Research Article Open Access
Microwave Hybrid Processing of Hydroxyapatite
Ehsani N1, Sorrell CC2and Ruys AJ3*
1Faculty of Materials and Manufacturing Technology, MUT University of Technology, Tehran 84817-75631, Iran
2Graduate School for Biomedical Engineering, University of New South Wales, Sydney, NSW 2052, Australia
3Centre for Advanced Materials Technology, Department of Mechanical and Mechatronic Engineering, University of Sydney, Sydney, NSW, 2006, Australia
- Corresponding Author:
- Ruys AJ
Centre for Advanced Materials Technology
Department of Mechanical and Mechatronic Engineering
University of Sydney, Sydney, NSW, 2006, Australia
E-mail: Andrew.ruys@sydney.edu.au
Received date April 19, 2013; Accepted date April 19, 2013; Published date July 15, 2013
Citation: Ehsani N, Sorrell CC, Ruys AJ (2013) Microwave Hybrid Processing of Hydroxyapatite. J Biomim Biomater Tissue Eng 18:108. doi: 10.4172/1662-100X.1000108
Copyright: © 2013 Ehsani N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
The effects of microwave hybrid heating on the densification and decomposition behaviour of four commercially available pre-calcined HAp powders (Plasma Biotal P81, P88, P120 and P149) and a bead milled HAp powder (BM16) were studied. The results were compared with conventionally sintered samples. Samples microwave heated to 1100°C for 10 min at 10,000°C.h-1 were 15 to 40% denser than the samples conventionally heated at same temperature for 60 min at 300°C.h-1. Microwave heating reduced densification temperatures and soak times by as much as ~150°C-200°C and ~57 min, respectively. Overall, the microwave sintering cycle was 20-30 times faster.
Introduction
Hydroxyapatite [HAp] clearly has a great potential for biomedical applications because of its similarity in chemical composition and crystallographic structure to human hard tissue [1,2]. However, relatively poor strength and fracture toughness have restricted its extensive applications. A great deal of work has been done on the research and development of HAp ceramics as biomaterials [3-7]. One possible solution for improving the mechanical properties is microwave sintering of HAp [8-11].
Microwave sintering of HAp has been studied by Fang et al. [12-14] and Agrawal et al. [15]. They successfully made a number of transparent, white and porous HAp ceramics. Commercial microwave furnaces (500 and 1000 W) with cylindrical ZrO2 or rod-like SiC susceptors were used. Fully dense (100%) and transparent hydroxyapatite was produced by microwave sintering at 1100°C-1175°C [15], whereas conventionally produced samples from the same powder did not show complete transparency.
Microwave hybrid heating [MHH]
Microwaves form the part of the electromagnetic spectrum between radio waves and infrared light, occupying the frequency range 0.3 to 300 GHz, with the corresponding wave length range of 1 mm to 1 m [16]. Allocated frequencies for industrial, scientific and medical use are shown in Figure 1. Among these frequencies, only 9.15 MHz and 2.45 GHz are commonly used in industrial equipment [17]. Microwave heating results from the interaction between the microwaves and the material that is being heated, resulting in energy dissipation by various mechanisms [18,19].
Microwave hybrid heating is a unique combination of conventional and microwave heating techniques, developed specifically for materials with low loss factor and low thermal conductivity [20,21]. Significant microwave heating problems can be solved by this novel technique [22-25]. In the MHH technique, a strongly microwave absorbent material is used as a susceptor to raise the temperature of a weakly microwave absorbent sample above the critical temperature (Tcrit). Once this temperature is reached, the sample can directly absorb microwave radiation. Therefore, at low temperatures the susceptor absorbs microwave energy and transmits heat into the sample by conventional heat transfer mechanisms, primarily radiation [20,26]. This technique facilitates the attainment of very high heating rates in a 2.45 GHz multimode microwave cavity [26]. Good temperature homogeneity can be achieved in large samples during MHH. MHH ceramics have shown greater microstructural uniformity and superior mechanical properties compared with conventional sintering using similar heating cycles.
The most common susceptor materials are SiC and ZrO2. YxNi1-xO compositions have recently been studied as a new susceptor material for high temperatures and oxidising conditions by Komarenko et al. [27]. Only pure NiO was found suitable as a microwave susceptor.
| Powder | Surface Area (m2.g) | Particle Size (μ) |
|---|---|---|
| P81 | 19.9 | 6.97 |
| P88 | 20.5 | 3.16 |
| P120 | 41.3 | 1.84 |
| P149 | 18.6 | 5.10 |
| BM16 | 85.2 | 0.48 |
Table 1: Characteristics of HAp powders.
