miR-148a Transcriptionally Regulated by ZEB1 Suppresses Dermal Papilla Cell Proliferation and Promotes Cell Apoptosis via FGF7/MAPK Axis
Received: 20-Mar-2023 / Manuscript No. CMB-23-92350 / Editor assigned: 22-Mar-2023 / PreQC No. CMB-23-92350(PQ) / Reviewed: 12-Apr-2023 / QC No. CMB-23-92350 / Revised: 22-Apr-2023 / Manuscript No. CMB-23-92350(R) / Accepted Date: 24-Apr-2023 / Published Date: 01-May-2023 DOI: 10.4172/1165-158X.1000266
Abstract
Background: Alopecia Areata (AA) is a hair follicle-specific autoimmune disease. Dermal Papilla (DP) cells play key roles in development and growth of hair follicles. MicroRNAs (miRNAs) are closely related to cell activities of DP cells. Herein, we probe the function of miR-148a in regulating cell activities of DP cells.
Methods: The mRNA and protein expressions were assessed using qRT-PCR and western blot. DP cell proliferation was assessed by MTT and EdU assays. In addition, cell apoptosis was evaluated using flow cytometer.Finally, dual luciferase reporter assay was carried out to verify the binding relationship between ZEB1, miR-148a and fibroblast growth factor 7 (FGF7).
Results: Compared with the normal tissues, miR-148a was upregulated in AA skin tissues, while FGF7 was lowly expressed. Function assays displayed that inhibition of miR-148a could promote DP cell proliferation and suppressed cell apoptosis, while miR-148a overexpression showed the opposite effects. FGF7 was identified as a target gene of miR-148a. We subsequently displayed that FGF7 overexpression accelerated DP cell proliferation and suppressed cell apoptosis, which were abolished by RWJ64809 (mitogen-activated protein kinase (MAPK) inhibitor) treatment.
Additionally, FGF7 silencing or RWJ64809 treatment abrogated the effects of miR-148a inhibition on cell activities of DP cells. Finally, it was turned out that ZEB1 transcriptionally inhibited miR-148a expression.
Conclusion: MiR-148a might have great potential as therapeutic target for AA, since miR-148a inhibition induced by ZEB1 facilitated DP cell proliferation and suppressed apoptosis during AA progression through activating MAPK signaling pathway by regulating FGF7 expression.
Keywords
Alopecia areata; Dermal papilla cells; miR-148a; FGF7; MAPK signaling pathway
Abbreviations
Alopecia Areata (AA); MicroRNA (miR); Dermal Papilla (DP); Zinc Finger E-Box Binding Homeobox 1 (ZEB1); Fibroblast Growth Factor 7 (FGF7); Mitogen-Activated Protein Kinase (MAPK); Extracellular Regulated Protein Kinases (ERK); Immunofluorescence (IF); Ethynyl-2ʹ-deoxyuridine (EdU); 3-(4, 5-Dimethylthiazolyl2)-2, 5-diphenyltetrazolium bromide (MTT); 4',6-diamidino-2-phenylindole (DAPI); Quantitative real-time polymerase chain reaction (qRT-PCR); Standard Deviation (SD); Analysis of Variance (ANOVA).
Introduction
Alopecia Areata (AA) is a disease characterized by autoimmune non-scarring alopecia, with a prevalence of about 2% [1]. AA seriously affects the patient’s appearance, causing great troubles to the lives of patients [2]. However, the current treatment methods for AA, which mainly include local or systemic drug treatment, have serious side effects [3]. Dermal Papilla (DP) cells act as key roles in hair follicle growth, making it a potential treatment strategy for AA [4]. However, although early-passage DP cells have been shown to induce hair follicle regeneration both in vivo and in vitro, while the agglutinative growth characteristics of DP cells gradually weaken as the number of passages increases [5]. Therefore, it is important to find effective targets for maintaining the hair inducibility of DP cells.
MicroRNAs (miRNAs) refer to non-coding RNAs with a length of 22-25 nt, which participate in post‐transcriptionally regulating gene expressions [6]. As widely reported, miRNAs are involved in AA progression and cell function of DP cells. For instance, Sheng et al. identified 36 significantly differentially expressed miRNAs in AA patients, indicating miRNA dysregulation might be one of the main reasons that promoted AA development [7]. In addition, miR-195-5p was reported to suppress hair inducibility of DP cells via inhibiting Wnt/β-catenin pathway [8]. MiR-148a is reported to participate in regulating hair follicle growth and development [9]. Specifically, miR- 148a overexpression could markedly suppress the proliferation of DP cells [9]. Previous study displayed that miR-148a was a factor affecting cell function of DP cells; however, the potential mechanism by which miR-148a regulated cell phenotypes of DP cells remains unclear. Zinc Finger E-Box Binding Homeobox 1 (ZEB1) is a transcription factor that promotes metastasis and stem cell characterization [10]. As revealed by Kiratipaiboon, ZEB1 inhibition markedly inhibited the stemness of DP cells [11]. Meanwhile, it was previously reported that ZEB1 upregulation enhanced the stemness of DP cells [12]. Herein, by using bioinformatics prediction, it was found that ZEB1 had potential binding sites to miR-148a. Nevertheless, the regulatory relationship between ZEB1 and miR-148a in AA remains unknown, which deserves further research.
Fibroblast Growth Factor 7 (FGF7) is a secreted protein mainly located in epithelial cells. It is reported that FGF signal regulates the size of dermal papilla [13]. In addition, as previous described, FGF7 was highly expressed immortalized mouse DP cells [14], and FGF7 overexpression in DP cells could prolong the anagen period [15]. All the above evidence suggested that FGF7 was closely related to cell function of DP cells. However, there’s no report about the molecular mechanisms of FGF7 in regulating cell phenotypes of DP cells. As we all known many growth factors are regulated by MAPK pathway [16]. Previous study displayed that MAPK signaling pathway activation contributed to hair growth [17]. More importantly, we observed that FGF7 played an important role in glaucoma optic nerve damage through regulation of the MAPK signaling pathway [18]. However, whether FGF7 is able to regulate cell function of DP cells via MAPK pathway is largely unclear.
