Review Article Open Access
Mineral Trioxide Aggregate Use in Pediatric Dentistry: A Literature Review
Jihan Khan1, Azza El-Housseiny2 and Najlaa Alamoudi3*
1Department of Dentistry, University Health Services Center, King Abdulaziz University, Saudi Arabia
2Department of Pediatric Dentistry, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia
3Department of Pediatric Dentistry, King Abdulaziz University, Saudi Arabia
- *Corresponding Author:
- Najlaa Alamoudi
Department of Pediatric Dentistry
King Abdulaziz University, Saudi Arabia
Tel: +966 505622325
E-mail: nalamoudi@kau.edu.sa
Received Date: October 26, 2016; Accepted Date: November 15, 2016; Published Date: November 22, 2016
Citation: Khan J, El-Housseiny A, Alamoudi N (2016) Mineral Trioxide Aggregate Use in Pediatric Dentistry: A Literature Review. J Oral Hyg Health 4: 209. doi:10.4172/2332-0702.1000209
Copyright: © 2016 Khan J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Oral Hygiene & Health
Abstract
Mineral trioxide aggregate (MTA), is unique endodontic cement that was initially introduced as a material for root perforation repair. Over the years its use has expanded to include versatile applications in the field of pediatric dentistry. The purpose of this article was to conduct an updated review on mineral trioxide aggregate (MTA) and on its applications in the practice of pediatric dentistry.
Sources and data: Electronic databases, “PubMed”, “Cochrane Database” and “Google Scholar”, were used to identify relevant English-language studies and literature published in the period from 1993 to 2016. The scientific papers were then screened for their relevance to the intended objectives. A combination of the key search terms mineral trioxide aggregate, MTA, pulp therapy, clinical applications, and pediatric dentistry were used.
Study Selection: Abstracts and full text articles were used to identify studies describing the composition, manipulation, properties, types, and clinical features. In addition, controlled clinical trials of clinical applications and relevant laboratory research on its properties and safety were also included.
Conclusions: MTA is a unique material with various advantages. It has been used successfully by pediatric dentists in a variety of clinical applications. However, its drawbacks especially its high cost, discoloration potential, difficulty in handling, and long setting time cannot be overlooked. With the emergence of other novel tricalcium silicate based materials that overcome MTA’s key limitations, they are competing to be the next potential dentin substitutes for the various clinical application in which MTA has been used. Nevertheless, with the recent introduction of new improved MTA products, MTA-based materials are likely to yet remain at the heart of good pediatric dental practice for many years to come.
Keywords
Mineral trioxide aggregate; Clinical applications; Pediatric dentistry; Pulp therapy
Introduction
Over the years, various types of dental materials have been developed to serve different purposes and to be applied to multiple scenarios in the field of dentistry. Mineral trioxide aggregate (MTA), is a unique endodontic cement that was initially introduced as material for root perforation repair by Mahmoud Torabinejad at Loma Linda University in 1993 [1]. It is primarily composed of tricalcium silicate, tricalcium alluminate, tricalcium oxide, and bismuth oxide [2]. It is formulated of fine hydrophilic particles that solidify in the presence of moisture or blood [3]. MTA was approved by the U.S. Food and Drug Administration for endodontic use in 1998 [4].
Further research over the years on MTA resulted in expanding its applications to include its use as a retrograde filling material [5], repair material for internal and external root resorption [6], and as an apexification material [7,8]. Not to mention its other diverse applications in the practice of pediatric dentistry. In addition to apexification, it is used for the management of the coronal part of fractured roots, in pulpotomies of primary and permanent teeth, and in pulp capping of young permanent teeth [9-12].
In view of the versatile endodontic/restorative applications of this ever-evolving material in pediatric dentistry, an updated review of MTA is encouraged. The aim of this review was to conduct an updated search on the composition, manipulation, types, properties, disadvantages, and clinical applications of MTA in the practice of pediatric dentistry.
Materials and Methods
Electronic databases, “PubMed”, “Cochrane Database” and “Google Scholar”, were used to identify relevant English-language studies and literature published in the period from 1993 to 2016. Abstracts and full text articles were used to identify studies describing the composition, manipulation, properties, types, and clinical features. In addition, controlled clinical trials of clinical applications and relevant laboratory research on its properties and safety were also included. A combination of the key search terms mineral trioxide aggregate, MTA, pulp therapy, clinical applications, and pediatric dentistry were used.
Results
Two hundred and twenty-seven articles together with several references from certain review articles were reviewed. The clinical applications of MTA in pediatric dentistry were illustrated in fortyseven recent studies.
Discussion
Composition
MTA is a tri-mineral aggregate, which is composed of tricalcium silicate, tricalicum aluminate, tricalcium oxide, silicate oxide and bismuth oxide [2]. The material contains calcium oxide (50-75% by weight) and silicon dioxide (15–25%), when they are mixed, they result in the production of tricalcium silicate, dicalcium silicate, tricalcium aluminate and tetracalcium aluminoferrite [13]. When water is added, the cement will hydrate to form a silicate hydrate gel that will eventually solidify to form a hard structure [13]. This hydration reaction was found to result in calcium hydroxide (Ca(OH)2) as a byproduct [14]. In addition, calcium ions release with a resultant alkaline pH rise was also reported in several studies [15-17]. After mixing MTA, its pH value was found to be 10.2 and rises at 3 hours to 12.5 [18]. This resultant microenvironment with high pH contributes to the particularly appealing antibacterial properties of the material [19].
MTA basically resembles Portland cement in composition with the exception of bismuth oxide which is added to MTA for radio capacity [20,21]. MTA also has more uniform and finer particle size and less gypsum than Portland cement, this decreased gypsum reduces the cement setting time. Additionally, Portland cement has greater level of toxic heavy metals and aluminium compared to MTA [13,22]. Accelerators like sodium phosphate dibasic (Na2HPO4), calcium formate (Ca(HCO2)2), and calcium chloride (CaCl2) are included to reduce the setting time [23,24].
Various studies examined the composition of MTA. They generally revealed some dissimilarity related to the type of MTA, the equipment that was used for examining the composition of the material, or the type of liquid mixed with the MTA powder [14,21,25-32].
Types of MTA
There are two basic forms of MTA based on color, gray (GMTA) and white (WMTA). MTA was initially presented as gray Pro-root MTA, however, the potential for discoloration of GMTA lead to the development of WMTA in 2002 [18,33].
They principally differ in that WMTA was found to have lower quantities of iron, aluminum, and magnesium oxides than GMTA [25-27]. The reduced ferrous oxide (FeO) was suggested by Asgary and colleagues [25-27] to be the likely cause of the lighter color of WMTA. Other differences between WMTA and GMTA were reported in the literature. GMTA’s crystal size was found to be about 8 times larger than WMTA’s crystal size [27]. GMTA also has significantly longer setting time; less radiopacity; solubility; and pH value than WMTA [34,35]. Concerning the compressive strength, there are conflicting results with one study reporting significantly less compressive strength [35], while two other studies reported more compressive strength of WMTA compared to GMTA [36,37]. Furthermore, regarding their antimicrobial behavior, it was reported that GMTA achieved similar antimicrobial action against particular microorganisms at lower concentrations than WMTA [38,39]. However, Asgary and Kamran [40], reported similar antibacterial properties for both types of MTA.
Other variations include incorporating resin in the MTA mix to be used as a root canal sealing cement. The purpose was to improve material flow, dentine bonding, and setting time and so to reduce micro-leakage. An example is the MTA Fillapex (Angelus, Londrina, Brazil) [41]. However, it was found that adding resin to the materials results in the reduction of the desired free Ca(OH)2 (essential for continued root formation in immature permanent teeth) [42,43].
