage: London
- Guest G, MacQueen KM, Namey EE (2012) Applied thematic analysis. Thousand Oaks: Sage.
- Holloway I, Todres L (2003) The status of method: flexibility, consistency and coherence. Qual Res: 345–357.
Indexed at, Google Scholar, Crossref
- Jones GN, Sletten C, Mandry C, Brantley PJ (1995) Ozone level effect on respiratory illness: an investigation of emergency department visits. South Med J88: 1049-1056.
Indexed at, Google Scholar, Crossref
- Jones PW, Quirk FH, Baveystock CM, Littlejohns PA (1992) self-complete measure of health status for chronic airflow limitation:The St George's Respiratory Questionnaire. Am Rev Respir Dis145:1321-1327.
Indexed at, Google Scholar, Crossref
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, et al. (2009) Development and first validation of the COPD Assessment Test. Eur Respir J 34: 648-654.
Indexed at, Google Scholar, Crossref
- Jones PW, Quirk FH, Baveystock CM (1991) The St George’s Respiratory Questionnaire. Respir Med 85: 25-31.
Indexed at, Google Scholar, Crossref
- Mayring P (2014) Qualitative content analysis: Theoretical foundation, basic procedures and software solution. SSOAR 143.
- Mayring P (2015) Qualitative content analysis: Theoretical background and procedures. Methodol 365-380.
Indexed at, Google Scholar, Crossref
- Morgan B, Grigsby M, Siddharthan T, Kalyesubula R, Wise R, et al. (2018) Validation of the Saint George's Respiratory Questionnaire in Uganda. BMJ Open Respir Res 5: 276.
Indexed at, Google Scholar, Crossref
- Nardi PM (2015) Doing survey research. Routledge 4: 24-29.
- Ozoh O, Aderibigbe S, Ayuk A, Desalu O, Olufela E, et al. (2019) The prevalence of asthma and allergic rhinitis in Nigeria: A nationwide survey among children, adolescents and adults. PLoS One 14:50-51.
Indexed at, Google Scholar, Crossref
- Paap MC, Lange L, van der Palen J, Bode C (2016) Using the Three-Step Test Interview to understand how patients perceive the St. George's Respiratory Questionnaire for COPD patients (SGRQ-C). Qual Life Res 25:1561-1570.
Indexed at, Google Scholar, Crossref
- Sofaer S (1999) Qualitative methods: what are they and why use them? Health Serv Res 35:1101–1118.
- WHO (2014) Burden of Disease from Ambient and Household Air Pollution?
- Woodward M (2005) Epidemiology: Study design and data analysis. 2nd Ed. Chapman & Hall, London.
- Mayring P (2014) Qualitative content analysis: Theoretical foundation, basic procedures and software solution. SSOAR 143: 45-46.
- Mayring S (2016) Qualitative content analysis: Theoretical background and procedures. Methodol 365-380.
Indexed at, Google Scholar, Crossref
- Grigsby M, Siddharthan T, Kalyesubula R, Wise R, et al. (2018) Validation of the Saint George's Respiratory Questionnaire in Uganda. BMJ Open Respir Res 5: 276.
Citation: Ihedike C, Ling J, Mooney J (2022) Modification and Validation of Respiratory Health Questionnaire Derived from ST. George's Respiratory Questionnaire Abuja, Nigeria. J Comm Pub Health Nursing, 8: 348. DOI: 10.4172/2471-9846.1000348
Copyright: © 2022 Ihedike C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3094
- [From(publication date): 0-2022 - Oct 02, 2025]
- Breakdown by view type
- HTML page views: 2613
- PDF downloads: 481