Experimental Procedure
Raw materials
Four commercially available pre-calcined HAp powders, including P81, P88, P120 and P149 (Plasma Biotal Ltd., Tideswell, Derbyshire, England), were used. BM16 powder was prepared by bead milling P149 HAp powder, with sixteen passes. Table 1 shows the average particle size and specific surface area of the HAp powders used in this work. For sintering studies, the powders were uniaxially pressed into pellets of 12.7 mm diameter.
Microwave hybrid heating: A 1.5 kW, 2.45 GHz variable-power microwave furnace (Ceramic Engineering, Sydney, Australia), with proportional power control (amplitude variation) and built-in mode stirrer, was used at the 65% power level for all experiments.
HAp is only weakly microwave-absorbent at low temperatures. It therefore cannot be heated from room temperature without a susceptor. However, like most ceramics, its microwave absorption capability increases with temperature [28]. Therefore, a microwave hybrid heating procedure was required to heat the HAp samples from room temperature to the critical temperature (Tcrit), above which HAp can efficiently absorb microwave radia cylindrical ZrO2 or rod-like SiC susceptors tion.
A cylindrical clay-bonded silicon carbide susceptor (Ø= 60 mm, h=20 mm) was positioned around the sample. The susceptor was placed inside an alumina brick (120 × 20 × 80 mm), which was surrounded by a layer (240 × 240 × 200 mm) of fibrous aluminosilicate insulation (Kaowool, Morganite Industrial Products, Sydney, Australia). This arrangement is shown schematically in Figure 1. For each experimental run, one sample was placed at the centre of the susceptor. The temperature was measured with an infrared pyrometer, which was calibrated during the cool-down cycle using a Pt - Pt13Rh thermocouple. A standard heating rate of ˜10,000°C.h-1 and soak time of 10 min were used. The mode stirrer was used at all times to produce a more uniform microwave field distribution across the cavity.
Conventional heating: An electric furnace (300 × 200 × 120 mm) with SiC elements (Gallenkamp, London, UK) was used for the conventional heating of the control samples. A standard heating rate of 300°C.h-1 (the furnace maximum) and soak time of 60 min were used in all cases. No serious overshooting was observed by such a high heating rate. Two sets of experimental trials were carried out.
Comparative densification and decomposition behaviour: Pellets from each of the HAp powders were sintered at a range of temperatures from 900°C to 1400°C in 100°C steps by microwave and conventional heating. After sintering, the apparent density and %HAp yield of the pellets were measured according to the methods described by Ruys et al. [29].
Effect of microwave soak time: To investigate the effect of microwave soak time, one pellet from each powder was microwave sintered at 1100°C using soak times of 1, 3, 10 and 30 min. No conventionally sintered controls were used in this study. After sintering, the apparent density of the pellets was measured to determine the effects of microwave soak time on the densification behaviour of HAp ceramics.
Results and Discussion
Comparative densification behaviour
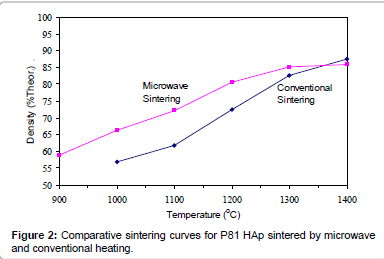
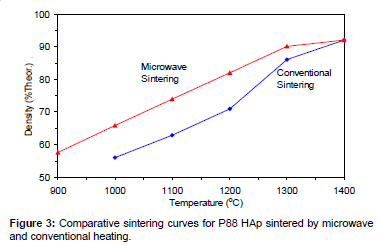
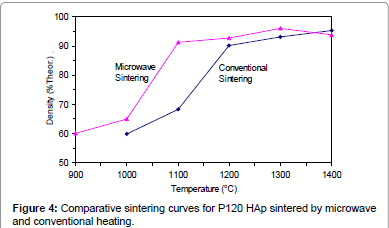
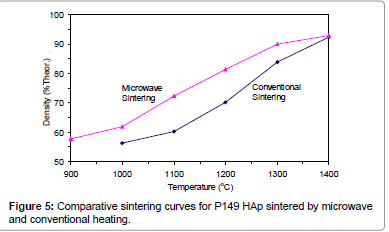
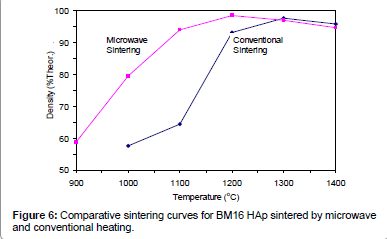
Figures 2-6 present the sintering curves of the HAp powders sintered by microwave and conventional heating. They reveal that, with microwave sintering, densification began at ˜900°C-1000°C, which was ˜100°C-150°C lower than for conventional sintering. These data show that the maximum densities were achieved at ˜1200°C-1400°C (the sintering plateau temperature for microwave heating) for microwave heating as compared with ˜1300°C-1400°C (the sintering plateau temperature for conventional heating) for conventional heating. In other words, microwave heating below the conventional heating plateau temperature significantly improved densification. However, the densities obtained above the conventional heating plateau temperature were similar for microwave or conventional heating.