The NeoMTA (NuSmile, Huston, USA), on the other hand, is a pure MTA and does not contain resin. It is marketed as a cost effective MTA intended to be used for pediatric pulp therapy. The powder is provided in a lined vial that is re-sealable which facilitates the use of only the needed amount of MTA in each treatment thereby enhancing costeffectiveness. The liquid used for mixing is a gel which makes it easier to mix and apply. In addition, it has a non-staining formulation and a fast setting time. All these features are desirable while delivering treatment to the pediatric patient [44]. Another comparable material is the Neo MTA Plus (Avalon Biomed Inc., Bradenton, FL) it also possesses a powder-gel formula and is promoted to be used in pulpotomies because it does not stain the tooth structure by replacing bismuth oxide with tantalum oxide for opacity [42].
Manipulation and setting reaction
To prepare MTA, its powder is mixed with sterile water in a ratio of 3:1 (powder to liquid) [45]. It was found that using either doxycycline or chlorhexidine (CHX) instead of distilled water, had no effect on MTA’s sealing ability [31]. A metal or plastic spatula can be used for mixing on a glass slab or paper pad to form the consistency of putty like paste [46]. Initial mixing of the material yields a colloidal gel that eventually hardens to form a solid structure [45]. A paper point, plugger, ultrasonic condensation, or carriers with special designs and messing guns can be used to deliver the MTA mix to the desired location [46].
The mean setting time for MTA is 165 ± 5 minutes [18,45]. Moisture from the surrounding tissues or from moistened cotton pellet will help the setting reaction [18,47]. Nevertheless, excess moisture can lead to ‘soupy’ mix [48,49]. Using CaCl2, Ca(HCO2)2, and Na2HPO4 as accelerators was found to significantly lower MTA’s setting time, however, it lowers the compressive strength significantly as well [23,24]. On the other hand, using 2% lidocaine anesthetic solution and saline lengthened the setting time without significantly affecting the compressive strength [23].
The mixing time of MTA is very crucial. Prolonged mixing can lead to dehydration of the mix. The mixing time was recommended to be less than 4 minutes [50]. Also, it is preferred to use the mix immediately after mixing to prevent the dehydration and drying into a sandy mixture [51].
Hand instruments or ultrasonic condensation can be used for the placement of MTA into the desired location. Parashos et al. [52] found improved compaction and flow of MTA with ultrasonic, however, it can adversely affect the properties of MTA when used in excess. Two seconds of ultrasonication per increment was the best time to give more desirable values in microhardness, sealing, and absence of radiographic voids. In a recent study, the ultrasonic technique decreased the setting time of MTA [53].
Concerning condensation pressure, it is better to avoid excess pressure while condensing MTA. Increasing the condensation pressure could decrease the MTA’s surface hardness because it could lessen the spaces available for water ingress which is necessary for the cement hydration [54].
In one study, it was found that placement of glass ionomer cement (GIC) over WMTA after 45 minutes for one-visit perforation repair had no effect on the calcium salts’ formation in the two materials’ interface nor on the setting of the WMTA [55]. In addition, the presence of WMTA did not disturb the GIC setting [56].
The addition of GIC powder to the MTA mix has been suggested to improve the setting time and handling characteristics of MTA. One study showed improved setting times but poor compressive strength and pH when compared to MTA powder alone [57]. Another study examined the effect of adding the GIC to the MTA mix (for the purpose of improving the setting time) on the resultant amount of calcium released from the mixture, which is the base for the biological actions of MTA. It was found that adding MTA and GIC at a proportion of 2:1 by volume did not significantly impact the calcium release from the mixture. However, further clinical evaluation was suggested [58].
Properties of MTA
Chemical and physical properties
Key features of the chemical and physical properties of MTA are summarized in Table 1. MTA’s compressive strength was reported to be affected by several factors like, the type of MTA, the type of liquid used to mix the material, the environment’s pH value, the condensation pressure on the material, and the condition of MTA storage [36,37,47,54]. Etching WMTA with phosphoric acid (37%) resulted in a significant reduction in the compressive strength of the MTA. Therefore, it was recommended to postpone acid-etch composite restorations after the placement of MTA for a minimum of 96 hours [59].
| Property | Features |
|---|---|
| Compressive strength | It was reported to be 40.0 MPa at 24 hours and it increases up to 67.3MPa at 21 days in the presence of moisture [2]. |
| Flexural strength | Increase by two sided hydration of MTA [60]. |
| Push-out strength | Increase by moisture [61]. |
| Marginal adaptation and sealing ability | Enhanced in the presence of moisture [62], good sealing of MTA is achieved with a thickness of around 4 mm [62,63]. |
| Solubility | Low or no solubility [2,64-66]. However, increased water: powder ratio in the MTA mix may increase its solubility and porosity[67]. |
| Displacement | The thickness of MTA directly effects its displacement when applied as an apical barrier [18]. An MTA apical barrier with a thickness of 4 mm offers significantly more resistance to displacement compared to a thickness of 1 mm [68]. |
| Retentive Strength | Low, it is not a suitable luting agent [69]. |
| Radiopacity | The mean value of MTA’s radiopacity was found to be 7.17mm of an equivalent thickness of aluminum [2]. |
| Reaction with other dental materials | MTA does not react or interfere with any other restorative material [55,56,70]. |
Table 1: Summary of the chemical and physical properties of MTA.
The flexural strength is the material’s capability to withstand deformation. It is highly recommended to place a wet cotton pellet on top of the placed MTA for the first 24 hours to increase the flexural strength then remove it to avoid the subsequent decrease in the flexural strength 72 hours after MTA receives moisture [18].
The push-out strength is the material’s ability to withstand dislodgement. It is a crucial factor for MTA since soon after repair, the material can be dislodged by the tooth function. It was found that the push-out strength of MTA is lower than that of the intermediate restorative material (IRM) or Super ethoxybenzoic acid (EBA) [71].
Both the maximum compressive and push-out strength of MTA was found to be reached several days after mixing [18]. While the sealing ability and marginal adaptation of MTA, was found to be better than the other conventional materials used for root-end filling (attributed to the MTA expansion in its setting reaction) [62,72], the retentive strength of MTA, however, was found to be significantly inferior to GIC or zinc phosphate cement as a luting cement [69]. Additionally, the adaptation of MTA to dentin may be affected by the presence of residual Ca(OH)2 in the cavity, reducing the MTA’s sealing ability either by reacting chemically with MTA or by becoming a mechanical barrier [12].
Concerning the Reaction of MTA with other dental materials, when GIC was placed over MTA it did not affect the setting reaction of the MTA or the GIC [55,56]. In addition, sufficient bond strength was also maintained when a resin composite or a compomer was placed over WMTA using the total-etch one-bottle adhesive [73]. However, acid etching before the first 96 hours after mixing MTA was found to affect the surface micro hardness and compressive strength of MTA [59].
Biologic properties
Concerning the antibacterial and antifungal properties, numerous investigations were conducted with conflicting results. Some studies reported that MTA has limited antimicrobial effect [74-76], while others reported that it has antifungal effects [77,78]. These conflicting results may be due to variability in the source of the prepared material, the species of the microorganisms that are tested, as well as the type and concentration of the MTA used [18].
