Mitochondrial ABC Transporters and Iron Metabolism
Received: 03-Feb-2018 / Accepted Date: 12-Feb-2018 / Published Date: 16-Feb-2018 DOI: 10.4172/2161-0681.1000338
Abstract
Mitochondrial are a key organelle in iron metabolism and many metabolic processes involved in iron homeostasis occur in the mitochondria. Eukaryotic cells have developed different transport mechanisms to deal with coordinating movement of iron and iron-related molecules across membranes. Some of those transport mechanisms involve ATP-binding cassette (ABC) transporters. There are four mitochondrial ABC transporters Abcb6, Abcb7, Abcb8 and Abcb10. Abcb6 is localized to the outer membrane of mitochondria where it is involved in porphyrin transport. Abcb7, Abcb8 and Abcb10 are localized to the inner mitochondrial membrane and the exact molecule transported by each is still unclear. Here, we provide a brief review of what is known about each transporter and its role in mitochondrial iron homeostasis. We describe the human diseases associated with known mutations in the genes encoding these proteins and discuss the possible importance of these transporters in immune cell function.
Keywords: ABC transporters; Mitochondria; Iron; Heme
Introduction
ATP-binding cassette (ABC) transporters belong to a large family of membrane proteins that are found in all kingdoms of life and require ATP hydrolysis to transport substrates across membranes [1,2]. They are involved in a large spectrum of biological processes such as transporting ions, lipids, peptides, metabolites, porphyrins and drugs. ABC transporters that are localized to the mitochondria of metazoans include Abcb6 , Abcb7 , Abcb8 and Abcb10 (Figure 1). These proteins belong to the half transporter B subfamily, thus the ABCB group [3-5]. They contain an N-terminal transmembrane domain and a C-terminal nucleotide binding domain and form homodimers for transport. The mitochondrial ABC transporters are encoded by nuclear genes and contain a varying length mitochondrial targeting sequence that is clipped off in the mitochondrial matrix. The structures of a few of these transporters has been solved although many of their exact substrates transported remain to be determined clues as to what these transporters export from the mitochondria and the biochemical pathways they affect come from biochemical and genetic studies [6-8]. All mitochondrial Abcb transporters have been linked to oxidative stress and are characterized to be involved in iron and/or heme biological processes [9-12].
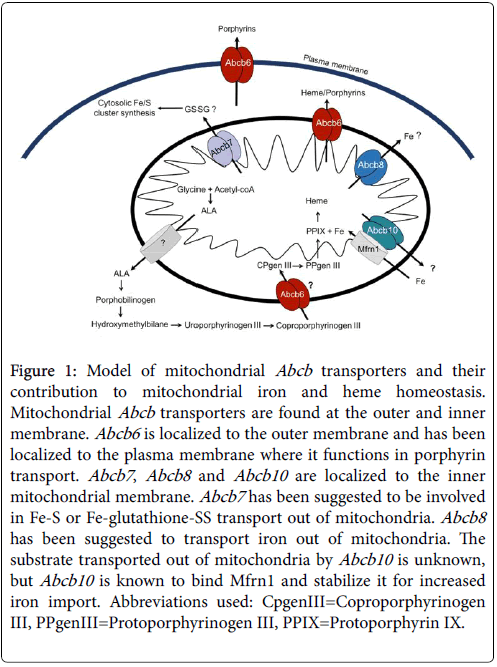
Figure 1: Model of mitochondrial Abcb transporters and their contribution to mitochondrial iron and heme homeostasis. Mitochondrial Abcb transporters are found at the outer and inner membrane. Abcb6 is localized to the outer membrane and has been localized to the plasma membrane where it functions in porphyrin transport. Abcb7 , Abcb8 and Abcb10 are localized to the inner mitochondrial membrane. Abcb7 has been suggested to be involved in Fe-S or Fe-glutathione-SS transport out of mitochondria. Abcb8 has been suggested to transport iron out of mitochondria. The substrate transported out of mitochondria by Abcb10 is unknown, but Abcb10 is known to bind Mfrn1 and stabilize it for increased iron import. Abbreviations used: CpgenIII=Coproporphyrinogen III, PPgenIII=Protoporphyrinogen III, PPIX=Protoporphyrin IX.