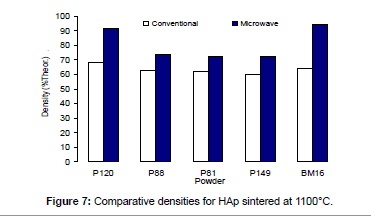
The densities attained after sintering at 1100°C using microwave and conventional sintering are shown in Figure 7. These data show that microwave heating significantly improved densification for each of the powders trialled. Overall, the improvement in densification by microwave heating at 1100°C was of the order of ˜11-30%. Although the soaking time for the microwave and conventionally processed samples are different, the comparison is valid below the composition temperature, where no decomposition occurs. At higher temperature, however, using identical soaking times would be more reliable.
Therefore the main difference between microwave and conventional heating in this trial was the fact that densification occurred at lower temperatures for the former. This is advantageous in the case of HAp since lower sintering temperatures reduce the risk of decomposition. Therefore, it appears that microwave sintering offers a solution to the problem of trying to achieve maximal densification of HAp without the decomposition.
Enhanced densification, and lower sintering temperatures compared to conventional heating have already been reported for microwave heating of ceramics [30-40]. The mechanisms and reasons for the enhanced sintering of ceramics by microwave heating are not completely understood [41]. However the following mechanisms are proposed:
High diffusion rate: It has been suggested that the lower sintering temperatures and the higher sintering rates during microwave heating are caused by enhanced diffusion rates induced by the electric field [42-45]. The diffusion rate enhancement by microwaves has been reported for several materials [44-48]. It has also been found that the diffusion rates were dependent, at least in part, on the input power level. Janney and Kimery [41] found that the activation energy for diffusion of 18O in sapphire was 410 kJ/mol for microwave heating and 710 kJ/mol for conventional heating. The presence of a microwave effect in the single crystal suggests that there would be a significant direct interaction between the crystals in polycrystalline ceramics and microwaves, and so microwave coupling cannot be attributed solely to grain boundary effects.
Preferential grain boundary coupling: Grain boundary coupling could have an important effect on the microwave sintering of ceramics. It has been suggested that the enhanced densification of ceramics by microwave sintering could be caused by accelerated diffusion rates within the grain boundaries [49]. This would be caused by preferential interaction of the microwaves with the interfaces, leading to higher temperatures in these regions. Several different mechanisms have been proposed for this temperature heterogeneity.
As previously have been suggested by Booske et al. [49,50], the loss tangent of the grain boundaries is higher than within the grains, and consequently microwave absorption and the temperature are higher at the grain boundaries. The electric field intensity in the areas of low dielectric constant is higher than that in the areas of high dielectric constant [51]. Since the dielectric constant of the pores at the grain boundaries (ε´air=0.1) is much lower than the dielectric constant of the ceramic grains (ε´ alumina=0.9), the electric field intensity between grains is much higher. Therefore, the field strength (E) and temperature are greater at the grain boundaries.
Another possible mechanism is that microwave radiation might generate dielectric breakdown at the mutual contact points of the green ceramic particles [52,53]. This phenomenon, which is common in polycrystalline high-dielectric constant materials, can lead to temperature gradients between the surface and centre of the grains. Meek et al. [54] found that the structure of the surface and the centre of the particles were crystalline in conventionally heated samples. However, in the microwave-heated samples, the structure of the centre was crystalline while the structure of the surface was amorphous. The existence of an amorphous layer on the surface of the microwave sintered grains suggests the presence of a liquid phase at the particle surfaces during microwave sintering. This would enhance densification.
It has also been suggested that, in microwave sintering, the temperature between particles is higher than the apparent or measured temperature and the supposed formation temperature of the liquid phase [55]. This temperature heterogeneity will decrease the liquid viscosity in the grain boundaries. Moreover, the vibration rate and amplitude of the liquid molecules will be increased by preferential coupling of the microwaves with the liquid formed in the grain boundaries. This also will reduce the liquid viscosity. The presence of a liquid with low viscosity increases the rate of grain rearrangement, thereby enhancing the densification rate.