An in vitro study comparing the antibacterial actions of WMTA and GMTA at different concentrations by the tube dilution test found that increasing the concentration of MTA increases its antibacterial effect. However, higher concentrations of WMTA than GMTA were needed to yield similar antibacterial effects [38]. Likewise, another in vitro test found that the antifungal effect of WMTA was enhanced by increasing its concentration [79]. The use of CHX to mix with the MTA powder significantly increased the antimicrobial activity of the material; however, it decreased the compressive strength [36]. In addition, the type of CHX whether liquid or gel was found to have different effects on the setting of MTA [23,36].
With regards to biocompatibility, bacterial and cell culture assay studies showed that MTA was not mutagenic or cytotoxic [80,81]. Studies which have examined MTA samples in the form of intraosseous and subcutaneous implants in animals found minimal inflammatory responses in the soft tissue and bone [12,82,83]. Lessa et al. [84], however, pointed out that when MTA is applied directly to the pulp tissue in a humid environment it can be solubilized which could permit leaching or dissolution of some of the MTA chemical elements with potential toxic effects. In addition, Balto [85] studied the effect of ProRoot MTA on the morphology of human fibroblasts and found that morphological alterations were observed in some cells. Indicating the potential toxicity of some of the elements released from MTA.
In return, an in vivo study that analyzed the effect of a hemostatic agent, which is plant-based, “Ankaferd Blood Stopper” (ABS) on the biocompatibility of MTA during apical surgery with the purpose to control blood contamination in the surgical crypt, it was found that ABS-contaminated MTA did not affect the biocompatibility of MTA [86].
In relation to mineralization, Myers et al. [87] established that MTA induces formation of a dentin bridge similar to Ca(OH)2. It was concluded by Faraco et al. [88] that the dentin bridge formed with MTA is relatively faster and with good structural integrity than with Ca(OH)2. Also, MTA results in higher mineralization compared to Ca(OH)2 [89].
MTA has the potential to stimulate the cementoblasts and therefore, to produce cementum [2]. It also allows the periodontal ligament (PDL) fibers to overgrow over its surface. It was also reported that MTA interacts favorably with bone-forming cells that were found to remain viable and release collagen [90]. These properties of MTA indicate the regenerative potential of the material.
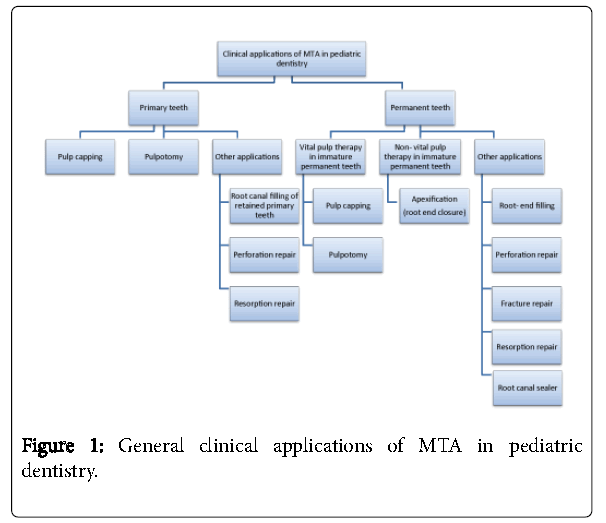
Clinical applications in pediatric dentistry
MTA has been used in a wide variety of clinical applications in the pulp therapy of both primary and permanent teeth (Figure 1).
MTA use in primary teeth
Pulp capping: The American academy of pediatric dentistry (AAPD) recommended the use of MTA as a direct pulp capping material to be placed on pinpoint mechanical or traumatic pulp exposures in primary teeth with normal pulps when conditions for a favorable response are optimal [91]. There are few studies that evaluated the use of MTA for pulp capping in primary teeth [92,93]. The results from a case report and a case series showed that MTA can be successfully used for direct pulp capping of primary molars [92,93]. In a prospective clinical study, Tuna and Olmez [94] reported clinical and radiographic success after 24 months fallowing pulp capping of primary molar teeth with either Ca(OH)2 or MTA .
Pulpotomy: The AAPD recommended the use of MTA for pulpotomies of primary teeth with normal pulps or reversible pulpitis when caries removal results in pulp exposure or after a traumatic pulp exposure [91]. Clinical trials have revealed that MTA’s performance is equal or superior to formocresol (FC), ferric sulfate, and might be considered as one of the favored pulpotomy materials [9,95-102]. The introduction of MTA for pulpotomy overcame the drawbacks of FC such as its potential toxicity, caustic nature, and tissue irritation and inflammation upon contact with soft tissue [101]. Farsi et al. [9] found that pulpotomized primary molars treated with MTA had significantly more success than those treated with FC. Sushynski et al. [101] conducted a randomized controlled trial and concluded that MTA demonstrated significantly better radiographic outcomes as a pulpotomy medicament compared to diluted FC which also exhibited a higher frequency of internal root resorption in the FC-treated molars. A recent systematic review and meta-analysis comparing MTA and FC used for primary molar pulpotomy, found that MTA was more effective and showed higher success rate than FC at six to 24-months follow-ups [103]. However, Marghalani et al. [104] systematic review and meta-analysis comparing long-term (24 months) clinical and radiographic success of MTA and FC as a pulp-dressing material in pulpotomy treatment in primary molars showed comparable success rates.
Biodentine is a novel calcium-silicate based bioactive material that is similar to MTA. It has been introduced as a “dentine replacement” material, its mechanical properties resemble those of natural dentine. It has been used in a variety of clinical applications similar to those in which MTA is used [105,106]. In a study that compared the clinical and radiographic success of MTA, Biodentine, and Laser when used as pulpotomy agents in primary molar teeth, it was found that all three showed similar clinical and radiographic success at their three and six months’ evaluations [106].
Other applications: MTA was also reported to be used as a root canal filling of retained primary teeth [107] and in furcation perforation repair and resorption repair of primary teeth [46,108].
Vital pulp therapy in immature permanent teeth
The objective of apexogenesis in vital immature young permanent teeth is to allow the continued physiologic root development and apex formation by placing an appropriate dressing medicament in the appropriate vital pulp therapy procedure such as indirect or direct pulp capping, and partial pulpotomy for carious exposures and traumatic exposures (Cvek pulpotomy) [91]. Various studies have investigated the use of MTA as a dressing material for vital pulp therapy in permanent teeth.
Pulp capping: Regarding the use of MTA for indirect pulp capping in permanent teeth. Leye Benoist et al. [109] conducted a randomized controlled trial to compare between MTA and Ca(OH)2 when used as an indirect pulp-capping material, at three months, a significantly higher success rate was observed in the MTA group, though, at six months there was no statistically significant difference in the dentine thickness between the two groups. They recommended that further histological studies are required for supporting these results.
AAPD recommended the use of MTA (or Ca(OH)2) for direct pulp capping in permanent teeth for small carious or mechanical exposures in teeth with normal pulps [91]. There are numerous prospective studies that were conducted to compare MTA to Ca(OH)2 in healthy teeth capped after mechanical pulp exposures and subsequently validated by histopathological examination and they demonstrated that MTA resulted in a thicker dentinal bridge, less pulpal inflammation and more favorable pulpal condition than Ca(OH)2 [110,111]. However, Iwamoto et al. [112] found no significant difference between MTA and Ca(OH)2, while Accorinte et al. [113] reported that initial healing was better with MTA, but, subsequent healing was similar in both.
In a study comparing between direct pulp capping using either MTA or Biodentine in healthy permanent molars which were planned to be extracted for orthodontic purposes, no significant difference was found between MTA and Biodentine upon histopathological examination of the pulp-dentin complex after 6 weeks [114].