Iron and heme are essential co-factors for many biological pathways such as Fe-S clusters synthesis, oxidative phosphorylation pathway in the mitochondria and also DNA synthesis. The heme biosynthesis pathway takes place in both the mitochondria and cytosol requiring tight regulation and transport of iron, porphyrin intermediates and heme across the mitochondrial membrane. In this review, we discuss what is known about each mitochondrial ABC transporter including information learned from eukaryote homologues, potential substrates transported, the relationship to iron metabolism and speculate on how these transporters might affect immune function.
Abcb6
Abcb6 was originally identified in a screen for genes associated with drug resistance in the liver [13]. Abcb6 has been localized to the outer mitochondrial membrane [14-16], the plasma membrane [16], Golgi apparatus [17], endoplasmic reticulum [18] and the endocytic Pathway [19,20]. Its function at each of these locations has been a source of much controversy. Abcb6 was first described as a mitochondrial porphyrin transporter that imports coproporhyrinogen III (CPgenIII) from the cytosol into the mitochondria but is also capable of binding heme and protoporphyrin IX (PPIX) [15]. That Abcb6 is important in red cell heme biosynthesis was further supported by studies done in the Abcb6 knockout mouse, however, the importance was only revealed under conditions of hematopoietic stress [21]. These studies further demonstrated that ATP-driven porphyrin import into mitochondria was completely lost in the absence of Abcb6 but that non-ATP-dependent porphyrin import was unaffected. This suggests that under “steady-state” porphyrin synthesis, red cells can compensate for the loss of Abcb6 by increased expression of other porphyrin synthesis and iron acquisition genes.
The role of Abcb6 at the plasma membrane has been associated with the new blood type Langereis (Lan) [22]. Individuals lacking Abcb6 at the plasma membrane show increased porphyrin accumulation supporting studies that suggest that Abcb6 functions as a plasma membrane porphyrin exporter [23,24]. Additionally, using a mouse model of porphyria, Fukuda et al. demonstrated that the loss of Abcb6 in combination with a ferrochelatase-deficient mouse resulted in increased PPIX accumulation [24].
The role of Abcb6 at other locations remains unknown. The fact that Abcb6 is found in the ER and Golgi may simply reflect its transit during biosynthesis and its presence in the endocytic pathway may reflect mechanisms to downregulate plasma membrane porphyrin export or some level of mitophagy where damaged mitochondria are degraded by the lysosome. Boswell-Casteel et al. provide a more detailed review of the role of Abcb6 in iron and heme processes and its impact in human disease and drug Treatments [25]. Further studies will be necessary to determine if the other locations for Abcb6 are important in heme or porphyrin homeostasis.
Abcb7
The first identification of Abcb7 was as a candidate gene for Xlinked sideroblastic anemia with spinocerebellar ataxia [26-28], suggesting a role for Abcb7 in iron/heme biological processes. Abcb7 is localized to the inner mitochondria membrane and studies have directly shown that reductions in Abcb7 give rise to reduced heme levels in developing red cells [29]. Studies on the Abcb7 yeast homologue Atm1 have provided a great deal of insights into its function. Lack of Atm1 results in accumulation of iron in the mitochondria and decreased cytosolic Fe-S clusters while mitochondrial Fe-S cluster are unaffected [30,31]. That cytosolic Fe-S clusters were affected in human Abcb7 mutants was confirmed by Bekri et al. [26] These studies as well as others suggest that Atm1/ Abcb7 may be a Fe-S cluster exporter [31].