This theory has been confirmed by Tiegs et al. [56-59], who have shown that preferential coupling of microwaves with the grain boundaries in microwave heated Si3N4-based ceramics leads to improved densification. Ferber et al. [60] established that the improved creep resistance of microwave-heated (Si3N4-6Y2O3-2Al2O3) was due to the preferential coupling of microwave radiation with the intergranular phases. It has been suggested that relatively small temperature gradients across the grains can induce a driving force to encourage densification [54].
Figures 3-7 shows that the temperature for the onset of densification for either microwave or conventional heating was lower for the powders with smaller particle size and higher surface area (such as BM16 and P120). The sintering characteristics of a powder depend on the surface area, particle size, particle size distribution, morphology and reactivity [61]. For a given chemical reactivity, densification at a given temperature will be enhanced with a finer particle size, higher surface area, and appropriate size distribution for dense particle packing. The temperature at which the maximum density was achieved also depended on the particle size and surface area of the powders. In case of BM16 impurities also have possibly affected the density. It was lower for the powders with a smaller particle size and higher surface area.
Figure 7 shows that the densification enhancement of microwave heating was strongly dependent on the particle size of the powders. The densification enhancement for the fine HAp powders, such as BM16 and P120, was much greater than that for the coarser HAp powders, such as P81, P88 and P149. Compared with conventional sintering, microwave sintering of fine powder (BM16) at 1100°C led to a ˜30% (64.5% versus 94.5% dense) densification enhancement whereas it led to only a ˜11% increase in densification of coarse powder (62.8% versus 73.9% dense). This enhanced effect with finer powders suggests that the particle size of the powders and, by association, the grain boundary area strongly influenced the degree of microwave densification. These accords with the theories discussed previously, where preferential coupling of grain boundaries with microwaves is one of the densification enhancement mechanisms of microwave sintering [49,51]. Since the fine powders have a higher surface area and higher grain boundary area, they are more susceptible to preferential grain boundary coupling.
Comparative decomposition behaviour: The %HAp yields of the samples sintered by microwave and conventional heating are shown in Table 2. The data reveal that the decomposition effect was slightly higher for microwave sintering than for conventional sintering. HAp decomposition is a diffusion controlled mechanism [62]. Since microwave heating presumably enhances diffusion rates [44-56], it is possible that it could enhance HAp decomposition. However, the data suggest that the effect of microwave heating on densification was much more significant than that on decomposition.
Tables 2 and Table 3, and Figure 6 show that, compared with conventional sintering, microwave sintering of BM16 powder at 1100°C led to a ˜30% (64.5% versus 94.5% dense) densification enhancement whereas, it led to only a 5% increase in decomposition (76% versus 81%). The fact that densification is enhanced much more than decomposition is a positive result, which points to practical benefits in processing of reinforced HAp biomaterials. The reason for this strong enhancement of densification (compared with a weak enhancement of decomposition) is that the decomposition rate during microwave heating was probably increased by the induced higher diffusion rate, whereas densification was enhanced by both the induced higher diffusion rate and the preferential grain boundary coupling mechanism. Therefore, any disadvantage in decomposition should be partially compensated by advantages in densification.
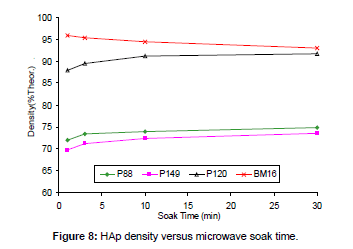
Effect of microwave soak time: A number of combinations of powder type and soak time were trialled at a soak temperature of 1100°C. The results are shown in Figure 8. These data indicate that, for three pure HAp powders (P88, P120 and P149), increasing soak times led to a slight improvement in densification level, although little improvement occurred for soak times above 3 to 10 min. Therefore, a 3 min soak time at 1100°C was all that was required for effective densification. This suggests that increasing the soak time at temperatures below Tonset will increase the density only marginally.
| TEMP (°C) | 900°C | 1000°C | 1100°C | 1200°C | 1300°C | 1400°C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CONV | MICRO | CONV | MICRO | CONV | MICRO | CONV | MICRO | CONV | MICRO | CONV | MICRO | |
| P120 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| P88 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 91.0 | |
| P81 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 88.0 | |
| P149 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 83.0 | |
| BM16 | 85.0 | 83.0 | 75.0 | 81.0 | 76.0 | 78.0 | 68.5 | 70.0 | 66.0 | 63.0 | 61.0 | |
Table 2: Comparative %HAp yield achieved by conventional and microwave sintering.