Herein, we probed the role of miR-148a in regulating cell function of DP cells. In AA progression, miR-148a inhibition induced by ZEB1 promotes the proliferation of DP cells and inhibited apoptosis through regulation of the FGF7/MAPK axis. Our research provided a new treatment strategy and potential therapeutic targets for AA.
Materials and Method
Clinical samples collection
AA skin tissues were collected from 8 AA patients who treated in the outpatient department of Shandong Provincial Hospital affiliated to Shandong First Medical University. The normal skin tissues were collected from 9 individuals selected randomly from the outpatient department with no history of AA dermatological condition. This study was passed the review of Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University and all participants signed informed consent.
Cell culture and treatment
Human DP cells and 293T cells were purchased from Promocell (Heidelberg, Germany) and ATCC (VA, USA), respectively. All cells were cultured in DMEM (Gibco, MD, USA) mixed with 10% FBS (Gibco) at 37°C with 5% CO2. To inhibit MAPK, DP cells were subjected to 10 μmol/L RWJ64809 (MedChemExpress, NJ, USA) for 24 h.
Cell transfection
The overexpression plasmid of FGF7 (oe-FGF7), the short hairpin RNA of FGF7 (sh-FGF7), the short hairpin RNA of ZEB1 (sh-ZEB1), mimics/inhibitor of miR-148a and their negative controls were transfected into cells with Lipofectamine™ 3000 (Invitrogen, CA, USA). The above plasmids were all obtained from GenePharma (Shanghai, China).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells and tissues with TRIzol (Thermo Fisher Scientific, MA, USA). RNA was reverse transcribed into cDNA with cDNA synthesis kit (Toyobo, Tokyo, Japan). For miRNA, the cDNA was synthesized with the first-strand cDNA synthesis kits (Sangon, Shanghai, China). Then, SYBR (Thermo Fisher Scientific) was employed for qRT-PCR assay. The relative expressions of miRNA and mRNA were respectively normalized by U6 and GAPDH and calculated by 2−ΔΔCt method. The primers used in the study were listed as follows (5’-3’):
miR-148a (F): 5’-CGGCTCAGTGCACTACAGAA-3’
miR-148a (R): 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAAAG- 3’
ZEB1 (F): 5’-GATGATGAATGCGAGTCAGATGC -3’
ZEB1 (R): 5’-ACAGCAGTGTCTTGTTGTTGT-3’
FGF7 (F): 5’-CACAGTGGTACCTGAGGATCG-3’
FGF7 (R): 5’-AATTCCAACTGCCACTGTCC-3’
U6 (F): 5’-CTCGCTTCGGCAGCACA-3’
U6 (R): 5’-AACGCTTCACGAATTTGCGT-3’
GAPDH (F): 5’-CTGACTTCAACAGCGACACC-3’
GAPDH (R): 5’-GTGGTCCAGGGGTCTTACTC-3’
Western blot
The proteins were isolated with RIPA, and then BCA kit was used to detect the protein concentration. And proteins were separated by SDSPAGE, which further transferred to a PVDF membrane (Millipore, MA, USA). The membranes were subsequently blocked with 5% skimmed dry milk for 1 h at room temperature. Then, membranes were incubated overnight at 4℃ with antibodies against Bax (Abcam, 1:1000, ab32503), Bcl-2 (Abcam, 1:1000, ab32124), cleaved caspase 3 (Abcam, 1:1000, ab32042), AKT (Abcam, 1:1000, ab8805), p-AKT (Abcam, 1:1000, ab38449), extracellular regulated protein kinases (ERK) (Abcam, 1:1000, ab32537), p-ERK (Abcam, 1:1000, ab76299), FGF-7 (Abcam, 1:1000, ab131162), ZEB1 (Abcam, 1:1000, ab203829) and GAPDH antibody (Abcam, 1:10000, ab8245). Membranes were then incubated with secondary antibody (Abcam, 1:10000, ab7090, ab97040) for 60 min. The membranes were visualized and imaged by GEL imaging system (Bio-Rad, CA, USA).
Flow cytometry
The Annexin V-FITC apoptosis detection kit (Beyotime) was performed to test cells apoptosis. Cells were re-suspended in 500 μL of 1X Annexin-binding buffer (Beyotime) and then incubated with 10 μL Annexin V-FITC and 5 μL PI stain (Beyotime) for 10 min protect from light. Samples were immediately analyzed using flow cytometry.
3-(4, 5-Dimethylthiazolyl2)-2, 5-diphenyltetrazolium bromide (MTT) assay
Cells were incubated with 5 mg/mL MTT (Sangon) for 4 h at 37℃. Then DMSO (Sigma-Aldrich, MO, USA) was added and the absorbance at 490 nm was examined using a microplate reader (Bioteke, Beijing, China).
Ethynyl-2ʹ-deoxyuridine (EdU) assay
DP cells were plated in 96-well plates (Corning, NY, USA) and incubated with EdU (Sangon) for 2 h at 37°C. Cells were subsequently fixed, permeabilized and incubated with the staining solution for 30 min. Hoechst 33342 was employed to indicate the cells by staining their nucleus. Cells were observed with a fluorescence microscope (Olympus, Tokyo, Japan).
Dual luciferase reporter gene assay
We predicted the target of gene binding site using bioinformatics software. Wild-type (wt) and mutant-type (mut) reporter plasmids of FGF7 3’-UTR sequences were cloned into pmirGLO vector (GenePharma). Then, 293T cells were co-transfected with wt-FGF7 or mut-FGF7 plasmids and miR-148a mimics or mimics NC by Lipofectamine™ 3000 (Invitrogen). Meanwhile, wt and mut reporter plasmids of miR-148a promoter sequences were cloned into pGL3 vector (HonorGene). And 293T cells were co-transfected with wt-miR- 148a or mut-miR-148a plasmids and sh-ZEB1 or sh-NC. The luciferase activity was evaluated by a dual-luciferase reporter assay system (Promega, WI, USA).