In yeast, Fe-S clusters are made in the mitochondria and require Atm1 to deliver Fe-S to the cytosol for assembling cytosolic and nuclear Fe-S cluster proteins [32-34]. These results support a role for Atm1/Abcb7 in cytosolic Fe-S cluster assembly, but what Atm1/Abcb7 transports is not clearly defined. In vitro vesicle and crystal structure studies using Abcb7 homologues suggest that a substrate for Abcb7 is glutathione and possibly glutathione-disulfide [7,8,35], which correlates with a cytosolic defect in Fe-S clusters in the absence of Abcb7 as glutathione is used for Fe-S cluster maturation in the cytosol. What has yet to be determined is if Fe-S clusters are also transported by Abcb7 and its homologues or if another mitochondrial protein is responsible for this process.
Abcb8
Abcb8 was first described as a mitochondrial ABC transporter in 1999 [36], however, its role in mitochondrial homeostasis and iron metabolism was not elucidated until Ichikawa et al. deleted Abcb8 in the mouse heart [37]. Loss of Abcb8 resulted in severe cardiomyopathy, increased mitochondrial iron accumulation and increased reactive oxygen species. Moreover, mitochondrial isolated from Abcb8 cardiomyocytes exhibited increased iron levels concomitant with decreased activity of cytosolic Fe-S clusters proteins including xanthine oxidase, glycerol-3-phosphate and cytosolic aconitase suggesting a role for Abcb8 in iron homeostasis and possibly iron export. Further evidence for Abcb8 as a mitochondrial iron exporter was observed in doxorubicin-mediated cardiotoxicity studies where overexpression of Abcb8 could reduce both mitochondrial iron accumulation and reactive oxygen species [38]. A role for Abcb8 in red blood cells or other tissues has not been determined.
Abcb10
Abcb10 was first identified as a downstream target of GATA1 , a major transcription factor for terminal erythroid differentiation and named ABC-me (for mitochondrial erythroid) [39]. Abcb10 knockout in mouse is embryonic lethal due to anemia, supporting an essential role in erythropoiesis [40], however, Abcb10 is expressed ubiquitously suggesting a broader function than erythropoiesis. Abcb10 is localized to the inner mitochondrial membrane and has been shown to form a complex with Mitoferrin1 (Mfrn1) and Ferrochelatase (Fech) to enhance heme synthesis [41,42]. Decreases in Abcb10 levels lead to reduced Mfrn1 protein levels and decreased iron import into mitochondria and consequently a reduction in heme biosynthesis. Abcb10 has also been shown to have a protective effect against oxidative stress [11,40]. There has been some controversy regarding the role of Abcb10 and the possible substrate transported. Yamamoto et al. reported increased protoporphyrin IX (PPIX) accumulation in hematopoietic cells in a hematopoietic-specific targeted deletion of Abcb10 [43] suggesting a defect in iron import or a defect in ferrochelatase function. In contrast, other groups have not observed PPIX accumulation when Abcb10 levels are greatly reduced [40,44-46]. One study suggested that Abcb10 was a delta-aminolevulinic acid (ALA) exporter, the product of the first reaction in heme synthesis that occurs in the mitochondria [44].
Recent studies, however, demonstrated that Abcb10 is not the ALA exporter [45,46]. Seguin et al. determined that significant reductions in Abcb10 in zebrafish and murine erythroleukemia cells did not result in a defect in ALA transport but rather total ALA synthesis was decreased due to reduced levels of Alas2 activity [45]. Further, it was shown that loss of Abcb10 resulted in a decrease in hemoglobinization transcripts due to increased occupation of Bach1 (a transcriptional repressor [47]) on the beta-Globin promoter. Overexpression of Gata1 or Alas2 was able to suppress Bach1 repression and partially rescue hemoglobinization transcripts levels in Abcb10 -silenced MEL cells. Surprisingly, hemin or ALA addition did not have an effect on the transcripts levels, suggesting that the substrate transported by Abcb10 is an important signal for hemoglobinization optimization. The substrate for Abcb10 has yet to be identified. Qui et al. determined that glutathione was able to regulate Abcb10 ATP binding and hydrolysis activity; oxidized glutathione GSSG activated while reduced glutathione GSH inhibited Abcb10 ATP hydrolysis activity [46]. Further studies are necessary to determine the substrate transported by Abcb10 to define its role in mitochondrial iron and heme homeostasis and red cell hemoglobinization induction.