| POWDER | P120 | P88 | P81 | P149 | M16 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TEMP (°C) | CONV | MICRO | CONV | MICRO | CONV | MICRO | CONV | MICRO | CONV | MICRO |
| 900 | 60.1 | 57.5 | 58.9 | 57.8 | 58.9 | |||||
| 1000 | 59.8 | 65.0 | 56.1 | 65.8 | 56.9 | 66.4 | 56.4 | 62.0 | 57.7 | 79.5 |
| 1100 | 68.3 | 91.2 | 62.8 | 73.9 | 61.7 | 72.1 | 60.2 | 72.4 | 64.5 | 94.5 |
| 1200 | 90.1 | 92.6 | 70.9 | 82.0 | 72.5 | 80.6 | 70.1 | 81.4 | 93.1 | 98.4 |
| 1300 | 93.1 | 95.9 | 86.1 | 90.1 | 82.6 | 85.2 | 83.9 | 82.0 | 97.7 | 96.9 |
| 1400 | 94.2 | 93.8 | 92.2 | 92.2 | 87.5 | 85.8 | 92.4 | 87.9 | 95.9 | 94.7 |
Table 3: Comparative density of HAp powders sintered by conventional and microwave heating.
However, for the contaminated HAp powder (BM16), longer soak times reduced the density. This was probably owing to decomposition effect of impurities since the data in Table 2 shows that the decomposition temperature (Tonset) of BM16 powder was much lower than 1100°C. Since decomposition is a time-dependent phenomenon [63], longer soak times led to more decomposition, which aerated the microstructure and prevented densification.
This was confirmed by the decomposition data, which show that the %HAp yield decreased with respect to soak time from 82.5% (1 min soak) to 78.9% (3 min), 76.3% (10 min) and 72.1% (30 min). Therefore, it can be assumed that increasing soak times will increase the density only at temperatures below Tonset. Excessive soak times at temperatures above Tonset will in fact be detrimental and decrease density through decomposition effects.
Conclusion
1. Samples microwave-heated to 1100°C at 10,000oC.h-1 were ˜11 to 30% denser than the samples conventionally heated at the same temperature for 60 min at 300°C.h-1.
2. With microwave heating, densification temperatures were ˜100°C-150°C lower than for conventional heating. The lower densification temperature for microwave heating offers a practical solution to the problem of HAp decomposition before attaining maximum sintered density.
3. Enhanced densification and lower sintering temperatures compared to conventional heating are assumed to be due to a) enhanced diffusion rates induced by the electric field and b) preferential grain boundary coupling with microwave radiation.
4. Owing to the preferential grain boundary coupling mechanism, densification enhancement by microwave heating (compared with conventional heating) was more pronounced when the powder was finer. It was ˜30% for the fine powder (BM16) and ˜11% for the coarse powder (p88).
5. The %HAp yields of the samples sintered by microwave and conventional heating were slightly lower for microwave sintering than for conventional sintering. However, the effect of microwave heating on densification was much more significant than that on decomposition.
6. The optimal microwave soak time at 1100°C was ˜3 min for a heating rate of 10,000°C/h. Longer soak times at temperatures below Tonset led to little densification improvement. Longer soak times above Tonset instead led to more decomposition, which prevented densification.
7. Overall, the microwave sintering cycle was 20-30 times faster than for conventional sintering.
References
- Suchanek W, Yoshimura M (1998) Processing and Properties of Hydroxyapatite-Based Biomaterials for Use as Hard Tissue Replacement Implant. J Mater Res 13: 94-117.
- Goller G, Oktar FN, Agathopoulos S, Tulyaganov DU, Ferreira JM, et al. (2006) Effect of sintering temperature on mechanical and microstructural properties of bovine hydroxyapatite (BHA). J Sol-Gel Sci Tech 37: 111-115.
- Monmaturapoj N, Yatongchai C (2010) Effect of Sintering on Microstructure and Properties of Hydroxyapatite Produced by Different Synthesizing Methods. J Metals Mater Min 20: 53-61.
- Kim HW, Kong YM, Koh YH, Kim HM, Ko JS (2003) Pressureless Sintering and Mechanical and Biological Properties of Fluor-hydroxyapatite Composites with Zirconia. J Am Ceram Soc 86: 2019-2026.
- Weinand WR, Goncalves FF, Lima WM (2006) Effect of Sintering Temperature in Physical-Mechanical Behaviour and in Titanium-Hydroxyapatite Composite Sinterability. Materials Science Forum 530-531: 249-254.
- Prokopiev O, Sevostianov I (2006) Dependence of the mechanical properties of sintered hydroxyapatite on the sintering temperature, Mater Sci Eng A 431: 218-227.
- Shi SL, Pan W (2007) Machinable Ti 3 SiC 2 / Hydroxyapatite Bioceramic Composites by Spark Plasma Sintering. J Am Ceram Soc 90: 3331-3333.