Data analysis
All the tests conducted in this work were repeated at least three times. GraphPad Prism 8.0 was applied for statistical data analysis and the measurement data were expressed as means ± SD. Betweengroup differences and multi-group comparisons were determined using Student’s t test and one-way ANOVA, respectively. P < 0.05 was considered to represent a significant difference.
Results
miR-148a inhibition promoted DP cell proliferation and suppressed apoptosis
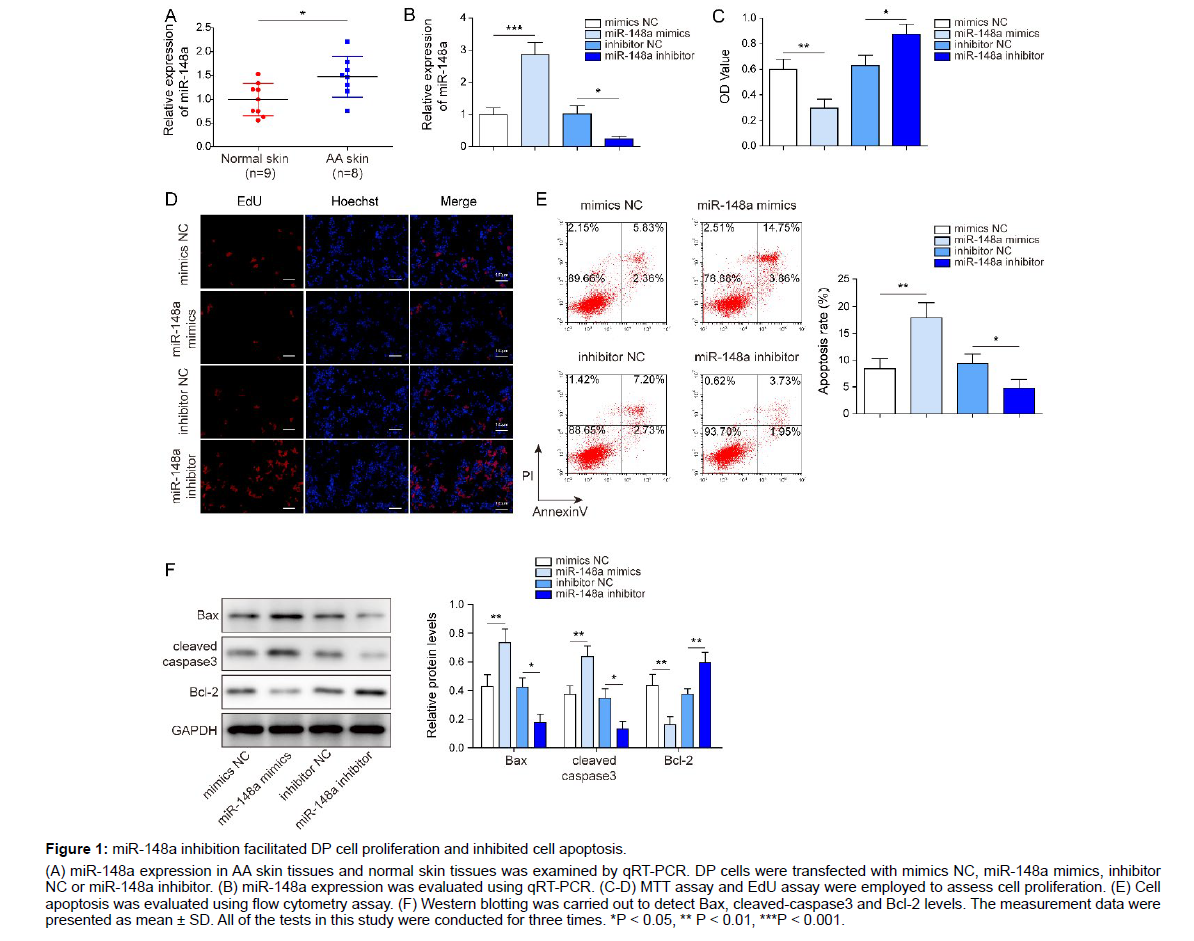
We firstly observed that miR-148a expression markedly increased in AA skin tissues in comparison with normal skin tissues (Figure 1A).
Figure 1: miR-148a inhibition facilitated DP cell proliferation and inhibited cell apoptosis.
(A) miR-148a expression in AA skin tissues and normal skin tissues was examined by qRT-PCR. DP cells were transfected with mimics NC, miR-148a mimics, inhibitor NC or miR-148a inhibitor. (B) miR-148a expression was evaluated using qRT-PCR. (C-D) MTT assay and EdU assay were employed to assess cell proliferation. (E) Cell apoptosis was evaluated using flow cytometry assay. (F) Western blotting was carried out to detect Bax, cleaved-caspase3 and Bcl-2 levels. The measurement data were presented as mean ± SD. All of the tests in this study were conducted for three times. *P < 0.05, ** P < 0.01, ***P < 0.001.