Observational studies in which MTA was placed over carious exposures in permanent teeth showed promising results [10,115]. In a nine year fallow up of an observational study in which MTA was placed over carious exposures in permanent teeth, showed favorable outcomes of 97.6% of the treated teeth with complete root formation in all of the treated immature teeth with open apices [115].
In a retrospective study comparing MTA with Ca(OH)2 when used for direct pulp capping over carious exposures in permanent teeth, it was found that the Ca(OH)2 treated teeth had significantly higher failure risks than those treated with MTA [116].
Pulpotomy: The use of MTA as a pulpotomy dressing material in permanent teeth have been widely investigated. In a study that examined the use of MTA versus Ca(OH)2 for the pulpotomy of premolar teeth that were scheduled for extraction after four and eight weeks for orthodontic treatment, MTA showed a more homogenous dentin bridge formation and less pulpal inflammation than Ca(OH)2 [117]. El-Meligy and Avery [11] conducted a prospective study using either MTA or Ca(OH)2 for the pulpotomy of immature permanent teeth, the results showed that there was no statistically significant difference neither clinically nor radiographically between the two groups. Similar results were seen in other studies where pulpotomy was done in cariously exposed permanent molars using MTA or Ca(OH)2, both demonstrating comparable success for two to three years fallow up periods [118,119].
Similarly, in a recent systematic review of pulp capping and partial pulpotomy by Fransson et al. [120], it was concluded that Ca(OH)2 or MTA frequently result in the formation of a hard tissue barrier when applied on an exposed pulp, however, the scientific evidence is insufficient in that MTA promotes more frequent hard tissue formation than Ca(OH)2.
Non vital pulp treatment in immature permanent teeth / Apexification (root end closure)
The objective of apexification is to induce root end closure in an incompletely formed non-vital permanent tooth. Root end closure can be achieved by the use of an apical barrier such as MTA and the remaining canal space can then be filled with Gutta percha. In cases with a thin canal wall, the canal can be filled with MTA or composite resin to support the tooth against fracture [91].
Ca(OH)2 has been widely used for apexification. But then again, this method requires the placement of Ca(OH)2 for a long time in the root canal to accomplish the apical hard tissue barrier formation [121,122]. The use of MTA as an apical barrier was introduced in 1999 [123] and has subsequently become the material of choice for this procedure mainly because of its shorter treatment time as a one visit procedure [124,125]. Many studies have reported the clinical success of MTA in the apexification of non-vital immature permanent teeth [8,126,127]. El-Meligy and Avery [7] compared the apexification with either Ca(OH)2 or MTA, and found that there was no statistically significant difference between the 2 groups, clinically or radiographically. Similarly, a systematic review and meta-analysis on the apexification of immature teeth with Ca(OH)2 or MTA, showed that there was no significant discrepancy between them in the rate of clinical success and apical barrier formation [122].
In a recent study comparing MTA and Ca(OH)2 for the treatment of immature non-vital permanent incisors by either ultrasonic or hand filing of the canal, it was found that ultrasonic filing plus MTA placement needed the shortest mean time for the formation of the apical hard tissue barrier, however, Ca(OH)2 was better in the elongation of the apical root length regardless of the method of filing used [128].
Other applications in permanent teeth
Root- end filling: Efficiently filling the root canal to secure good sealing and leakage prevention is the key element for the success of periapical endodontic surgery. In a review by Kim and Kratchman [5] on the practice and materials used in modern endodontic surgery, they stated that MTA was the most biocompatible root-end filling material and that it can be used in endodontic surgery with predictable outcomes. In a meta-analysis on the materials used as fillers in periapical surgery, it was found that MTA offers the best seal with superior biocompatibility and high clinical success rates, and that it is the only root-end filling material that stimulates tissue regeneration in comparison to IRM, amalgam, and Super EBA [129].
Perforation repair: Perforation repair with various dental materials, such as amalgam, gutta-percha, GIC, zinc EBA cement, and IRM, have been researched over the years with varying degrees of success [130]. With the introduction of MTA, promising results were believed to be accomplished with its use for perforation repairs [131]. In a systematic review and meta-analysis by Siew et al. [130] on the treatment outcome of non-surgically repaired root perforation it was reported that the overall pooled success rate was 72.5%, and the success rate was enhanced to 80.9% by the use of MTA but the difference was not statistically significant.
A comparable success rate was seen by Mente et al. [132] who investigated the outcome of 64 teeth that were managed with MTA for the repair of root perforations with a long term fallow up period of 12– 107 months. It was found that 86% of the teeth healed and that MTA demonstrated good long-term root perforation sealing ability regardless of the perforation location.
Fracture repair: Fallowing root fracture of permanent teeth, the coronal non-vital portion of the fractured tooth can be sealed with MTA [12]. Many case reports demonstrated the use of MTA mainly for horizontal root fractures’ repair without any problems or symptoms. [133-137].
Resorption repair: Several case reports have described successful treatment of external root resorption using MTA as the material to fill the resorbed area of the tooth surface with surgical, nonsurgical methods, or by a combination of these approaches [138-140].
Concerning the treatment of internal resorption, successful use of MTA for the repair of the resorption defects by surgical and nonsurgical procedures in the management of such cases has been reported in some case reports [141-143].
Root canal sealer: In a review by Parirokh and Torabinejad [144] it was suggested that MTA can be used as a root canal sealer, it induces closure of the foramen of the canal by the deposition of new cementum but cautions should be taken from overfilling as it might have adverse effects on periapical tissues. Thakur et al. [145], in 2013, compared the clinical and radiological outcome of MTA or epoxy resin as a root canal sealer with ZOE sealer and found no significant difference between them at six months. Further studies are needed to test MTA as a root canal sealer.
Disadvantages of MTA
The main disadvantages of MTA include its high cost, which has been one of its major limitation, its discoloration potential, difficulties in handling the material, prolonged setting time, presence of toxic elements in its composition, difficulty to remove after setting, and it does not have a solvent [12,144].
Unfortunately, the use of MTA as a dressing for pulpotomies in molars [146], for filling pulp chambers of immature teeth [147], and as an apical barrier [148], has resulted in the discoloration of the crown. The reason of discoloration is debatable, but it has been linked mainly to the interaction between bismuth oxide and the collagen of the tooth tissue [149] and sodium hypochlorite, which is usually used in root canal therapy [150].
In concern of the toxic elements in MTA, mainly in its arsenic content several investigations were conducted and found that the amount of arsenic released is very low [151,152]. In addition, other factors further minimize the arsenic release from the material into the tissue, such a s the small amounts of the MTA that are actually used clinically, its insoluble nature [64], and the FeO present in its composition with its subsequent stabilizing effect on the arsenic content [25,152].
In an effort to overcome these disadvantages other tricalcium silicate based materials have been introduced to the market [150]. Bioaggregate (Verio Dental, Vancouver, Canada) and Biodentine (Septodont, Saint-Maur-des-Foss es, France) are tricalcium silicate based materials that have been developed to overcome the drawbacks of MTA while maintaining its desired characteristics and clinical applications [150,152]. Compared to MTA, Biodentine is a cost effective material that is easier to handle, has a faster setting time (set at about 10-12 minutes), is less soluble, and results in a better seal. It also has alternative radiopacifiers to overcome the discoloration related to bismuth oxide present in MTA [102,152].
In a study to assess the color stability of MTA Plus compared to Neo MTA plus and Biodentine when used in pulpotomies in immature permanent teeth in the presence of sodium hypochlorite solution, it was found that MTA Plus showed discoloration while Neo MTA Plus and Biodentine did not show discoloration [42].