- Nath S, Basu B, Sinha A (2006) A Comparative Study of Conventional Sintering with Microwave Sintering of Hydroxyapatite Synthesized by Chemical Route. Trends Biomater Artif Organs 19: 93-98.
- Harabi A, Belamri D, Karboua N, Mezahi FZ (2011) Sintering of bioceramics using a modified domestic microwave oven: Natural hydroxyapatite sintering. J Thermal Analysis and Calorimetry 104: 383-388.
- Curran DJ, Fleming TJ, Towler MR, Hampshire S (2010) Mechanical properties of hydroxyapatite-zirconia compacts sintered by two different sintering methods. J Mater Sci Mater Med 21: 1109-1120.
- Teoreanu I, Preda M, Melinescu A (2008) Synthesis and characterization of hydroxyapatite by microwave heating using CaSO4.2H2O and Ca(OH)2 as calcium source. J Mater Sci Mater Med 19: 517-523.
- Fang Y, Agrawal DK, Roy DM, Roy R (1994) Microwave Sintering of Hydroxyapatite Ceramics. J Mater Res 9: 180-187.
- Fang Y, Agrawal DK, Roy DM, Roy R (1992) Fabrication of Porous Hydroxyapatite Ceramics by Microwave Processing.J Mater Res 7: 490-494.
- Fang Y, Agrawal DK, Roy DM, Roy R (1991) Rapid Sintering of Hydroxyapatite Ceramics by Microwave Processing. Ceramic Transactions 21: 349-356.
- Agrawal DK, Fang Y, Roy DM, Roy R (1992) Fabrication of Hydroxyapatite Ceramics by Microwave Processing. Mater Res Soc Proc 269: 231-236.
- Binner JG (1990) Microwave Processing of Ceramics. Brit Ceram Proc 45: 97-108.
- Sutton WH (1993) Key Issues in Microwave Process Technology. Ceramic Transactions Microwaves: Theory and Applications in Materials Processing II. 36: 3-18.
- Meek TT, Blake RD, Petrovic JJ (1987) Microwave Sintering of Al2O3 and Al2O3-SiC Whisker Composites. Ceram Eng Sci Proc 8: 861-71.
- Das S, Mukhopadhyay AK, Datta S, Basu D (2009) Prospects of microwave processing: An overview. Bull Mater Sci 32: 1-13.
- Sutton WH (1992) Microwave Processing of Ceramics - An Overview. MRS Proceedings 269: 3-20.
- Del Regno GE (2006) Susceptor for hybrid microwave sintering system, hybrid microwave sintering system including same and method for sintering ceramic members using the hybrid microwave sintering system. US Patent No: 7112769.
- Lasri J, Ramesh PD, Schachter L (2000) Energy Conversion during Microwave Sintering of a Multiphase Ceramic Surrounded by a Susceptor". J Am Ceram Soc 83: 1465-1468.
- Janney MA, Calhoun CL, Kimrey HD (1992) Microwave Sintering of Solid Oxide Fuel Cell Materials: I, Zirconia-8 mol% Yttria. J Am Ceram Soc 75: 341-346.
- Janney MA, Calhoun CL, Kimrey HD (1991) Microwave Sintering of Zirconia- 8 mol% Yttria. Microwaves: Theory and Applications in Materials Processing 21: 311-318.
- De AS, Ahmad I, Whitney ED, Clark DE (1991) Microwave (Hybrid) Heating of Alumina at 2.45 GHz: II. Effect of Processing Variables, Heating Rate and Particle Size. Microwaves: Theory and Applications in Materials Processing 21: 329-340.
- Dé AS, Ahmad I, Whitney ED, Clark DE (1991) Microwave (Hybrid) Heating of Alumina at 2.45 GHz: I. Microstructural Uniformity and Homogeneity. In: Clark DE, Gac FD, Sutton WH (Eds.), Microwaves: Theory and Applications in Materials Processing I, American Ceramic Society, Westerville, OH, 319-28.
- Komarenko P, Clark DE (1993) Microwave Susceptor Materials in the (MgxNi1-x)O System for High-Temperature Use in Air. In: Clark DE, Tinga WR, Laia JR (Eds.), Microwaves: Theory and Applications in Materials Processing II, Jr. American Ceramic Society, Westerville, OH, 351-58.
- Yamashita K, Owada H, Nakagawa H, Umegaki T, Kanazawa T (1986) Trivalent-Cation-Substituted Calcium Oxyhydroxyapatite. J Am Ceram Soc 69: 590-94.
- Ruys AJ, Zeigler KA, Standard OC, Brandwood A, Milthorpe BK, et al. (1992) Hydroxyapatite Sintering Phenomena: Densification and Dehydration Behaviour. In: Bannister MJ (Ed.), Adding the Value, CSIRO Publications, Melbourne, 2: 605-10.
