Research Article Open Access
Microvascular Perfusion Changes in the Contralateral Gastrocnemius Following Unilateral Eccentric Exercise
| Noelle M Selkow1, Daniel C Herman2, Zhenqi Liu3, Jay Hertel3, Joseph M Hart3 and Susan A Saliba3 | |
| 1School of Kinesiology and Recreation, Illinois State University, USA | |
| 2Primary Care Sports Medicine Fellow, University of Florida, USA | |
| 3University of Virginia, USA | |
| Corresponding Author : | Noelle M Selkow Assistant Professor School of Kinesiology and Recreation Illinois State University, Campus Box 5120 Normal, Il 61761, USA Tel: 309-438-1875 Fax: 309-438-5559 E-mail: nselkow@ilstu.edu |
| Received May 16, 2013; Accepted June 26, 2013; Published June 28, 2013 | |
| Citation: Selkow NM, Herman DC, Liu Z, Hertel J, Hart JM, et al. (2013) Microvascular Perfusion Changes in the Contralateral Gastrocnemius Following Unilateral Eccentric Exercise. J Nov Physiother 3:163. doi: 10.4172/2165-7025.1000163 | |
| Copyright: © 2013 Selkow NM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
Context: Eccentric exercise increases local blood flow and volume as a result of increased metabolic demand. It is not known how eccentric exercise affects microvascular perfusion in the musculature of the opposite limb over this 48 hour period.
Objective: To quantify microvascular perfusion immediately after eccentric exercise to the gastrocnemius in the opposite limb and over 48 hours following the exercise.
Design: Descriptive laboratory study. Setting: Laboratory.
Patients or Other Participants: Six healthy participants volunteered (1M, 5F; Age: 22.4 ± 2.1 years; Height: 165.2 ± 16.6 cm; Weight: 64.5 ± 25.1 Kg).
Intervention(s): A unilateral, eccentric exercise was performed to a randomized leg. Each subject performed 100 calf-lowering repetitions in the sequence of 50 repetitions, 5 min rest and 50 repetitions.
Main outcome measure: Micro vascular perfusion measurements (blood volume (dB), blood flow (dB/sec), and blood flow velocity (1/sec)) of the contralateral gastrocnemius were taken using contrast-enhanced ultrasound (CEU) at baseline, immediately after exercise, and 48 hours after exercise. Pain using a visual analog scale was also recorded baseline, 24, and 48 hours after exercise to determine the onset of delayed onset muscle soreness (DOMS).
Results: There was a significant increase in blood volume immediately after exercise (9.77 ± 3.19 dB) from baseline (6.18 ± 2.05 dB) (p=.023). There was a significant increase in blood flow immediately after exercise (3.53 ± 0.86 dB/sec) from baseline (2.40 ± 0.69 dB/sec) (p=.010). There was no change in blood flow velocity (p=0.487). Blood flow (p=0.003) and volume (p=0.002) remained significantly higher at 48 hours from baseline, with no change in blood flow velocity (p=0.316). Pain significantly increased.
Conclusion: Micro vascular perfusion increased immediately after exercise in the contralateral gastrocnemius. This increase was maintained following a single bout of eccentric exercise over a 48 hour period in the presence of DOMS.