To further probe the potential role of miR-148a in hair growth, DP cells were transfected with mimics NC, miR-148a mimics, inhibitor NC or miR-148a inhibitor, and cell proliferation and apoptosis were evaluated. Firstly, results of qRT-PCR displayed that miR- 148a expression in DP cells was markedly reduced by miR-148a inhibitor transfection, while miR-148a expression was markedly elevated by miR-148a mimics transfection (Figure 1B), suggesting the transfection was successful. Results of MTT assay and EdU assay demonstrated that DP cell proliferation was remarkably suppressed by miR-148a overexpression, while the proliferation was promoted by miR-148a inhibitor transfection (Figure 1C, D). In addition, result of flow cytometry assay subsequently revealed that miR-148a mimics transfection led to elevated cell apoptosis of DP cells, while miR-148a inhibition resulted in reduced cell apoptosis (Figure 1E). Then, results of western blot displayed that miR-148a overexpression significantly increased the levels of pro-apoptotic proteins (Bax and cleaved caspase3) and reduced the level of anti-apoptotic protein (Bcl-2); while miR-148a inhibition presented the opposite effects (Figure 1F). All these results suggested that miR-148a inhibition accelerated DP cell proliferation, while suppressed cell apoptosis.
miR-148a knockdown activated MAPK signaling by upregulating FGF7
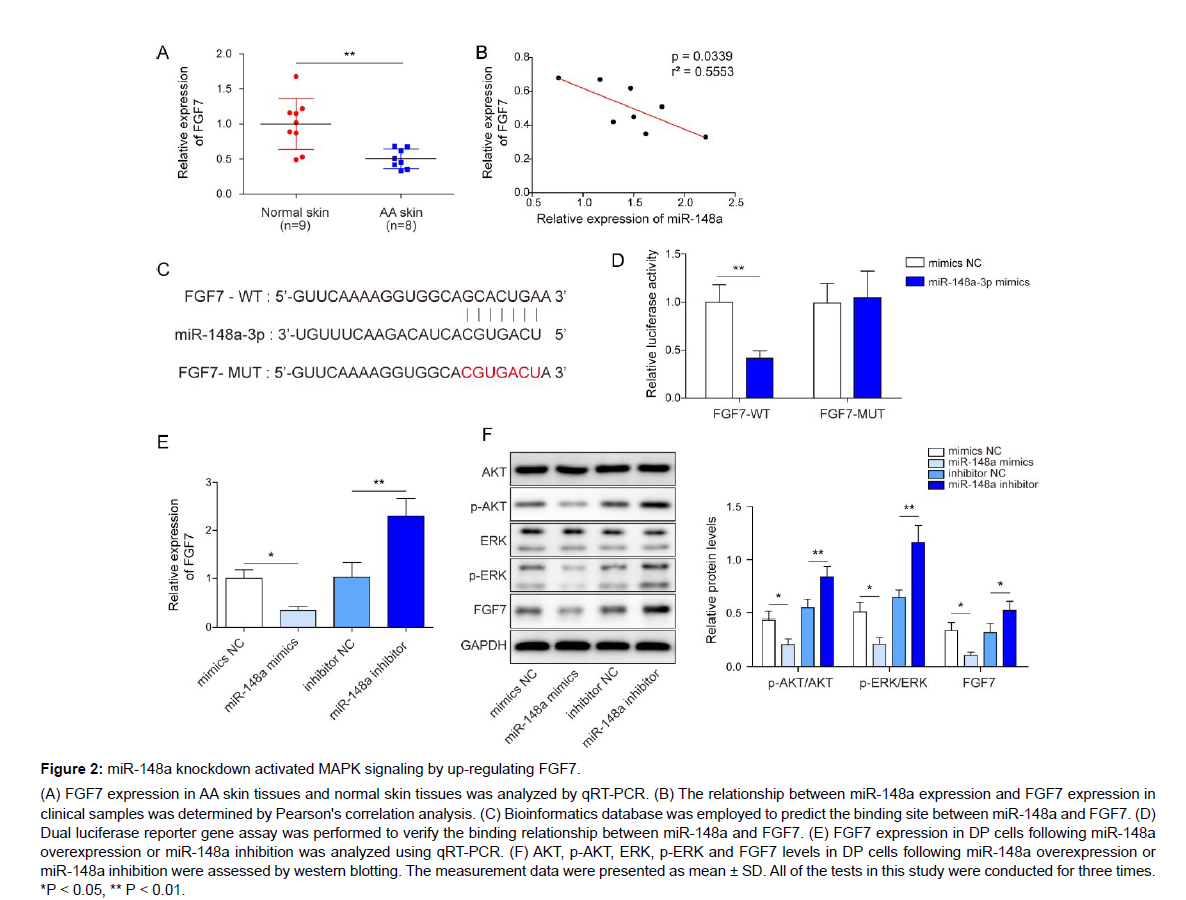
Next, we aimed to probe the downstream molecules and signaling pathways of miR-148a in regulating cell function of DP cells. FGF7 was reported to be highly expressed immortalized mouse DP cells [14]. We firstly observed that FGF7 was significantly downregulated in AA skin tissues (Figure 2A). Subsequently, we analyzed the correlation between miR-148a and FGF7, and the results found that miR-148a expression was negatively correlated with FGF7 expression (Figure 2B). By using bioinformatics database, miR-148a was predicted to have a binding site to FGF7 (Figure 2C). To further verify this binding relationship, dual luciferase reporter gene assay was carried out, and the results displayed that miR‐148a directly bound to FGF7 (Figure 2D). In addition, FGF7 expression was markedly inhibited by miR-148a mimics transfection, while its expression was promoted by miR-148a inhibition (Figure 2E, F). Previous study displayed that MAPK signaling pathway activation contributed to hair growth [17]. Herein, it was found that p-AKT and p-ERK levels were obviously reduced by miR-148a mimics transfection, while p-AKT and p-ERK levels were markedly elevated by miR-148a inhibitor transfection (Figure 2F). In conclusion, miR-148a inhibition could activate MAPK signaling by increasing FGF7 expression.
Figure 2: miR-148a knockdown activated MAPK signaling by up-regulating FGF7.
(A) FGF7 expression in AA skin tissues and normal skin tissues was analyzed by qRT-PCR. (B) The relationship between miR-148a expression and FGF7 expression in clinical samples was determined by Pearson's correlation analysis. (C) Bioinformatics database was employed to predict the binding site between miR-148a and FGF7. (D) Dual luciferase reporter gene assay was performed to verify the binding relationship between miR-148a and FGF7. (E) FGF7 expression in DP cells following miR-148a overexpression or miR-148a inhibition was analyzed using qRT-PCR. (F) AKT, p-AKT, ERK, p-ERK and FGF7 levels in DP cells following miR-148a overexpression or miR-148a inhibition were assessed by western blotting. The measurement data were presented as mean ± SD. All of the tests in this study were conducted for three times. *P < 0.05, ** P < 0.01.
