Research Article Open Access
Microtuber Induction of Two Potato (Solanum tuberosum L.) Varieties
| Miheretu Fufa1* and Mulugeta Diro2 | ||
| 1Oromia Agricultural Research Institute, Adami Tullu Agricultural Research Center, Plant Biotechnology Team, P.O. Box 35, Zeway, Ethiopia | ||
| 2Southern Agricultural Research Institute, Ethiopia | ||
| Corresponding Author : | Miheretu Fufa Oromia Agricultural Research Institute Adami Tullu Agricultural Research Center Plant Biotechnology Team P.O. Box 35, Zeway, Ethiopia Tel: +251 116 187 346 E-mail: miheretufufag@gmail.com |
|
| Received November 19, 2013; Accepted February 27, 2014; Published March 01, 2014 | ||
| Citation: Fufa M, Diro M (2014) Microtuber Induction of Two Potato (Solanum tuberosum L.) Varieties. Adv Crop Sci Tech 2:122. doi: 10.4172/2329-8863.1000122 | ||
| Copyright: © 2014 Fufa M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Two potato varieties namely, ‘Hunde’ and ‘Ararsa’ were tested for their microtuber induction under five levels of sucrose (40, 60, 80, 100 and 120 gl-1) in completely randomized design with 2×5 factorial combinations. The objective was to determine optimum concentration of sucrose for microtuber induction. In both varieties, among the five concentrations of sucrose, MS medium supplemented with 60 gl-1 sucrose exhibited a better response than the other concentrations in mean values of microtuber number, diameter, and weight and was found optimum. Accordingly, this medium produced, after 42.57 ± 0.58 days of culture, an average value of (1.97 ± 0.02) microtuber number, (3.60 ± 0.04 mm) microtuber diameter, and (0.08 ± 0.002 g) weight of microtuber in the variety Ararsa. On the other hand, it gave, after 35.67 ± 0.58 days of culture, mean value of (2.90 ± 0.031) microtuber number, (2.95 ± 0.01 mm) microtuber diameter, and (0.06 ± 0.001 g) weight of microtuber in the variety Hunde.
| Keywords | |
| In vitro tuberization; Microtuber; Potato; Sucrose | |
| Introduction | |
| The potato (Solanum tuberosum L.) is an important food and cash crop [1] having the first rank in the world from none grain crops to ensure food security [2]. It is a high biological value crop that gives an exceptionally high yield and more nutrients per unit area per unit time than any other major crops [3]. | |
| The tubers produced through the conventional propagation are characterized by low multiplication rate and susceptibility to pathogens [3]. Microtubers are an ideal propagating material for producing highquality seed potatoes [4]. Microtubers, on the other hand, has several merits over in vitro plantlets due to their little size, reduced weight and vigorous nature. | |
| Microtubers offer advantages of small space accommodation, ease of transport and storage for long time in addition to solving the problems of transplanting of plantlets [5,6]. Moreover, microtubers are utilized for minituber production in greenhouses and, less commonly, are directly field-planted [7]. These properties make microtubers an ideal propagating material [4] for producing high-quality seed potatoes [8]. | |
| Microtubers are also useful in other applications, including germplasm storage and exchange or as experimental research tools in the areas of plant metabolism, germplasm selection and evaluation, transformation, somatic hybridization or molecular farming, and for in vitro selection of agronomically important characters, such as maturity and abiotic stress tolerance [9]. | |
| Moreover, microtubers and field grown tubers have strong and consistent similarity in their morphology and biochemical features. This makes the induction, growth and development of microtubers a valuable model system [10]. | |
| In general, the use of microtuber technology appears to have enormous potential in seed tuber production, breeding programs, germplasm conservation and research. The technology helps to reduce the time necessary to supply seed tuber [7] of greatly improved quality in large scale with low cost. | |