Mitochondrial ABC Transporters and Human Disease
Almost all organisms require iron for life as Fe-S clusters and heme synthesis, all processes that occur in the mitochondria, are essential. Because of this fact, the inability to regulate mitochondrial iron homeostasis would be predicted to affect all mammalian cells. Evidence to support the importance of mitochondrial Abcb transporters has been shown for Abcb8 and Abcb10 in cardiomyocytes [37,44], Abcb6 and Abcb10 in red cells [21,40-43,45,46,48,49] and for Abcb6 in liver [23,24]. In addition, Abcb6 is highly expressed in megakaryocytes and platelet production is elevated in the absence of Abcb6 [49]. Furthermore, Abcb6 null platelets are hyperactive in platelet-mediated development of atherosclerosis and show increased leukocyte interactions without changes in peripheral leukocyte numbers [50-55]. Deletion of Abcb7 in mouse leads to a decrease in platelets and white blood cells. These results suggest some regulatory mechanism for porphyrin/heme synthesis is important in immune cell function although no studies have addressed this possibility. Human disease that are associated with the loss of the Abcb mitochondrial transporters are listed in Table 1 [5].
| Gene | Human disease | Reference |
|---|---|---|
| ABCB6 | Familial pseudohyperkalemia | [56,57] |
| Dyschromatosis universalis hereditaria | [58,59] | |
| Microphthalmia, isolated with coloboma | [60] | |
| ABCB7 | X-linked sideroblastic anemia with cerebella ataxia (XLSA/A) | [26,27] |
| Refractory Anemia with Ring Sideroblasts (RARS) | [61] |
Table 1: Human diseases associated with mutations in mitochondrial Abcb transporters.
Conclusion
To our knowledge, a role for Abcb transporters in other organ systems or cellular functions has not been reported. A role for ABC transporters has been suggested for immune cell functions including antigen processing and presentation, natural killer and T cell development transporters. Cells that are highly proliferative such as activated neutrophils, T and B cells would require high levels of ATP, which is generated in the mitochondria through the function of several Fe-S cluster proteins. The roles of Abcb transporters in specific tissues and cells can now be addressed by either target deletion in mice or by CrispR-mediated deletion in cell lines. These future studies will help elucidate the substrates transported by Abcb proteins and their contributions to mitochondrial and cellular homeostasis.
Acknowledgments
The authors apologize to those studies that were not cited due to limited space. This work is supported by NIH R01 grant DK052380 and U54 P&F grant DK110858 to DMW.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Higgins CF (2001) ABC transporters: physiology, structure and mechanism--an overview. Res Microbiol 152: 205-210.
- Higgins CF, Linton KJ (2001) Structural biology. The xyz of ABC transporters. Science 293: 1782-1784.
- Schaedler TA, Faust B, Shintre CA, Carpenter EP, Srinivasan V, et al. (2015) Structures and functions of mitochondrial ABC transporters. Biochem Soc Trans 43: 943-950.
- Sarkadi B, Homolya L, Szakacs G, Varadi A (2006) Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 86: 1179-1236.
- Zutz A, Gompf S, Schagger H, Tampe R (2009) Mitochondrial ABC proteins in health and disease. Biochim Biophys Acta 1787: 681-690.
- Shintre CA, Pike AC, Li Q, Kim JI, Barr AJ, et al. (2013) Structures of ABCB10, a human ATP-binding cassette transporter in apo- and nucleotide-bound states. Proc Natl Acad Sci USA 110: 9710-9715.