FGF7 overexpression promoted the proliferation of DP cells, while suppressed cell apoptosis
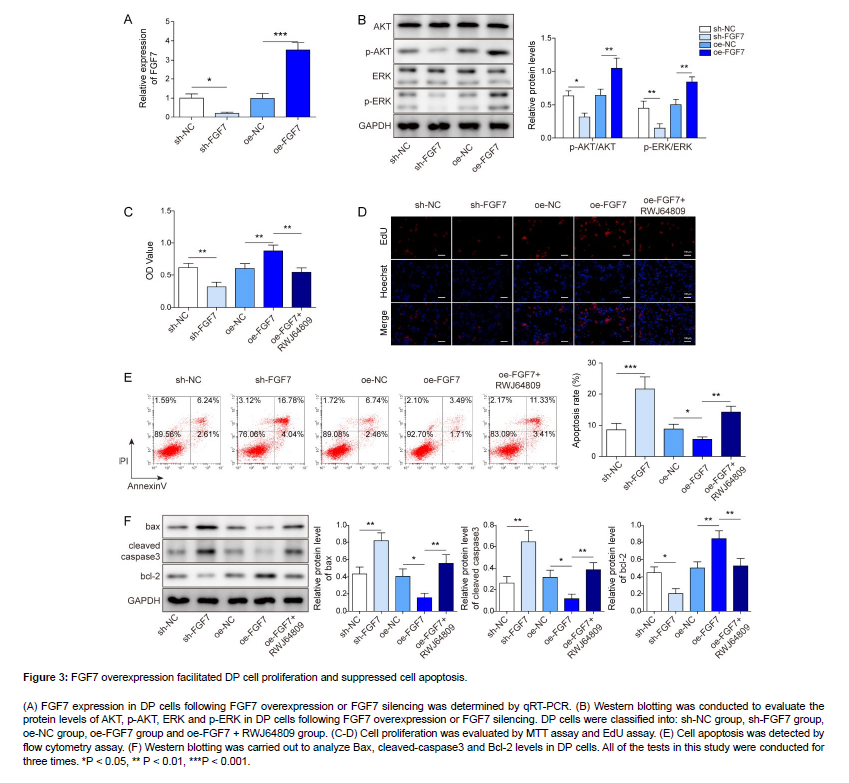
As revealed in Figure 3A, B, sh-FGF7 transfection resulted in reduced FGF7 expression as well as p-AKT and p-ERK levels, while oe-FGF7 transfection presented the opposite effects. To probe the potential function of FGF7/MAPK axis in regulating cell phenotypes of DP cells, DP cells were co-treated with oe-FGF7 and RWJ64809. Functional experiments revealed that cell proliferation of DP cells was obviously suppressed by FGF7 knockdown; the proliferation was promoted by FGF7 overexpression, while RWJ64809 (MAPK inhibitor) treatment reversed the promoting effect of FGF7 overexpression on cell proliferation (Figure 3C, D). As displayed in Figure 3E, sh-FGF7 transfection remarkably enhanced cell apoptosis of DP cells; FGF7 overexpression significantly suppressed cell apoptosis, which was eliminated by RWJ64809 treatment. In addition, FGF7 knockdown resulted in elevated Bax and cleaved-caspase3 levels and reduced Bcl-2 level; as expected, FGF7 overexpression led to reduced Bax and cleaved-caspase3 levels and elevated Bcl-2 level, while it was abolished by RWJ64809 treatment (Figure 3F). Taken together, FGF7 overexpression promoted DP cell proliferation and suppressed cell apoptosis via activating MAPK signaling.
Figure 3: FGF7 overexpression facilitated DP cell proliferation and suppressed cell apoptosis.
(A) FGF7 expression in DP cells following FGF7 overexpression or FGF7 silencing was determined by qRT-PCR. (B) Western blotting was conducted to evaluate the protein levels of AKT, p-AKT, ERK and p-ERK in DP cells following FGF7 overexpression or FGF7 silencing. DP cells were classified into: sh-NC group, sh-FGF7 group, oe-NC group, oe-FGF7 group and oe-FGF7 + RWJ64809 group. (C-D) Cell proliferation was evaluated by MTT assay and EdU assay. (E) Cell apoptosis was detected by flow cytometry assay. (F) Western blotting was carried out to analyze Bax, cleaved-caspase3 and Bcl-2 levels in DP cells. All of the tests in this study were conducted for three times. *P < 0.05, ** P < 0.01, ***P < 0.001.