| Keywords |
| Contrast-enhanced ultrasound; Delayed onset muscle soreness; Exercised-induced muscle damage |
| Introduction |
| Repeated contractions of skeletal muscle, as in exercise, have been shown to increase local [1-6] and systemic blood flow [7,8]. This increase in blood flow is proportional to the metabolic demands of the muscle tissue being exercised [9]. Even with relatively low force contractions, blood flow is elevated [3]. Microvascular perfusion changes in an isolated muscle have been studied previously using concentric, isometric, and eccentric contractions. Eccentric exercise is often used as model for examining the effects of muscle damage and inflammation [10,11]. Recently, we examined the effect of a single bout of unilateral, eccentric exercise to the gastrocnemius. We found that microvascular perfusion increased immediately after exercise, [12] and this increase was sustained over 48 hours [13]. Understanding systemic microvascular perfusion characteristics in a healthy population during eccentric exercise will help explain the metabolic load of this type of exercise and quantifying the vascular changes in healthy individuals is important prior to an examination in pathological populations. Understanding normative values in a healthy population will allow for deficiencies to be detected in pathological individuals. If the deficiencies are vascular in nature, treatments could be administered that affect vascular responses. |
| Microvascular perfusion immediately following low force isometric and concentric exercises have been examined in skeletal muscle [2-4,14,15] more than eccentric exercise at the local level. While blood volume and flow increase following exercise, it is unclear what the vascular response in the unexercised limb is. When injury occurs, particularly at a joint, deficits in strength and muscle activation have been shown in the contralateral limb [16-18]. Clinicians aim to address these deficits during rehabilitation, but the cause of these contralateral deficits remain unknown. It is possible that during injury, blood flow increases systemically contributing to some of the deficits present. |
| There have been several measurements techniques to estimate blood flow in the extremities of humans and animal models, however, these tools monitor the arteriole function and total flow, but cannot differentiate immediate or ongoing responses at the capillary level [19- 21]. Contrast-enhanced ultrasound (CEU) is a non-invasive method to measure microvascular volume in muscle [15]. Infused micro bubbles act as the contrast agent through continuous intravenous infusion at a constant rate and concentration [22]. Based on the principle that ultrasound does not transmit through air and media of differing densities have greater contrast, the micro bubbles can be visualized with a diagnostic ultrasound imager [23]. |
| While blood flow has been shown to increase during isometric contractions of the lower limb using CEU, [3] and immediately following eccentric exercise, [12] the systemic response in the contralateral limb has not been assess using CEU. Therefore, the purpose of our study was to examine the immediate effects of unilateral, fatiguing eccentric exercise on microvascular perfusion in the contralateral gastrocnemius and at 48 hours. Our hypothesis was that blood flow and volume would increase immediately post-exercise due to the systemic increase in perfusion from exercise. However, at 48 hours, microvascular perfusion would return to baseline levels, since the exercised leg would only experience DOMS. |
| Methods |
| Design |
| This study was a descriptive laboratory study. The independent variable was time (baseline, immediate post-exercise, and 48 hours post exercise). The dependent variables were blood flow, blood volume, and blood flow velocity. |
| Subjects |
| Six healthy volunteers participated in the study (1M, 5F; Age: 22.4 ± 2.1 years; Height: 165.2 ± 16.6 cm; Weight: 64.5 ± 25.1 Kg) without a lower extremity injury in the past six months or lower extremity surgery in the past year. Other exclusion criteria were history of cardiovascular disease, abnormal ECG, heart murmur, or pregnancy. All participants read and signed informed consent and the study was approved by the University’s investigational review board (IRB-HSR #15227). Sample size was calculated at 9 subjects using blood flow data from the exercised limb baseline to post-exercise, when power equals .80 and alpha is set at p=0.05 [24]. While our sample size is small, this was the control group to a larger study [13] and significant differences were found. |
| Instruments |
| CEU measurements were recorded using the SONOS 7500 ultrasound machine (Philips Medical Systems, Andover, MA). The S3 adult echo transducer was used to image the muscle with an imaging ratio of 1.3:3.6 MHz. The mechanical index was set at 1.5. |
| The contrast agent Definity® (Lantheus Medical Imaging, N. Billerica, MA) is an FDA approved contrast agent containing octafluorpropane gas-filled albumin. The definity microbubbles are supplied in 1.5 ml volume vials and were mixed with 0.9% sodium chloride in a ratio of 0.3 ml microbubbles per 7 ml saline. A maximum of 2 vials (3.0 ml) can be safely infused per day. The solution was infused intravenously at a rate of 1.5 ml.min-1. |