- Srinivasan V, Pierik AJ, Lill R (2014) Crystal structures of nucleotide-free and Glutathione-bound mitochondrial ABC transporter Atm1. Science 343: 1137-1140.
- Lee JY, Yang JG, Zhitnitsky D, Lewinson O, Rees DC (2014) Structural basis for heavy metal detoxification by an Atm1-type ABC exporter. Science 343: 1133-1136.
- Senbongi H, Ling F, Shibata T (1999) A mutation in a mitochondrial ABC transporter results in mitochondrial dysfunction through oxidative damage of mitochondrial DNA. Mol Gen Genet 262: 426-436.
- Chloupkova M, LeBard LS, Koeller DM (2003) MDL1 is a high copy suppressor of ATM1: evidence for a role in resistance to oxidative stress. J Mol Biol 331: 155-165.
- Liesa M, Luptak I, Qin F, Hyde BB, Sahin E, et al. (2011) Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation 124: 806-813.
- Kispal G, Csere P, Guiard, B, Lill R (1997) The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett 418: 346-350.
- Furuya KN, Bradley G, Sun D, Schuetz EG, Schuetz JD (1997) Identification of a new P-glycoprotein-like ATP-binding cassette transporter gene that is overexpressed during hepatocarcinogenesis. Cancer Res 57: 3708-3716.
- Mitsuhashi N, Miki T, Senbongi H, Yokoi N, Yano H, et al. (2000) MTABC3, a novel mitochondrial ATP-binding cassette protein involved in iron homeostasis. J Biol Chem 275: 17536-17540.
- Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, et al. (2006) Identification of a mammalian mitochondrial porphyrin transporter. Nature 443: 586-589.
- Paterson JK, Shukla S, Black CM, Tachiwada T, Garfield S, et al. (2007) Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry 46: 9443-9452.
- Tsuchida M, Emi Y, Kida Y, Sakaguchi M (2008) Human ABC transporter isoform B6 (ABCB6) localizes primarily in the Golgi apparatus. Biochem Biophys Res Commun 369: 369-375.
- Fukuda Y, Aguilar-Bryan L, Vaxillaire M, Dechaume A, Wang Y, et al. (2011) Conserved intramolecular disulfide bond is critical to trafficking and fate of ATP-binding cassette (ABC) transporters ABCB6 and sulfonylurea receptor 1 (SUR1)/ABCC8. J Biol Chem 286: 8481-8492.
- Kiss K, Brozik A, Kucsma N, Toth, A, Gera M, et al. (2012) Shifting the paradigm: the putative mitochondrial protein ABCB6 resides in the lysosomes of cells and in the plasma membrane of erythrocytes. PLoS One 7: e37378.
- Kiss K, Kucsma, N, Brozik A, Tusnady GE, Bergam P, et al. (2015) Role of the N-terminal transmembrane domain in the endo-lysosomal targeting and function of the human ABCB6 protein. Biochem J 467: 127-139.
- Ulrich DL, Lynch J, Wang Y, Fukuda Y, Nachagari D, et al. (2012) ATP-dependent mitochondrial porphyrin importer ABCB6 protects against phenylhydrazine toxicity. J Biol Chem 287: 12679-1269.
- Helias V, Saison C, Ballif BA, Peyrard T, Takahashi J, et al. (2012) ABCB6 is dispensable for erythropoiesis and specifies the new blood group system Langereis. Nat Genet 44: 170-173.
- Matsumoto K, Hagiya Y, Endo Y, Nakajima M, Ishizuka M, et al. (2015) Effects of plasma membrane ABCB6 on 5-aminolevulinic acid (ALA)-induced porphyrin accumulation in vitro: tumor cell response to hypoxia. Photodiagnosis Photodyn Ther 12: 45-51.