FGF7 knockdown or RWJ64809 treatment abolished the effects of miR-148a knockdown on cell activities of DP cells
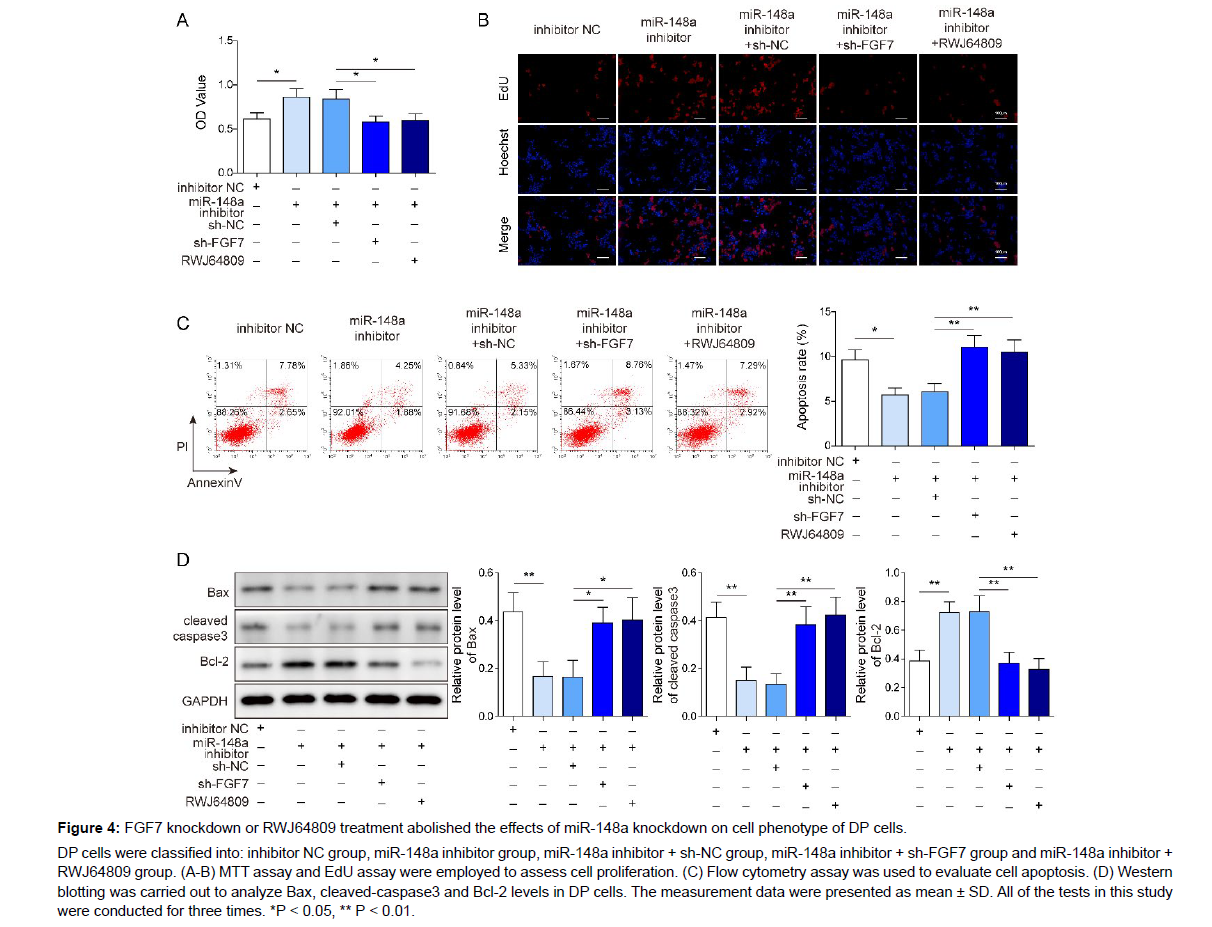
To confirm the potential role of miR-148a/FGF7/MAPK axis in regulating cell phenotypes of DP cells, DP cells were co-transfected with miR-148a inhibitor and sh-FGF7 or co-treated with miR-148a inhibitor and RWJ64809. As displayed in Figure 4A-C, miR-148a inhibition resulted in elevated cell proliferation and reduced cell apoptosis of DP cells, while FGF7 knockdown or RWJ64809 treatment attenuated the effects of miR-148a inhibitor on cell activities of DP cells. In addition, miR-148a inhibitor transfection significantly reduced Bax and cleaved-caspase3 levels and elevated Bcl-2 level, while FGF7 knockdown or RWJ64809 treatment abolished the effects of miR-148a inhibitor (Figure 4D). In summary, miR-148a inhibition promoted DP cell proliferation and suppressed cell apoptosis via activation of FGF7- mediated MAPK signaling.
Figure 4: FGF7 knockdown or RWJ64809 treatment abolished the effects of miR-148a knockdown on cell phenotype of DP cells.
DP cells were classified into: inhibitor NC group, miR-148a inhibitor group, miR-148a inhibitor + sh-NC group, miR-148a inhibitor + sh-FGF7 group and miR-148a inhibitor + RWJ64809 group. (A-B) MTT assay and EdU assay were employed to assess cell proliferation. (C) Flow cytometry assay was used to evaluate cell apoptosis. (D) Western blotting was carried out to analyze Bax, cleaved-caspase3 and Bcl-2 levels in DP cells. The measurement data were presented as mean ± SD. All of the tests in this study were conducted for three times. *P < 0.05, ** P < 0.01.
ZEB1 transcriptionally inhibited miR-148a expression
ZEB1 is a transcription factor which is closely related to the stemness of DP cells [12]. To investigate the function of ZEB1 in regulating AA progression, ZEB1 knockdown was induced in DP cells by transfecting sh-ZEB1 into DP cells. As revealed in Figure 5A, B, ZEB1 expression level in DP cells was markedly reduced by sh-ZEB1 transfection, suggesting the transfection was successful. It was predicted that ZEB1 had potential binding sites to miR-148a promoter by using JASPARE database (Figure 5C). Dual-luciferase reporter gene assay subsequently displayed that ZEB1 knockdown increased the luciferase activity presented by wt-miR-148a (Figure 5D), revealing that ZEB1 directly bound with miR-148a. In addition, it was observed that miR-148a expression in DP cells was markedly increased by ZEB1 knockdown (Figure 5E). In conclusion, ZEB1 transcriptionally inhibited miR-148a expression in DP cells.
Figure 5: ZEB1 transcriptionally inhibited miR-148a expression.
(A-B) ZEB1 expression level in DP cells after sh-NC or sh-ZEB1 transfection was assessed using qRT-PCR and western blotting. (C) The potential binding sites between ZEB1 and miR-148a promoter was predicted by bioinformatics. (D) Dual-luciferase reporter gene assay was performed to analyze the interaction between ZEB1 and miR- 148a promoter. (E) miR-148a expression in DP cells after sh-NC or sh-ZEB1 transfection was assessed using qRT-PCR. The measurement data were presented as mean ± SD. All of the tests in this study were conducted for three times. *P < 0.05, ** P < 0.01.