| The VAS was used as the primary tool for pain quantification. A 100 mm line, with no markings, except no pain at the left and worst pain at the right end of the continuum was used. Subjects were asked to mark a vertical dash on the horizontal line indicating level of pain relative to the continuum. A line without numerical markings was used so the patient was less apt to remember previous markings. |
| Eccentric exercise protocol |
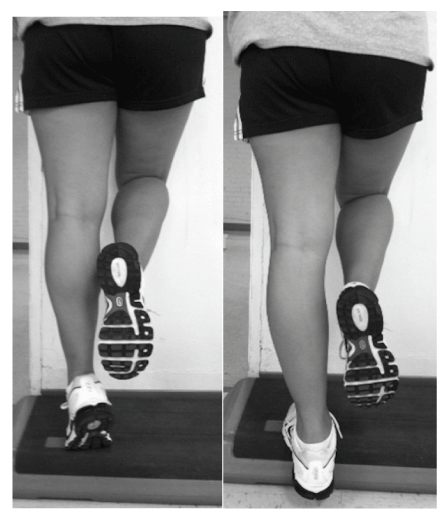
| There are several eccentric exercise protocols to induce DOMS in the calf since this is a commonly utilized mechanism for creating an inflammatory Model [25-28]. We chose to use a modified version of the method described by Tegeder et al. [28] since this utilized a unilateral exercise procedure for the calf. Subjects stood on one foot on an aerobic step with the heel of the exercising leg off the edge of the step. The subjects lifted the heel of the leg that was randomized to do the exercise and then lowered the heel slowly for 3 seconds until they could not lower the heel any further. A metronome was set at 60 beats per minute for uniform timing of the exercise. The control leg was used to shift the weight distribution off the exercised leg, so it could be returned to the starting position without doing a concentric contraction on that extremity (Figure 1). A random number generator was used to determine which leg was exercised. The exercise protocol consisted of 2 sets of 50 eccentric contractions of the gastrocnemius separated by a 5-minutes rest interval. |
| Procedures |
| Participants met with the lead investigator and physician for the initial screening. Height, weight, blood pressure, pulse, respirations, and heart sounds were recorded. A general health history and lower extremity health history questionnaire were completed. If no exclusion criteria were identified, the participant had a 12-lead ECG assessment and metabolic blood draw. Female participants had a serum pregnancy test to make sure they were not pregnant. Results were obtained within 48 hours and were reviewed by a physician. Once the physician cleared the participant, he or she was enrolled in the exercise portion of the study. |
| All participants stayed overnight at the GCRC the night before testing began. Participants were instructed to stay in bed except to use the bathroom and lights were turned off at 11 pm. between 5 and 6 am the next morning, a registered nurse prepared the preferred arm for an intravenous catheter for micro bubble infusion in an antecubital vein using sterile techniques. A 3-lead ECG and pulse oxiometer was attached to the participant for continuous monitoring of heart rhythm and oxygen saturation until the subject was discharged. Subjects were positioned prone. The medial gastrocnemius of the contralateral limbs was marked over the area of greatest girth with a 2.5 cm×2.5 cm square (size of the transducer head). A solution of 0.9 ml of microbubbles were mixed with 21 ml of saline and infused at a rate 1.5 ml/min. After steady state was reached (about 2 min), CEU measurements began. The ultrasound transducer was placed over the mark on the gastrocnemius after applying an ample amount of ultrasound gel to the transducer. Images were triggered to pulse intervals of the ECG, with one image taken every 1 beat, 2 beats, 3 beats, 4 beats, 5 beats, 8 beats, 12 beats, 16 beats, and 20 beats, for three images at every pulse interval, resulting in 27 images. Infusion of the micro bubbles stopped after the last image was collected. Measurements took between 3-5 min, because of the dependence on heart rate. |
| Participants were monitored for any cardiovascular effects or symptoms associated with the micro bubble infusion for 5 minutes before performing the eccentric exercise protocol. The examiner taking the CEU measurements instructed the subject on how to perform the exercise and allowed the subject to practice up to 10 repetitions with feedback. Once the exercise was finished, participants were positioned prone and infusion of the micro bubbles began again. CEU measurements were recorded as before. |
| At 48 hours, the subject returned to the GCRC where the subject was instructed to lay quietly for 2 hours to ensure a resting perfusion level. The CEU measurements were captured as previously described. The subject was monitored for 30 minutes before being discharged. |
| Data reduction |
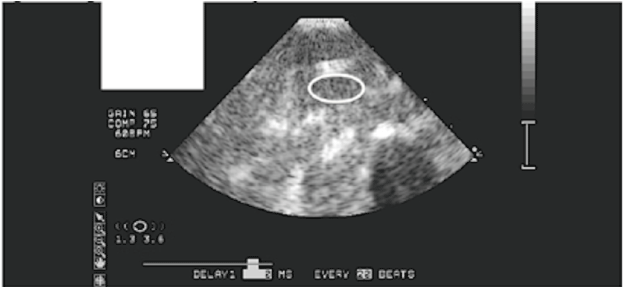
| Images were analyzed using specialized software developed for the CEU procedures. QLAB (Philips Healthcare, Andover, MA) was used to assess blood volume and blood flow velocity using replenishment kinetics for a specific region of interest (Figure 2). Blood flow is calculated as the product of blood volume and blood flow velocity. The equation of y=A (1-exp-βt), where y equals video intensity (VI) at pulse interval t, VI plateau (blood volume) is A, and β is the rate of rise in VI (blood flow velocity), is used [22]. |
| Statistical Analysis |
| Three separate 1×3 repeated measures ANOVAs (SPSS 17, Chicago, IL) were used to analyze microvascular perfusion over 48 hours. ADD is VAS planned effect size calculations were performed on significant findings. |