- Xu G, Lloyd IK, Carmel Y, Olorunyolemi T, Wilson OC Jr. (2001) Microwave sintering of ZnO at ultra-high heating rates. J Mater Res 16: 2850-58.
- Fang Y, Roy DM, Cheng J, Roy R, Agrawal DK (1993) Microwave Sintering of Hydroxyapatite-Based Composites. In: Clark DE, Tinga WR, Laia JR (Eds.), Microwaves: Theory and Applications in Materials Processing II, American Ceramic Society, Westerville, OH, 397-406.
- Janney MA, Kimrey HD (1988) Microstructure Evolution in Microwave Sintered Alumina. In: J. Bleninger J, Handwerker C (Eds.), Advances in Sintering, American Ceramic Society, Westerville, OH.
- Fanslow GE (1991) Microwave Enhancement of Chemical and Physical Reactions. Mater Res Soc Proc 189. In: Synder WB Jr, Sutton WH, Iskander MF, Johnson DL (Eds.), Microwave Processing of Materials II, Materials Research Society, Pittsburgh, PA, 43-48.
- Dé A, Ahmad I, Whitney ED, Clark DE (1990) Effect of Green Microstructure on Microwave Processing of Alumina: Effect of Particle Size.Ceram Eng Sci Proc 11: 1743-53.
- Janney MA, Jackson ML, Kimrey HD (1993) Microwave Sintering of ZrO2-12 mol% CeO2. In: . Edited by Clark DE, Tinga WR, Laia JR Jr. (Eds.), Microwaves: Theory and Applications in Materials Processing II, American Ceramic Society, Westerville, OH, 101-108.
- Zhang J, Yang Y, Cao L, Chen S, Shong X, et al. (1994) Microwave Sintering of Nanocrystalline ZrO2 Powders. Mater Res Soc Proc 347. In: Iskandar MF, Lauf RJ, Sutton WH (Eds.), Microwave Processing of Materials IV, Materials Research Society, Pittsburgh, PA, 591-597.
- Nightingale SA, Hannink RHJ, Street S (1993) Fabrication and Characterization of Microwave Sintered Zirconia Ceramics. In: Badwal SPS, Bannister MS, Hannink RHJ (Eds.), Science and Technology of Zirconia V.Technomic Publishing Co. Inc, Lancaster, PA, 299-309.
- Holcombe CE, Dykes NL (1991) Microwave Sintering of Titanium Diboride. J Mater Sci 26: 3730-38.
- Wroe FCR, Samuels J (1990) Microwave Sintering of Advanced Ceramics. Brit Ceram Proc 50. In: Thompson DP (Ed.), Engineering Ceramics, British Ceramic Society, 39-51.
- Katz JD, Blake RD, Petrovic JJ, Sheinberg H (1988) Microwave Sintering of Boron Carbide. Mater Res Soc Proc 124. In: Sutton WH, Brooks MH, Chabinsky IJ (Eds.), Microwave Processing of Materials I, Materials Research Society, Pittsburgh, PA, 219-26.
- Janney MA, Kimrey HD (1991) Diffusion-Controlled Processes in Microwave-Fired Oxide Ceramics. Mater Res Soc Proc 189. In: Synder WB Jr., Sutton WH, Iskander MF, Johnson DL (Eds.), Microwave Processing of Materials II, Materials Research Society, Pittsburgh, PA, 215-28.
- Demirskyi D, Agrawal D, Ragulya A (2011) Neck growth kinetics during microwave sintering of nickel powder. J Alloys Compounds 509: 1790-95.
- Ahmad I, Clark DE (1991) Effect of Microwave Heating on Solid State Reactions of Ceramics. In: Clark DE, Gac FD, Sutton WH (Eds.), Microwaves: Theory and Applications in Materials Processing I, American Ceramic Society, Westerville, OH, 605-12.
- Fathi Z, Ahmad I, Simmons JH, Clark DE, Loding AR (1991) Surface Modification of Sodium Aluminosilicate Glasses Using Microwave Energy. In: Clark DE, Gac FD, Sutton WH (Eds.), Microwaves: Theory and Applications in Materials Processing I, American Ceramic Society, Westerville, OH, 623-30.
- Fathi Z, Clark DE, Hutcheon R (1992) Surface Modification of Ceramics using Microwave Energy. Mater Res Soc Proc 269. In: Beatty RL, Sutton WH, Iskandar MF (Eds.), Microwave Processing of Materials III, Materials Research Society, Pittsburgh, PA, 347-52.