Discussion
Much evidence has confirmed that DP cells play key roles in regulating hair induction by affecting hair follicle circulation [19]. Recently, miRNAs are confirmed to be key regulators for cell activities of DP cells [20, 21]. Herein, we focused on the regulation of miR-148a on DP cell proliferation and apoptosis. miR-148a silencing promoted the proliferation and suppressed cell apoptosis of DP cells through targeting FGF7 to activate MAPK signaling pathway.
It’s widely reported that miRNAs are crucial for regulating DP cells mediated hair follicle regeneration [22]. For instance, miR-205 could promote cell apoptosis of DP cells [21]. In addition, miR-133b overexpression was reported to suppress DP cell proliferation [23]. MiR-148a, located on chromosome 7p15.2, is reported to regulate the progress of various human malignancies [24]. In addition to the biological roles of miR-148a in tumors, a recent study revealed that miR-148a suppressed DP cell proliferation [9], indicating the potential effects of miR-148a in regulating cell activities of DP cells. However, the precise mechanism by which miR-148a regulated cell phenotypes of DP cells is not fully explained. Herein, our results displayed that miR-148a expression markedly increased in AA skin tissues. Besides, functional experiment revealed that miR-148a inhibition accelerated DP cell proliferation and suppressed cell apoptosis, while miR-148a overexpression presented the opposite effects. Some studies have revealed the interactive regulatory network of transcription factors and miRNAs in diseases [25]. SP1 negatively regulates miR-335 by combining with miR-335 promoter to participate in ovarian cancer metastasis and prognosis [26]. ZEB1 is a transcription factor which is closely related to the stemness of DP cells [11]. In the current research, our results revealed that ZEB1 transcriptionally inhibited miR-148a expression in DP cells. Therefore, all our results indicated a key role of ZEB1/miR-148a axis in regulating cell activities of DP cells.
As widely reported, miRNAs regulate post-transcriptional genes expressions by binding to its targets, leading to mRNAs degradation or translational inhibition [27]. Next, we focused on the downstream of miR-148a. Herein, we confirmed that FGF7 was the target of miR-148a. It was also observed that FGF7 expression was enhanced/suppressed by miR-148a inhibition/overexpression. FGF7, a keratinocyte growth factor, is expressed specifically in mesenchyme [28]. As previously reported, activation of FGF signaling contributes to the increased size of the DP [13]. FGF7 upregulation in DP cells is conducive to DP cell growth [29]. It was also reported that ruxolitinib could directly stimulate anagen-re-entry signals in DP cells through promoting FGF7 expression in DP cells [30]. However, the relationship between miR- 148a and FGF7 in regulating cell activities of DP cells is still unclear. In the current study, function assays displayed that FGF7 overexpression accelerated DP cell proliferation and suppressed cell apoptosis, while FGF7 silencing presented the opposite effects. In addition, FGF7 knockdown eliminated the effects of miR-148a knockdown on cell activities of DP cells. Taken together, miR-488 inhibition promoted the proliferation and suppressed the apoptosis of DP cells via directly targeting FGF7. ERK1/2 and AKT are related to cell proliferation and apoptosis, and are the main pathways of the MAPK pathway [31]. Recently, accumulated evidence has revealed that MAPK signaling pathway is closely related to hair follicle growth and hair growth. For example, Xiao et al. displayed that MAPK signaling pathway activation participated in the enhancement of fatty acids on hair follicle cell proliferation [32]. Additionally, Kim et al. displayed that quercitrin facilitated DP cell proliferation and suppressed cell apoptosis through activating MAPK pathway [17]. It was also reported that MAPK signaling pathway was the downstream pathway of FGF7 in regulating apoptosis of ganglion cells [18]. However, how MAPK signaling pathway can be regulated by miR-148a in DP cells is unknown. Herein, we found that miR-148a inhibition could markedly elevate p-AKT and p-ERK levels, while miR-148a overexpression showed the opposite effects. As expected, FGF7 overexpression could markedly elevate p-AKT and p-ERK levels, while FGF7 silencing presented the opposite effects. In addition, RWJ64809 (MAPK inhibitor) treatment reversed the effects of FGF7 overexpression or miR-148a inhibition on cell activities of DP cells. Therefore, we came to the conclusion that miR- 148a inhibition facilitated DP cell proliferation and suppressed cell apoptosis through activating MAPK signaling pathway by interfering with FGF7.
We conclude that ZEB1/miR-148a/FGF7/MAPK is a novel regulatory mechanism for the regulation of DP proliferation and apoptosis. Therapeutically inhibition of miR-148a or overexpression of FGF7 may facilitate DP cell proliferation and suppress cell apoptosis. Thus, our study suggests that miR-148a/FGF7 may be new therapeutic targets for AA treatment.
Declaration
Ethical approval
This study was passed the review of Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University and all participants signed informed consent.
Conflict of Interest
All authors agree with the presented findings, have contributed to the work, and declare no conflict of interest.
Data Availability Statements
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
The authors express their gratitude to for his help for directing our article. This work was supported by grants from Shandong Medical and Health Science and Technology Development Plan (No.2013WS0101) and Natural Science Foundation of Shandong Province (ZR2021QH361).
Authors' Contribution
Bai-He Wang: Guarantor, concepts, design, review Fan Wu: Experimental studies, data acquisition Jie Zhang: Experimental studies, preparation, editing Sheng-Nan Wang: Data acquisition, data analysis, editing Yuan-Yuan Li: Study concepts, study design, data analysis, preparation, editing
References
- Strazzulla LC, Wang EHC, Avila L, Sicco KL, Brinster N,et al. (2018) Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol 78: 1-12.
- Pratt CH, Kind LE, Messenger AG, Christiano AG, Sundberg JP (2017) Alopecia areata. Nat Rev Dis Primers 3: 17011.
- Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J, et al. (2010) Alopecia areata update: part II. Treatment. J Am Acad Dermatol 62: 191-202, quiz 203-204.
- Randall VA, Hull SP, Nutbrown M, Calver NS, Parkin SM, et al. (1995) Is the dermal papilla a primary target in alopecia areata? J Invest Dermatol 104(5 Suppl): 7s-8s.