- Fukuda Y, Cheong PL, Lynch J, Brighton C, Frase S, et al. (2016) The severity of hereditary porphyria is modulated by the porphyrin exporter and Lan antigen ABCB6. Nat Commun 7: 12353.
- Boswell-Casteel RC, Fukuda Y, Schuetz JD (2017) ABCB6, an ABC Transporter Impacting Drug Response and Disease. AAPS J 20: 8.
- Bekri S, Kispal G, Lange H, Fitzsimons E, Tolmie J, et al. (2000) Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron- sulfur protein maturation. Blood 96: 3256-3264.
- Shimada Y, Okuno S, Kawai A, Shinomiya H, Saito A, et al. (1998) Cloning and chromosomal mapping of a novel ABC transporter gene (hABC7), a candidate for X-linked sideroblastic anemia with spinocerebellar ataxia. J Hum Genet 43: 115-122.
- Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, et al. (1999) Mutation of a putative mitochondrial iron transporter gene (ABC7) in X- linked sideroblastic anemia and ataxia (XLSA/A). Hum Mol Genet 8: 743-749.
- Taketani S, Kakimoto K, Ueta H, Masaki R, Furukawa T (2003) Involvement of ABC7 in the biosynthesis of heme in erythroid cells: interaction of ABC7 with ferrochelatase. Blood 101: 3274-3280.
- Csere P, Lill R, Kispal G (1998) Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Lett 441: 266-270.
- Kispal G, Csere P, Prohl C, Lill R (1999) The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. Embo J 18: 3981-3989.
- Li J, Cowan JA (2015) Glutathione-coordinated [2Fe-2S] cluster: a viable physiological substrate for mitochondrial ABCB7 transport. Chem Commun (Camb) 51: 2253-2255.
- Paul VD, Lill R (2015) Biogenesis of cytosolic and nuclear iron-sulfur proteins and their role in genome stability. Biochim Biophys Acta 1853: 1528-1539.
- Braymer JJ, Lill R (2017) Iron-Sulfur Cluster Biogenesis and Trafficking in Mitochondria. J Biol Chem.
- Schaedler TA, Thornton JD, Kruse I, Schwarzlander M, Meyer AJ, et al. (2014) A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly. J Biol Chem 289: 23264-23274.
- Hogue DL, Liu L, Ling V (1999) Identification and characterization of a mammalian mitochondrial ATP-binding cassette membrane protein. J Mol Biol 28: 379-389.
- Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, et al. (2012) Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc Natl Acad Sci USA 109: 4152-4157.
- Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, et al. (2014) Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124: 617-630.
- Shirihai OS, Gregory T, Yu C, Orkin SH, Weiss MJ (2000) ABC-me: a novel mitochondrial transporter induced by GATA-1 during erythroid differentiation. Embo J 19: 2492-2502.
- Hyde BB, Liesa M, Elorza AA, Qiu W, Haigh SE, et al. (2012) The mitochondrial transporter ABC-me (ABCB10), a downstream target of GATA-1, is essential for erythropoiesis in vivo. Cell Death Differ 19: 1117-1126.
- Chen W, Dailey HA, Paw BH (2010) Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood 116: 628-630.
- Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, et al. (2009) Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci U S A 106: 16263-16268.
- Yamamoto M, Arimura H, Fukushige T, Minami K, Nishizawa Y, et al. (2014) Abcb10 role in heme biosynthesis in vivo: Abcb10 knockout in mice causes anemia with protoporphyrin IX and iron accumulation. Mol Cell Biol 34: 1077-1084.
- Bayeva M, Khechaduri A, Wu R, Burke MA, Wasserstrom JA, et al. (2013) ATP-binding cassette B10 regulates early steps of heme synthesis. Circ Res 113: 279-287.
- Seguin A, Takahashi-Makise N, Yien YY, Huston NC, Whitman JC, et al. (2017) Reductions in the mitochondrial ABC transporter Abcb10 affect the transcriptional profile of heme biosynthesis genes. J Biol Chem.