| Results |
| There was a significant main effect for time for blood volume (p=0.023) and blood flow (p=0.010), with no significant difference in blood flow velocity (p=0.316). There were significant increases in blood volume (p=0.001) and blood flow (p<0.001) immediately postexercise (9.77 ± 3.19 dB and 3.53 ± 0.86 dB/sec), respectfully in the contralateral limb compared to baseline (6.18 ± 2.05 dB and 2.40 ± 0.69), with no change in blood flow velocity (p=0.487). The effect size for blood volume was 1.34 (0.09, 2.60) and blood flow was 1.41 (0.15, 2.68). The increases in contra lateral blood volume (p=0.002) and blood flow (p=0.003) were maintained at 48 hours (9.41 ± 1.90 dB and 3.51 ± 0.47 dB/sec) compared to baseline, with again no change in blood flow velocity (p=0.411). The effect size for blood volume was 1.62 (0.32, 2.92) and blood flow was 1.86 (0.51, 3.22). There were no changes in blood volume (p=0.814), blood flow (p=0.962), or blood flow velocity (p=0.493) between post-exercise and 48 hours for the contra lateral limb. |
| VAS scores for the exercised limb were significantly different at 24 and 48 hours from baseline (p<.001), as well as between 24 and 48 hours (p<0.001). The effect sizes are all above 5. Baseline was 0.0 ± 0.0 mm, 24 hours was 18.38 ± 5.01 mm and 48 hours was 52.5 ± 5.37 mm. |
| Discussion |
| Following eccentric exercise to a single limb, the contralateral limb resulted in increased blood volume and blood flow immediately after exercise and at 48 hours post exercise. From previous research in our lab [12] immediately after eccentric exercise, blood volume and blood flow increased in the exercise leg by 42% and 80%, respectfully. From this study, the contra lateral leg increased 17% and 35% for blood volume and blood flow, respectfully. This finding supports earlier work by Seals [7] and Taylor et al. [8] that identified vasodilatation of the contra lateral limb after exercise initiation. Blood flow velocity did not change in the contra lateral limb after exercise and at 48 hours. Since this limb was not exercised, recruitment of capillaries is not necessary, as would be in exercised muscle [14]. |
| The increases in blood volume and blood flow of the contra lateral limb were maintained over the 48 hour period. Since eccentric exercise was performed in our study, we feel that the sustained increase in micro vascular perfusion was from an inflammatory response in the form of DOMS from the exercised leg. This local inflammatory response leads into a systemic response called the acute-phase response, which is mediated by circulating cytokines from the injured tissue, including IL-6 and TNF-α [29]. This may lead to a systemic response in increased blood flow. Pain scores were also elevated over 48 hours, indicating DOMS was achieved in the exercised leg and a form of muscle damage occurred. |
| Another explanation could be from the added work performed by the contra lateral limb for locomotion. Several of our subjects could not bear full weight on the exercised limb and had limited dorsiflexionin the exercised limb. While this was not directly measured, side-toside differences were noted. Subjects reported having to limp because they could not lower the heel all the way to the ground due to pain. Loss of function occurs immediately after eccentric exercise, where the greatest deficits in force production and range of motion are noted [30]. The reduced range of motion may allow for a position of optimal healing when new capillaries and new connective tissue are forming 48 hours after injury [31]. There were no functional deficits or pain observed in the contralateral limb. Examining micro vascular perfusion of the upper extremity may have been a better model for assessing systemic blood flow. The mechanisms behind micro vascular perfusion increases systemically remain unknown. |
| While we controlled for ice applications and analgesic use, a limitation of our study was that we could not control for activities of daily living within the 48 hours between CEU assessments. They were told to go about activities of daily living, but to refrain from exercise or taking over the counter pain medication. Some subjects walked to and from class, potentially accumulating up to 30 minutes, while other subjects worked desk jobs. We asked each subject to report use of NSAIDs or additional treatments, but all reported none were used. |
| Conclusion |
| Eccentric exercise increased microvascular perfusion immediately after exercise in the contralateral limb, which had not been examined before. The increased perfusion was maintained over 48 hours, so the prolonged increased in perfusion of the contralateral limb may have been due to an inflammatory response or the extra demands placed on the contralateral limb for support during walking. |
| Acknowledgements |
| Supported by NIH Grant RR00847 and the University of Virginia Curry School of Education. |
References |
|
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 14243
- [From(publication date):
July-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9649
- PDF downloads : 4594