In addition, recently other cements have been developed, such as calcium aluminate alfa-aluminate cement (CAAC), calcium aluminate alfa-aluminate plus cement (CAAC Plus), and wollastonite (a naturally occurring calcium silicate) and CAAC cement mixture (WOLCA) (Torabinejad Dental Research Center, Isfahan University of Medical Sciences (IUMS), Isfahan, Iran).
An animal study by Aminozarbian et al. [147], in 2012, found that CAAC Plus was not biocompatible while the biocompatibility of CAAC and WOLCA was comparable with that of MTA. Further studies are necessary to determine the biocompatibility of these materials.
Conclusion
MTA is a unique material with various advantages. It has been used successfully by pediatric dentists in a variety of clinical applications. Despite the many advantages of MTA, its drawbacks especially its high cost, discoloration potential, difficulty in handling, and long setting time cannot be overlooked. With the emergence of other novel tricalcium silicate based materials in the market, such as Biodentine, that overcome MTA’s key limitations, they are competing to be the next potential dentin substitute for the various clinical application in which MTA has been used. However, with the recent introduction of new improved MTA products, MTA-based materials are likely to remain at the heart of good pediatric dental practice for many years to come.
References
- Lee SJ, Monsef M, Torabinejad M (1993) Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod 19: 541-544.
- Torabinejad M, Hong CU, McDonald F, Ford TR (1995) Physical and chemical properties of a new root-end filling material. J Endod 21: 349-353.
- Casella G, Ferlito S (2006) The use of mineral trioxide aggregate in endodontics. Minerva Stomatol 55: 123-143.
- Schmitt D, Lee J, Bogen G (2001) Multifaceted use of ProRoot MTA root canal repair material. Pediatr Dent 23: 326-330.
- Kim S, Kratchman S (2006) Modern endodontic surgery concepts and practice: A review. J Endod 32: 601-623.
- Jacobovitz M, de Lima RK (2008) Treatment of inflammatory internal root resorption with mineral trioxide aggregate: A case report. IntEndod J 41: 905-912.
- El-Meligy OA, Avery DR (2006) Comparison of apexification with mineral trioxide aggregate and calcium hydroxide. Pediatr Dent 28: 248-53.
- Sarris S, Tahmassebi JF, Duggal MS, Cross IA (2008) A clinical evaluation of mineral trioxide aggregate for root-end closure of non-vital immature permanent incisors in children-a pilot study. Dent Traumatol 24: 79-85.
- Farsi N, Alamoudi N, Balto K, Mushayt A (2005) Success of mineral trioxide aggregate in pulpotomized primary molars. J ClinPediatr Dent 29: 307-311.
- Farsi N, Alamoudi N, Balto K, Al Mushayt A (2006) Clinical assessment of mineral trioxide aggregate (MTA) as direct pulp capping in young permanent teeth. J ClinPediatr Dent 31: 72-76.
- El-Meligy OA, Avery DR (2006) Comparison of mineral trioxide aggregate and calcium hydroxide as pulpotomy agents in young permanent teeth (apexogenesis). Pediatr Dent 28: 399-404.
- Srinivasan V, Waterhouse P, Whitworth J (2009) Mineral trioxide aggregate in paediatric dentistry. Int J Paediatr Dent 19: 34-47.
- Camilleri J, Pitt Ford TR (2006) Mineral trioxide aggregate: a review of the constituents and biological properties of the material. IntEndod J 39: 747-754.
- Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, et al. (2005) The constitution of mineral trioxide aggregate. Dent Mater 21: 297-303.
- Duarte MA, Demarchi AC, Yamashita JC, Kuga MC, FragaSde C (2003) pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 95: 345-347.
- Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I (2005) Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod 31: 97-100.
- Santos AD, Moraes JC, Araujo EB, Yukimitu K, Valerio Filho WV (2005) Physico-chemical properties of MTA and a novel experimental cement. IntEndod J 38: 443-447.
- Parirokh M, Torabinejad M (2010) Mineral trioxide aggregate: a comprehensive literature review--Part I: chemical, physical, and antibacterial properties. J Endod 36: 16-27.
- Tawil PZ, Duggan DJ, Galicia JC (2015) Mineral trioxide aggregate (MTA): its history, composition, and clinical applications. CompendContinEduc Dent 36: 247-252.
- Roberts HW, Toth JM, Berzins DW, Charlton DG (2008) Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater 24: 149-164.
- Asgary S, Parirokh M, Eghbal MJ, Brink F (2004) A comparative study of white mineral trioxide aggregate and white Portland cements using X-ray microanalysis. AustEndod J 30: 89-92.
- Dammaschke T, Gerth HU, Zuchner H, Schafer E (2005) Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater 21: 731-738.
- Kogan P, He J, Glickman GN, Watanabe I (2006) The effects of various additives on setting properties of MTA. J Endod 32: 569-572.
- Prasad A, Pushpa S, Arunagiri D, Sawhny A, Misra A, et al. (2015) A comparative evaluation of the effect of various additives on selected physical properties of white mineral trioxide aggregate. J Conserv Dent 18: 237-241
- Asgary S, Parirokh M, Eghbal MJ, Brink F (2005) Chemical differences between white and gray mineral trioxide aggregate. J Endod 31: 101-103.
- Song JS, Mante FK, Romanow WJ, Kim S (2006) Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 102: 809-815.
- Asgary S, Parirokh M, Eghbal MJ, Stowe S, Brink F (2006) A qualitative X-ray analysis of white and grey mineral trioxide aggregate using compositional imaging. J Mater Sci Mater Med 17: 187-191.
- Bortoluzzi AE, Broon JN, Duarte AHM, Demarchi OAC, Bramante MC (2006) The use of a setting accelerator and its effect on pH and calcium ion release of mineral trioxide aggregate and white Portland cement. J Endod 32: 1194-1197.
- Camilleri J (2007) Hydration mechanisms of mineral trioxide aggregate. IntEndod J 40: 462-470.
- Belio-Reyes IA, Bucio L, Cruz-Chavez E (2009) Phase composition of Pro Root mineral trioxide aggregate by X-ray powder diffraction. J Endod 35: 875-878.
- Arruda RA, Cunha RS, Miguita KB, Silveira CF, De Martin AS, et al. (2012) Sealing ability of mineral trioxide aggregate (MTA) combined with distilled water, chlorhexidine, and doxycycline. J Oral Sci 54: 233-239.
- Dorileo MC, Bandeca MC, Pedro FL, Volpato LE, Guedes OA, et al. (2014) Analysis of metal contents in Portland Type V and MTA-based cements. Scientific World J 2014: 983728.
- Kratchman SI (2004) Perforation repair and one-step apexification procedures. Dent Clin North Am 48: 291-307.
- Chng HK, Islam I, Yap AU, Tong YW, Koh ET (2005) Properties of a new root-end filling material. J Endod 31: 665-668.
- Islam I, Chng HK, Yap AU (2006) Comparison of the physical and mechanical properties of MTA and portland cement. J Endod 32: 193-197.
- Holt DM, Watts JD, Beeson TJ, Kirkpatrick TC, Rutledge RE (2007) The anti-microbial effect against enterococcus faecalis and the compressive strength of two types of mineral trioxide aggregate mixed with sterile water or 2% chlorhexidine liquid. J Endod 33: 844-847.
- Watts JD, Holt DM, Beeson TJ, Kirkpatrick TC, Rutledge RE (2007) Effects of pH and mixing agents on the temporal setting of tooth-colored and gray mineral trioxide aggregate. J Endod 33: 970-973.