- Cheng J, Qiu J, Zhou J, Ye N (1992) Densification Kinetics of Alumina during Microwave Sintering. Mater Res Soc Proc 269. In: Beatty RL, Sutton WH, Iskandar MF (Eds.), Microwave Processing of Materials III, Materials Research Society, Pittsburgh, PA, 323-28.
- Ahmad I, Clark DE (1993) Effect of Microwave Heating on the Mass Transport in Ceramics. In: Clark DE, Tinga WR, Laia JR (Eds.), Microwaves: Theory and Applications in Materials Processing II, Jr. American Ceramic Society, Westerville, OH, 36: 287-96.
- Fathi Z, Folz DC, Clark DE, Hutcheon R (1993) Surface Modification of Sodium Aluminosilicate Glasses Using Microwave Energy II. In: Clark DE, Tinga WR, Laia JR (Eds.), Microwaves: Theory and Applications in Materials Processing II, Jr. American Ceramic Society, Westerville, OH, 333-40.
- Booske JH, Cooper RF, Dobson I (1992) Mechanisms for Nonthermal Effects on Ionic Mobility During Microwave Processing of Crystalline Solids. J Mater Res 7: 495-501.
- Booske JH, Cooper RF, Dobson I, McCaughan L (1991) Models of Nonthermal Effects on Ionic Mobility during Microwave Processing of Crystalline Solids. In: Clark DE, Gac RD, Sutton WH (Eds.), Microwaves: Theory and Applications in Materials Processing I, American Ceramic Society, Westerville, OH, 185-92.
- Meek TT (1987) Proposed Model for the Sintering of a Dielectric in a Microwave Field. J Mater Sci Lett 6: 638-40.
- Metaxas AC, Meredith RJ (1983) Industrial Microwave Heating, Peter Peregrinus Ltd., London.
- Rilley B (1991) Microwave Treatment of Ceramic Materials. In: Vincenzini P, Elsevier BV (Eds.), Ceramics Today - Tomorrow’s Ceramics 1233-61.
- Meek TT, Zhang X, Rader M (1991) An Analysis of the Individual Grain Structure of an Oxide Heated Using Microwave Radiation and Heated Conventionally. In: Clark DE, Gac RD, Sutton WH (Eds.), Microwaves: Theory and Applications in Materials Processing I, American Ceramic Society, Westerville, OH, 81-94.
- Zhang J, Cao L, Xia F (1992) Microwave Sintering of Si3N4 Ceramics. Mater Res Soc Proc 269. In: Beatty RL, Sutton WH, Iskandar MF (Eds.), Microwave Processing of Materials III, Materials Research Society, Pittsburgh, PA, 329-34.
- Tiegs TN, Kiggans JO, Kimrey HD (1991) Microwave Sintering of Silicon Nitride. Ceram Eng Sci Proc 12: 1981-92.
- Tiegs TN, Ferber MK, Kiggans JO, More KL, Hubbard CM, et al. (1991) Microstructure Development During Microwave Annealing of Dense Silicon Nitride. In: Clark DE, Gac FD, Sutton WH (Eds.), Microwaves: Theory and Applications in Materials Processing I, American Ceramic Society, Westerville, OH.
- Tiegs TN, Ploetz KL, Kiggans JO, Yeckley RL (1993) Crystallization of Grain Boundary Phases in Silicon Nitride with Low Additive Contents by Microwave Annealing. In: Microwaves: Theory and Applications in Materials Processing II, Jr. American Ceramic Society, Westerville, OH.
- Tiegs TN, Kiggans JO, Lin HT, Willkens CA (1994) Comparison of Properties of Sintered Reaction-Bonded Silicon Nitride Fabricated by Microwave and Conventional Heating. Mater Res Soc Proc 347. In: Iskander MF, Lauf RJ, Sutton WH (Eds.), Microwave Processing of Materials IV. Materials Research Society, Pittsburgh, PA.
- Ferber MK, Tiegs TN, Jenkins MG (1991) Effect of Post-Sintering Microwave Treatments on the Mechanical Performance of Silicon Nitride.Ceram Eng Sci Proc 12: 9-10.
- Richerson DW (1992) Modern Ceramic Engineering, Second Edition. Marcel Dekker, New York.
- Zeigler KA, Ruys AJ, Sorrell CC (1992) Interdiffusion in Hydroxyapatite Composites. In: Bandyopadhyay S, Crosky AG (Eds.), Proceedings of the 3rd Australian Forum on Metal Matrix Composites, IMMA, Sydney 175-84.
- Ruys AJ, Wei M, Sorrell CC, Dickson MR, Brandwood A, et al. (1995) Sintering effects on the strength of hydroxyapatite. Biomaterials 16: 409-415.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 21607
- [From(publication date):
July-2013 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 16876
- PDF downloads : 4731