- Yang CC, Cotsarelis G (2010) Review of hair follicle dermal cells. J Dermatol Sci 57: 2-11.
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281-297. Google Scholar, Crossref, Indexed at
- Sheng Y, Qi S, Hu R, Zhao J, Rui W, et al. (2019) Identification of blood microRNA alterations in patients with severe active alopecia areata. J Cell Biochem 120: 14421-14430.
- Zhu N, Huang K, Liu Y, Zhang H, Lin E, et al. (2018) miR-195-5p Regulates Hair Follicle Inductivity of Dermal Papilla Cells by Suppressing Wnt/β-Catenin Activation. Biomed Res Int 2018: 4924356.
- Lv X, Gao W, Jin C, Wang L, Wang Y, et al. (2019) Preliminary study on microR-148a and microR-10a in dermal papilla cells of Hu sheep. BMC Genet 20: 70.
- Zhou Y, Lin F, Wan T, Chen A, Wang H, et al. (2021) ZEB1 enhances Warburg effect to facilitate tumorigenesis and metastasis of HCC by transcriptionally activating PFKM. Theranostics 11: 5926-5938.
- Kiratipaiboon C, Tengamnuay P, Chanvorachote P (2015) Glycyrrhizic acid attenuates stem cell-like phenotypes of human dermal papilla cells. Phytomedicine 22: 1269-78.
- Kiratipaiboon C, Tengamnuay P, Chanvorachote P (2016) Ciprofloxacin Improves the Stemness of Human Dermal Papilla Cells. Stem Cells Int 2016: 5831276.
- Yue Z, Jiang TX, Wu P, Widelitz RB, Chuong CM (2012) Sprouty/FGF signaling regulates the proximal-distal feather morphology and the size of dermal papillae. Dev Biol 372: 45-54.
- Guo H, Xing Y, Zhang Y, He L, Deng F, et al. (2018) Establishment of an immortalized mouse dermal papilla cell strain with optimized culture strategy. Peer J 6: e4306.
- Gentile P, Garcovich S (2019) Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 8.
- Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, et al. (2010) NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc Natl Acad Sci U S A 107: 20477-20482.
- Kim J, Kim SR, Choi YH, Shin JY, Kim CD, et al. (2020) Quercitrin Stimulates Hair Growth with Enhanced Expression of Growth Factors via Activation of MAPK/CREB Signaling Pathway. Molecules 25.
- Peng H, Sun YB, Hao JL, Lu CW, Bi MC, et al. (2019) Neuroprotective effects of overexpressed microRNA-200a on activation of glaucoma-related retinal glial cells and apoptosis of ganglion cells via downregulating FGF7-mediated MAPK signaling pathway. Cell Signal 54: 179-190.
- Zhang P, Kling RE, Ravuri SK, Kokai LE, Rubin JP, et al. (2014) A review of adipocyte lineage cells and dermal papilla cells in hair follicle regeneration. J Tissue Eng 5: 2041731414556850.
- Lin BJ, Lin GY, Zhu JY, Yin GQ, Huang D, et al. (2020) LncRNA-PCAT1 maintains characteristics of dermal papilla cells and promotes hair follicle regeneration by regulating miR-329/Wnt10b axis. Exp Cell Res 394: 112031.
- Liu G, Li S, Liu H, Zhu Y, Bai L, et al. (2020) The functions of ocu-miR-205 in regulating hair follicle development in Rex rabbits. BMC Dev Biol 20: 8.
- Andl T, Botchkareva NV (2015) MicroRNAs (miRNAs) in the control of HF development and cycling: the next frontiers in hair research. Exp Dermatol 24: 821-826.
- Deng W, Hu T, Han L, Liu B, Tang X, et al. (2021) miRNA microarray profiling in patients with androgenic alopecia and the effects of miR-133b on hair growth. Exp Mol Pathol 118: 104589.
- Li Y, Deng X, Zeng X, Peng X (2016) The Role of Mir-148a in Cancer. J Cancer 7: 1233-1241.
- Tang F, Jiang X, Liao S, Liu Y, He M (2022) Construction of a transcription factor-miRNA-mRNA interactive network elucidates underlying pathogenesis for osteosarcoma and validation by qRT-PCR. Medicine (Baltimore) 101: e31049.
- Wang S, Li Y, Sun S, Cai J, Cao J (2020) Sp1 promotes ovarian cancer cell migration through repressing miR-335 expression. Biochem Biophys Res Commun 524: 211-216.
- Ambros V (2004) The functions of animal microRNAs. Nature 431: 350-355.
- Beenken A, Mohammadi M (2009) The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8: 235-253.
- Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, et al. (2015) Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv 1: e1500973.
- Kim JE, Lee YJ, Park HR, Lee DG, Jeong KH, et al. (2020) The Effect of JAK Inhibitor on the Survival, Anagen Re-Entry, and Hair Follicle Immune Privilege Restoration in Human Dermal Papilla Cells. Int J Mol Sci 21.
- Kim DS, Kim SY, Chung JH, Kim KH, Eun HC, et al. (2002) Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell Signal 14: 779-785.
- Xiao L, Zhang X, Chen Z, Li Y, Li B, et al. (2020) ERK1/2 Pathway Is Involved in the Enhancement of Fatty Acids from Phaeodactylum tricornutum Extract (PTE) on Hair Follicle Cell Proliferation. Biomed Res Int 2020: 2916104.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Wang BH, Wu F, Zhang J, Wang SN, Li YY (2023) miR-148aTranscriptionally Regulated by ZEB1 Suppresses Dermal Papilla Cell Proliferationand Promotes Cell Apoptosis via FGF7/MAPK Axis. Cell Mol Biol, 69: 266. DOI: 10.4172/1165-158X.1000266
Copyright: © 2023 Wang BH. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3985
- [From(publication date): 0-2023 - Nov 18, 2025]
- Breakdown by view type
- HTML page views: 3572
- PDF downloads: 413