- Al-Hezaimi K, Al-Shalan TA, Naghshbandi J, Oglesby S, Simon JH, et al. (2006) Antibacterial effect of two mineral trioxide aggregate (MTA) preparations against Enterococcus faecalis and Streptococcus sanguis in vitro. J Endod 32: 1053-1056.
- Al-Hezaimi K, Naghshbandi J, Oglesby S, Simon JH, Rotstein I (2006) Comparison of antifungal activity of white-colored and gray-colored mineral trioxide aggregate (MTA) at similar concentrations against Candida albicans. J Endod 32: 365-367.
- Asgary S, Kamrani FA (2008) Antibacterial effects of five different root canal sealing materials. J Oral Sci 50: 469-474.
- Camilleri J (2014) Composition and Setting Reaction. In: JCamilleri (Ed.), Mineral trioxide aggregate in dentistry: From preparation to application. Springer Berlin Heidelberg, Berlin, Heidelberg.
- Camilleri J (2015) Staining potential of Neo MTA Plus, MTA Plus, and biodentine used for pulpotomy procedures. J Endod 41: 1139-1145.
- Camilleri J (2014) Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent Mater 30: 709-715.
- Xu Z (2016) Is MTA finally affordable for pediatric pulp therapy?PennWell Corporation.
- Torabinejad M, Watson TF, Pitt Ford TR (1993) Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod 19: 591-595.
- Macwan C, Deshpande A (2014) Mineral trioxide aggregate (MTA) in dentistry: A review of literature. J Oral Res Rev 6: 71-74.
- Chogle S, Mickel AK, Chan DM, Huffaker K, Jones JJ (2007) Intracanal assessment of mineral trioxide aggregate setting and sealing properties. Gen Dent 55: 306-311.
- Schwartz RS, Mauger M, Clement DJ, Walker WA (1999) Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc 130: 967-975.
- Naik S, Hegde AH (2005) Mineral trioxide aggregate as a pulpotomy agent in primary molars: an in vivo study. J Indian SocPedodPrev Dent 23: 13-16.
- Sluyk S, Moon P, Hartwell G (1998) Evaluation of setting properties and retention characteristics of mineral trioxide aggregate when used as a furcation perforation repair material. J Endod 24: 768-771.
- Torabinejad M, Chivian N (1999) Clinical applications of mineral trioxide aggregate. J Endod 25: 197-205.
- Parashos P, Phoon A, Sathorn C (2014) Effect of ultrasonication on physical properties of mineral trioxide aggregate. Biomed Res Int 2014: 4.
- Shahi S, Ghasemi N, Rahimi S, Yavari H, Janani M, et al. (2015) The effect of different mixing methods on working time, setting time, dimensional changes and film thickness of mineral trioxide aggregate and calcium-enriched mixture. Iran Endod J 10: 248.
- Nekoofar M, Adusei G, Sheykhrezae M, Hayes S, Bryant S, et al. (2007) The effect of condensation pressure on selected physical properties of mineral trioxide aggregate. IntEndod J 40: 453-461.
- Nandini S, Ballal S, Kandaswamy D (2007) Influence of glass-ionomer cement on the interface and setting reaction of mineral trioxide aggregate when used as a furcal repair material using laser Raman spectroscopic analysis. J Endod 33: 167-172.
- Ballal S, Venkateshbabu N, Nandini S, Kandaswamy D (2008) An in vitro study to assess the setting and surface crazing of conventional glass ionomer cement when layered over partially set mineral trioxide aggregate. J Endod 34: 478-480.
- Jeong YN, Yang SY, Park BJ, Park YJ, Hwang YC, et al. (2010) Physical and chemical properties of experimental mixture of mineral trioxide aggregate and glass ionomer cement. J Korean AcadConserv Dent 35: 344-352.
- Sawhney S, Vivekananda Pai AR (2015) Comparative evaluation of the calcium release from mineral trioxide aggregate and its mixture with glass ionomer cement in different proportions and time intervals - An in vitro study. Saudi Dent J 27: 215-219.
- Kayahan M, Nekoofar MH, KazandaĬ? M, Canpolat C, Malkondu O, et al. (2009) Effect of acidâ¬?¬źetching procedure on selected physical properties of mineral trioxide aggregate. IntEndod J 42: 1004-1014.
- Loxley EC, Liewehr FR, Buxton T, McPherson J (2003) The effect of various intracanal oxidizing agents on the push-out strength of various perforation repair materials. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 95: 490-494.
- Bates CF, Carnes DL, Carlos E (1996) Longitudinal sealing ability of mineral trioxide aggregate as a root-end filling material. J Endod 22: 575-578.
- Shipper G, Grossman E, Botha A, Cleatonâ¬?¬źJones P (2004) Marginal adaptation of mineral trioxide aggregate (MTA) compared with amalgam as a rootâ¬?¬źend filling material: a lowâ¬?¬źvacuum (LV) versus highâ¬?¬źvacuum (HV) SEM study. IntEndod J 37: 325-336.
- Vargas JW, Liewehr FR, Joyce AP, Runner RR (2004) A comparison of the in vitro retentive strength of glass-ionomer cement, zinc-phosphate cement, and mineral trioxide aggregate for the retention of prefabricated posts in bovine incisors. J Endod 30: 775-777.
- Tunc ES, Bayrak S, Egilmez T (2008) The evaluation of bond strength of a composite and a compomer to white mineral trioxide aggregate with two different bonding systems. J Endod 34: 603-605.
- Estrela C, Bammann LL, Estrela C, Silva RS, Pecora JD (2000) Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J 11: 3-9.
- Miyagak DC, de Carvalho EM, Robazza CR, Chavasco JK, Levorato GL (2006) In vitro evaluation of the antimicrobial activity of endodontic sealers. Braz Oral Res 20: 303-306.
- Yasuda Y, Kamaguchi A, Saito T (2008) In vitro evaluation of the antimicrobial activity of a new resin-based endodontic sealer against endodontic pathogens. J Oral Sci 50: 309-313.
- Mohammadi Z, Modaresi J, Yazdizadeh M (2006) Evaluation of the antifungal effects of mineral trioxide aggregate materials. AustEndod J 32: 120-122.
- Tanomaru-Filho M, Tanomaru JM, Barros DB, Watanabe E, Ito IY (2007) In vitro antimicrobial activity of endodontic sealers, MTA-based cements and Portland cement. J Oral Sci 49: 41-45.
- Al-Hezaimi K, Al-Hamdan K, Naghshbandi J, Oglesby S, Simon JH, et al. (2005) Effect of white-colored mineral trioxide aggregate in different concentrations on Candida albicans in vitro. J Endod 31: 684-686.
- Kettering JD, Torabinejad M (1995) Investigation of mutagenicity of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod 21: 537-542.
- Osorio RM, Hefti A, Vertucci FJ, Shawley AL (1998) Cytotoxicity of endodontic materials. J Endod 24: 91-96.
- Moretton TR, Brown CE, Legan JJ, Kafrawy AH (2000) Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J Biomed Mater Res 52: 528-533.
- Masuda YM, Wang X, Hossain M, Unno A, Jayawardena JA, et al. (2005) Evaluation of biocompatibility of mineral trioxide aggregate with an improved rabbit ear chamber. J Oral Rehabil 32: 145-150.
- Lessa FC, Aranha AM, Hebling J, Costa CA (2010) Cytotoxic effects of White-MTA and MTA-Bio cements on odontoblast-like cells (MDPC-23). Braz Dent J 21: 24-31.
- Balto HA (2004) Attachment and morphological behavior of human periodontal ligament fibroblasts to mineral trioxide aggregate: a scanning electron microscope study. J Endod 30: 25-29.