| In India, the advances in microtuber production is considered as second “green revolution” in agriculture and are expected to make farming more efficient, profitable and environmentally safe in addition to helping the farmers economically, socially and commercially. As a result, microtubers are used as an alternative in potato seed production [11]. | |
| Sucrose is the most critical stimulus for inducing microtubers at high concentration [6]. It is a cheap, safe and superior agent for microtuber induction [4]. No attempt was made for in vitro tuber induction in Ethiopia. Hence, the present study was initiated with the objective to determine optimum concentration of sucrose for microtuber induction. | |
| Materials and Methods | |
| Single nodal excision from one week old sprouts of the relatively clean tubers of ‘Ararsa’ and ‘Hunde’ potato varieties that were released by Sinana Agricultural Research Center in 2006 were used for microtuber induction experiment at Tissue Culture Laboratory of Jimma University College of Agriculture and Veterinary Medicine. The two varieties were tested for tuberization response under five levels of sucrose (40, 60, 80, 100 and 120 gl-1) in completely randomized design with 2×5 factorial combinations. | |
| The pH of the medium was adjusted at 5.8, agar (8 gl-1) was added and then the medium was autoclaved at 121°C for 20 minutes at 15 psi. Murashige and Skoog (MS) basal medium containing gebberellic acid (0.1 mgl-1), naphthalene acetic acid (0.01 gl-1) and sucrose (30 gl-1) was used for initiation. In the case of microtuber induction, the MS basal medium was prepared for each treatment combination. | |
| All the surface sterilization procedures were carried out under aseptic condition of laminar flow chamber following the procedure of Naik and Karihaloo [11]. One week old sprouts with buds were excised and used as initial explants. The excised explants were washed 3 times in running tap water with three drops of Tween-20. Then it was washed thoroughly three times with sterile distilled water and immersed in 70% ethyl alcohol for 10 seconds. The alcohol was removed by three times washing with sterile distilled water and sterilized with 10% NaOCl for 20 minutes. | |
| The excised explants were dissected into single nodes (2cm) on a sterile plate after removing the leaves. Six explants were cultured into 40 ml of an initiation culture medium in culture jar and incubated under a 16 h photoperiod at 24°C with a light intensity of 2500 lux. For 3-4 weeks, the sprouts were allowed to grow into plantlets having nodal segments. After decanting the multiplication medium, the plantlets were kept in a conditioning medium before being used for microtuber induction medium to avoid the carryover effects of hormones. Forty milliliters of microtuber induction medium was dispensed into each culture jar. The culture were transferred to the growth room and kept at a temperature of 18°C under dark condition. | |
| Data collection and analysis | |
| The date of formation of microtuber (first, second and third) was followed carefully and days (50%) to microtuber formation was recorded and used for analysis. The number of microtubers produced by each explant was counted and all the microtubers produced were harvested. The diameter (mm) of each microtuber was measured by Digital Caliper. Immediately after harvest, each microtuber was weighed on sensitive balance to get the mean microtuber weight (g). After fifteen days of light exposure, the microtubers were treated with gibberellic acid (GA3) and incubated in the dark before planting in the green house. The number of the microtubers germinated and established was counted to get their percent survival under in vivo. The data were subjected to the analysis of variance (ANOVA) at 5% level of significance using SAS statistical software [12]. The REGWQ multiple comparison procedure was used for separating significant means. | |
| Results and Discussion | |
| Analysis of variance revealed that sucrose and variety interaction had very highly significant effect (α=5%) on days to microtuber induction and on the average number, diameter (mm) and weight (g) of microtubers (Table 1). This implied that there is interdependence of sucrose and genotype on induction of microtuber of potato. Thus, the microtuber induction of genotypes varies with the level of sucrose. | |