- Qiu W, Liesa M, Carpenter EP, Shirihai OS (2015) ATP Binding and Hydrolysis Properties of ABCB10 and Their Regulation by Glutathione. PLoS One 10: e0129772.
- Igarashi K, Sun J (2006) The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal 8: 107-118.
- Tang L, Bergevoet SM, Bakker-Verweij G, Harteveld C L, Giordano PC, et al. (2012) Human mitochondrial ATP-binding cassette transporter ABCB10 is required for efficient red blood cell development. Br J Haematol 157: 151-154.
- Hosseini MJ, Shaki F, Ghazi-Khansari M, Pourahmad J (2014) Toxicity of copper on isolated liver mitochondria: impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochem Biophys 70: 367-38.
- Murphy AJ, Sarrazy V, Wang N, Bijl N, Abramowicz S, et al. (2014) Deficiency of ATP-binding cassette transporter B6 in megakaryocyte progenitors accelerates atherosclerosis in mice. Arterioscler Thromb Vasc Biol 34: 751-758.
- Pondarre C, Antiochos BB, Campagna DR, Clarke SL, Greer EL, et al. (2006) The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron-sulfur cluster biogenesis. Hum Mol Genet 15: 953-964.
- Lehnert E, Tampe R (2017) Structure and Dynamics of Antigenic Peptides in Complex with TAP. Front Immunol 8: 10.
- Pendse SS, Briscoe DM, Frank MH. (2003) P-glycoprotein and alloimmune T-cell activation. Clin Appl Immunol Rev 4: 3-14.
- Nowyhed HN, Chandra S, Kiosses W, Marcovecchio P, Andary F, et al. (2017) ATP Binding Cassette Transporter ABCA7 Regulates NKT Cell Development and Function by Controlling CD1d Expression and Lipid Raft Content. Sci Rep 7: 40273.
- Pallis M, Russell N (2000) P-glycoprotein plays a drug-efflux-independent role in augmenting cell survival in acute myeloblastic leukemia and is associated with modulation of a sphingomyelin-ceramide apoptotic pathway. Blood 95: 2897-2904.
- Andolfo I, Russo R, Manna F, De Rosa G, Gambale A, et al. (2016) Functional characterization of novel ABCB6 mutations and their clinical implications in familial pseudohyperkalemia. Haematologica 101: 909-917.
- Andolfo I, Alper SL, Delaunay J, Auriemma C, Russo R, et al. (2013) Missense mutations in the ABCB6 transporter cause dominant familial pseudohyperkalemia. Am J Hematol 88: 66-72.
- Liu H, Li Y, Hung KK, Wang N, Wang C, et al. (2014) Genome-wide linkage, exome sequencing and functional analyses identify ABCB6 as the pathogenic gene of dyschromatosis universalis hereditaria. PLoS One 9: e87250.
- Â Lu C, Liu J, Liu F, Liu Y, Ma D, et al. (2014) Novel missense mutations of ABCB6 in two chinese families with dyschromatosis universalis hereditaria. J Dermatol Sci 76: 255-258.
- Wang L, He F, Bu J, Zhen Y, Liu X, et al. (2012) ABCB6 mutations cause ocular coloboma. Am J Hum Genet 90: 40-48.
- Boultwood J, Pellagatti A, Nikpour M, Pushkaran B, Fidler C, et al. (2008) The role of the iron transporter ABCB7 in refractory anemia with ring sideroblasts. PLoS One 3: e1970.
Citation: Seguin A, Ward DM (2018) Mitochondrial ABC Transporters and Iron Metabolism. J Clin Exp Pathol 8: 338. DOI: 10.4172/2161-0681.1000338
Copyright: ©2018 Seguin A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5414
- [From(publication date): 0-2018 - Dec 20, 2024]
- Breakdown by view type
- HTML page views: 4653
- PDF downloads: 761