- Dincol ME, Ozbas H, Yilmaz B, Ersev H, Gokyay S, et al. (2016) Effect of the plant-based hemostatic agent Ankaferd Blood Stopper(R) on the biocompatibility of mineral trioxide aggregate. BMC Oral Health 16: 111.
- Myers K, Kaminski E, Lautenschlager E, Miller D (1996) The effects of mineral trioxide aggregate on the dog pulp. J Endod 22: 184-186.
- Faraco IM, Holland R (2001) Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol 17: 163-166.
- Téclès O, Laurent P, Aubut V, About I (2008) Human tooth culture: a study model for reparative dentinogenesis and direct pulp capping materials biocompatibility. J Biomed Mater Res B ApplBiomater 85: 180-187.
- Pelliccioni G, Ciapetti G, Cenni E, Granchi D, Nanni M, et al. (2004) Evaluation of osteoblast-like cell response to Proroot™ MTA (mineral trioxide aggregate) cement. J Mater Sci Mater Med 15: 167-173.
- American Academy of Pediatric Dentistry (2015-2016) Guideline on pulp therapy for primary and immature permanent teeth. Clinical Practice GuidlinesRefference Manual 37: 244-252.
- Bodem O, Blumenshine S, Zeh D, Koch M (2004) Direct pulp capping with mineral trioxide aggregate in a primary molar: A case report. Int J Paediatr Dent 14: 376-379.
- Caicedo R, Abbott PV, Alongi DJ, Alarcon MY (2006) Clinical, radiographic and histological analysis of the effects of mineral trioxide aggregate used in direct pulp capping and pulpotomies of primary teeth. Aust Dent J 51: 297-305.
- Tuna D, Olmez A (2008) Clinical longâ¬?¬źterm evaluation of MTA as a direct pulp capping material in primary teeth. IntEndod J 41: 273-278.
- Agamy HA, Bakry NS, Mounir MM, Avery R (2004) Comparison of mineral trioxide aggregate and formocresol as pulp-capping agents in pulpotomized primary teeth. Pediatr Dent 26: 302-309.
- Holan G, Eidelman E, Fuks AB (2005) Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatr Dent 27:129-136.
- Maroto M, Barberia E, Planells P, Garcia Godoy F (2005) Dentin bridge formation after mineral trioxide aggregate (MTA) pulpotomies in primary teeth. Am J Dent 18:151-154.
- Peng L, Ye L, Tan H, Zhou X (2006) Evaluation of the formocresol versus mineral trioxide aggregate primary molar pulpotomy: A meta-analysis. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 102: e40-e44.
- Maroto M, Barberia E, Vera V, Garcia-Godoy F (2007) Mineral trioxide aggregate as pulp dressing agent in pulpotomy treatment of primary molars: 42-month clinical study. Am J Dent 20: 283-286.
- Ng FK, Messer LB (2008) Mineral trioxide aggregate as a pulpotomy medicament: an evidence-based assessment. Eur Arch Paediatr Dent 9: 58-73.
- Sushynski JM, Zealand CM, Botero TM, Boynton JR, Majewski RF, et al. (2012) Comparison of gray mineral trioxide aggregate and diluted formocresol in pulpotomized primary molars: a 6- to 24-month observation. Pediatr Dent 34: 120-128.
- Singh H, Kaur M, Markan S, Kapoor P (2014) Biodentine: A promising dentin substitute. J Interdiscipl Med Dent Sci 2014.
- Shirvani A, Asgary S (2014) Mineral trioxide aggregate versus formocresolpulpotomy: A systematic review and meta-analysis of randomized clinical trials. Clin Oral Investig 18: 1023-1030.
- Marghalani AA, Omar S, Chen JW (2014) Clinical and radiographic success of mineral trioxide aggregate compared with formocresol as a pulpotomy treatment in primary molars: a systematic review and meta-analysis. J Am Dent Assoc 145: 714-721.
- Malkondu O, KarapinarKazandag M, Kazazoglu E (2014) A review on biodentine, a contemporary dentine replacement and repair material. Biomed Res Int 2014: 160951.
- Niranjani K, Prasad MG, Vasa AA, Divya G, Thakur MS, et al. (2015) Clinical evaluation of success of primary teeth pulpotomy using mineral trioxide aggregate((R)), laser and biodentine(TM)- an in vivo study. J ClinDiagn Res 9: Zc35-37.
- O'Sullivan SM, Hartwell GR (2001) Obturation of a retained primary mandibular second molar using mineral trioxide aggregate: a case report. J Endod 27: 703-705.
- El-Khodary HM, Farsi DJ, Farsi NM, Zidan AZ (2015) Sealing ability of four calcium containing cements used for repairing furcal perforations in primary molars: An in vitro study. J Contemp Dent Pract 16: 733-739.
- Benoist LF, Ndiaye GF, Kane AW, Benoist HM, Farge P (2012) Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal((R))) in the formation of a dentine bridge: a randomised controlled trial. Int Dent J 62: 33-39.
- Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS (2003) Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. IntEndod J 36: 225-231.
- Nair PNR, Duncan HF, Pitt Ford TR, Luder HU (2008) Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. IntEndod J 41: 128-150.
- Iwamoto CE, Adachi E, Pameijer CH, Barnes D, Romberg EE, et al. (2006) Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent 19: 85-90.
- Lourdes RAM, Holland R, Reis A, Bortoluzzi MC, Murata SS, et al. (2008) Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod 34: 1-6.
- Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, et al. (2013) Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod 39: 743-747
- Bogen G, Kim JS, Bakland LK (2008) Direct pulp capping with mineral trioxide aggregate: an observational study. J Am Dent Assoc 139: 305-315.
- Mente J, Hufnagel S, Leo M, Michel A, Gehrig H, et al. (2014) Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: long-term results. J Endod 40: 1746-1751.
- Chacko V, Kurikose S (2006) Human pulpal response to mineral trioxide aggregate (MTA): a histologic study. J ClinPediatr Dent 30: 203-209.
- Chailertvanitkul P, Paphangkorakit J, Sooksantisakoonchai N, Pumas N, Pairojamornyoot W, et al. (2014) Randomized control trial comparing calcium hydroxide and mineral trioxide aggregate for partial pulpotomies in cariously exposed pulps of permanent molars. IntEndod J 47: 835-842.
- Qudeimat MA, Barrieshi-Nusair KM, Owais AI (2007) Calcium hydroxide vs mineral trioxide aggregates for partial pulpotomy of permanent molars with deep caries. Eur Arch Paediatr Dent 8: 99-104.
- Fransson H, Wolf E, Petersson K (2016) Formation of a hard tissue barrier after experimental pulp capping or partial pulpotomy in humans: an updated systematic review. Int Endod J 49: 533-542.
- Chala S, Abouqal R, Rida S (2011) Apexification of immature teeth with calcium hydroxide or mineral trioxide aggregate: systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 112: e36-42.
- Witherspoon DE, Ham K (2001) One-visit apexification: technique for inducing root-end barrier formation in apical closures. Pract Proced Aesthet Dent 13: 455-460.
- Steinig TH, Regan JD, Gutmann JL (2003) The use and predictable placement of Mineral Trioxide Aggregate in one-visit apexification cases. Aust Endod J 29: 34-42.
- Simon S, Rilliard F, Berdal A, Machtou P (2007) The use of mineral trioxide aggregate in one-visit apexification treatment: a prospective study. IntEndod J 40: 186-197.
- Holden DT, Schwartz SA, Kirkpatrick TC, Schindler WG (2008) Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J Endod 34: 812-817.