| Effects of sucrose on days to tuberization, mean microtuber number and diameter | |
| At 40 gl-1 sucrose, both varieties did not produce microtubers. However, when 60 gl-1 sucrose was added to growth media, ‘Hunde’ produced microtubers in 36 days, which is significantly earlier than that of ‘Ararsa’ (43 days). Increasing concentration of sucrose from 60 to 80 gl-1 delayed microtuber formation in both varieties but more pronounced on Ararsa. This might be due to the marked variation in the responses of plant gene to changing sucrose status. Some genes are induced, some are repressed, and others are minimally affected [13]. | |
| Moreover, microtuber number and size get reduced, in both varieties, as the concentration of sucrose increased from 60 gl-1 to 80 gl-1 (Table 1). At 120 gl-1 of sucrose, both genotypes did not produce microtubers (Table 1). The absence of microtuber formation at high sucrose concentration might be due to the effect of supra optimal level of sucrose that can result in an unfavorable osmotic condition for water uptake, and then affected microtuber formation of the seedlings. | |
| Effects of sucrose on mean microtuber weight | |
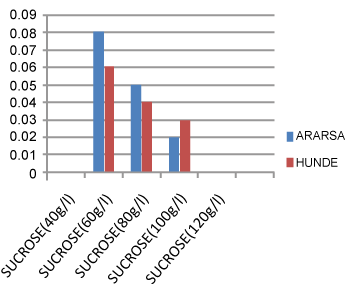
| A decreasing trend in mean weight (g) of microtuber was observed, in both varieties, as the level of sucrose increased (Figure 1). This might be, again, due to the effect of high sucrose level on osmotic condition of the culture for water uptake that affect cell turgidity [13] and hence microtuber weight. | |
| In both varieties, among the five concentrations of sucrose, MS medium supplemented with 60 gram litre-1sucrose exhibited a better response than the other concentrations in mean values of microtuber number, diameter, and weight and was found optimum. Accordingly, this medium produced, after 42.57 ± 0.58 days of culture, an average value of (1.97 ± 0.02) microtuber number, (3.60 ± 0.04 mm) microtuber diameter, and (0.08 ± 0.002 g) weight of microtuber in the variety ‘Ararsa’. On the other hand, it gave, after 35.67 ± 0.58 days of culture, mean value of (2.90 ± 0.031) microtuber number, (2.95 ± 0.01 mm) microtuber diameter, and (0.06 ± 0.001 g) weight of microtuber in the variety ‘Hunde’ (Table 1). | |
| The present result is in agreement with that of Aslam et al. [9] who found that a medium containing 6% sucrose was optimal in terms of minimum time of induction (34), mean tuber number (1.2) and weight (0.03 g) of microtubers per single nodal explant in cultivar Desiree. Imani et al. [14] also reported that MS medium supplemented with 60 gl-1 of sucrose as the best in producing the maximum number (4.20) and size (0.44 cm) of micro tubers. Iqbal et al. [4] also recorded similar results on mean numbers of tubers (4.8) on MS medium treated with 60 gl-1 sucrose. Kanwal et al. [15], on the other hand, reported that MS medium supplemented with 30 and 40 gl-1 sucrose did not produce microtubers. | |
| Conclusion | |
| A protocol for microtuber induction of potato varieties ‘Ararsa’ and ‘Hunde’ from single nodal explant has been developed. The result indicated that microtuber induction of potato was highly dependent on sucrose and genotype interaction. | |
| MS medium supplemented with 60 gl-1 exhibited fewer days to microtuber formation (35.67 ± 0.58/42.67 ± 0.58), better mean number (2.90 ± 0.031/1.97 ± 0.02), diameter (2.81 ± 0.015/3.60 ± 0.04), fresh weight (0.06 ± 0.001/0.08 ± 0.002) and dry weight (0.046 ± 0.001/0.063 ± 0.002) of microtubers in ‘Hunde’ and ‘Ararsa’ varieties, respectively. However, microtuber production needs further improvement as the size of microtubers produced was not large enough. Thus, trying different levels of BAP in combination with sucrose and extending the time of harvesting may be helpful to improve the size of the microtuber. | |
| References | |
References
|
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 16879
- [From(publication date):
April-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11787
- PDF downloads : 5092