- Lee LW, Hsieh SC, Lin YH, Huang CF, Hsiao SH, et al. (2015) Comparison of clinical outcomes for 40 necrotic immature permanent incisors treated with calcium hydroxide or mineral trioxide aggregate apexification/apexogenesis. J Formos Med Assoc 114: 139-146.
- Sanchez AFY, Leco-Berrocal MI, Martinez-Gonzalez JM (2008) Metaanalysis of filler materials in periapical surgery. Med Oral Patol Oral Cir Bucal 13: E180-185.
- Siew K, Lee AH, Cheung GS (2015) Treatment outcome of repaired root perforation: A systematic review and meta-analysis. J Endod 41:1795-1804.
- Holland R, Filho JA, de Souza V, Nery MJ, Bernabe PF, et al. (2001) Mineral trioxide aggregate repair of lateral root perforations. J Endod 27: 281-284.
- Mente J, Leo M, Panagidis D, Saure D, Pfefferle T (2014) Treatment outcome of mineral trioxide aggregate: repair of root perforations-long-term results. J Endod 40: 790-796.
- Bramante CM, Menezes R, Moraes IG, Bernardinelli N, Garcia RB, et al. (2006) Use of MTA and intracanal post reinforcement in a horizontally fractured tooth: a case report. Dent Traumatol 22: 275-278.
- Erdem AP, Ozdas DO, DincolE, Sepet E, Aren G (2009) Case series: Root healing with MTA after horizontal fracture. Eur Arch Paediatr Dent 10: 110-113.
- Yildirim T, Gencoglu N (2009) Use of mineral trioxide aggregate in the treatment of horizontal root fractures with a 5-year follow-up: report of a case. J Endod 35:292-295.
- Sheikh-Nezami M, Mokhber N, Shamsian K, Saket S (2009) Management of a midroot and complicated crown fracture: A case report. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 107: e65-67.
- Roig M, Espona J, Mercade M, Duran-Sindreu F (2011) Horizontal root fracture treated with MTA, a case report with a 10-year follow-up. Dent Traumatol 27: 460-463.
- White C, Bryant N (2002) Combined therapy of mineral trioxide aggregate and guided tissue regeneration in the treatment of external root resorption and an associated osseous defect. J Periodontol 73: 1517-1521.
- Baratto-Filho F, Limongi O, AraujoCde J, Neto MD, Maia SM, et al. (2005) Treatment of invasive cervical resorption with MTA: case report. AustEndod J 31: 76-80.
- Pace R, Giuliani V, Pagavino G (2008) Mineral trioxide aggregate in the treatment of external invasive resorption: a case report. IntEndod J 41: 258-266.
- Meire M, De Moor R (2008) Mineral trioxide aggregate repair of a perforating internal resorption in a mandibular molar. J Endod 34: 220-223.
- Silveira FF, Nunes E, Soares JA, Ferreira CL, Rotstein I (2009) Double 'pink tooth' associated with extensive internal root resorption after orthodontic treatment: a case report. Dent Traumatol 25: e43-47.
- Szeffer BM, Lagocka R, Trusewicz M, Lipski M, Radlinska JB (2015) Perforating internal root resorption repaired with mineral trioxide aggregate caused complete resolution of odontogenic sinus mucositis: a case report. J Endod 41: 274-278.
- Parirokh M, Torabinejad M (2010) Mineral trioxide aggregate: a comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. J Endod 36: 400-413.
- Thakur S, Emil J, Paulaian B (2013) Evaluation of mineral trioxide aggregate as root canal sealer: A clinical study. J Conserv Dent 16: 494-498.
- Liu H, Zhou Q, Qin M (2011) Mineral trioxide aggregate versus calcium hydroxide for pulpotomy in primary molars. Chin J Dent Res 14: 121-125.
- Ioannidis K, Mistakidis I, Beltes P, Karagiannis V (2013) Spectrophotometric analysis of coronal discolouration induced by grey and white MTA. Int Endod J 46: 137-144.
- Moore A, Howley MF, O'Connell AC (2011) Treatment of open apex teeth using two types of white mineral trioxide aggregate after initial dressing with calcium hydroxide in children. Dent Traumatol 27:166-173.
- Marciano MA, Costa RM, Camilleri J, Mondelli RF, Guimaraes BM, et al. (2014) Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J Endod 40: 1235-1240.
- Camilleri J (2014) Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J Endod 40: 436-440.
- Duarte MA, De Oliveira Demarchi AC, Yamashita JC, Kuga MC, De Campos Fraga S (2005) Arsenic release provided by MTA and Portland cement. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 99: 648-650.
- De-Deus G, de Souza MC, Sergio Fidel RA, Fidel SR, de Campos RC, et al. (2009) Negligible expression of arsenic in some commercially available brands of Portland cement and mineral trioxide aggregate. J Endod 35: 887-890.
- Poggio C, Lombardini M, Alessandro C, Simonetta R (2007) Solubility of root-end-filling materials: a comparative study. J Endod 33: 1094-1097.
- Mishra D, Farrell J (2005) Evaluation of mixed valent iron oxides as reactive adsorbents for arsenic removal. Environ SciTechnol 39: 9689-9694.
- Nayak G, Hasan MF (2014) Biodentine-a novel dentinal substitute for single visit apexification. Restor Dent Endod 39: 120-125.
- Aminozarbian MG, Barati M, Salehi I, Mousavi SB (2012) Biocompatibility of mineral trioxide aggregate and three new endodontic cements: An animal study. Dent Res J (Isfahan) 9: 54-59.
- Walker MP, Diliberto A, Lee C (2006) Effect of setting conditions on mineral trioxide aggregate flexural strength. J Endodont 32: 334-336.
- Gancedo-Caravia L, Garcia-Barbero E (2006) Influence of humidity and setting time on the push-out strength of mineral trioxide aggregate obturations. J Endod 32: 894-896.
- Valois CR, Costa ED (2004) Influence of the thickness of mineral trioxide aggregate on sealing ability of root-end fillings in vitro. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 97: 108-111.
- Danesh G, Dammaschke T, Gerth H, Zandbiglari T, Schafer E (2006) A comparative study of selected properties of ProRoot mineral trioxide aggregate and two Portland cements. Int Endod J 39: 213-219.
- Shie MY, Huang TH, Kao CT, Huang CH, Ding SJ (2009) The effect of a physiologic solution pH on properties of white mineral trioxide aggregate. J Endod 35: 98-101.
- Fridland M, Rosado R (2003) Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod 29: 814-817.
- Hachmeister DR, Schindler WG, Walker WA, Thomas DD (2002) The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod 28: 386-390.
Relevant Topics
- Advanced Bleeding Gums
- Advanced Receeding Gums
- Bleeding Gums
- Children’s Oral Health
- Coronal Fracture
- Dental Anestheia and Sedation
- Dental Plaque
- Dental Radiology
- Dentistry and Diabetes
- Fluoride Treatments
- Gum Cancer
- Gum Infection
- Occlusal Splint
- Oral and Maxillofacial Pathology
- Oral Hygiene
- Oral Hygiene Blogs
- Oral Hygiene Case Reports
- Oral Hygiene Practice
- Oral Leukoplakia
- Oral Microbiome
- Oral Rehydration
- Oral Surgery Special Issue
- Orthodontistry
- Periodontal Disease Management
- Periodontistry
- Root Canal Treatment
- Tele-Dentistry
Recommended Journals
Article Tools
Article Usage
- Total views: 31453
- [From(publication date):
November-2016 - Apr 24, 2025] - Breakdown by view type
- HTML page views : 28734
- PDF downloads : 2719