Short Communication Open Access
Microsatellite Instability (MSI) Testing in Extra-colonic Tumors
Sanam Husain* and Lewis Allen HassellUniversity of Oklahoma Health Sciences Center, Oklahoma City, OK USA
- *Corresponding Author:
- Sanam Husain
University of Oklahoma Health Sciences Center
Oklahoma City, OK USA
Tel: 405-973-7527
E-mail: sanamhusain@ouhsc.edu
Received date: February 05, 2015, Accepted date: March 11, 2015, Published date: March 15, 2015
Citation: Husain S, Hassell LA (2015) Microsatellite Instability (MSI) Testing in Extra-colonic Tumors. J Clin Exp Pathol 5:221. doi: 10.4172/2161-0681.1000221
Copyright: © 2015 Husain S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Faulty DNA repair due to defects in the mismatch repair genes results in microsatellite instability (MSI). Microsatellites are short tandem repeats that are present throughout the genome and are sensitive to errors during the cell cycle. Malfunctioning of the DNA repair mechanisms many occur as a result of germ line mutations in the MMR genes (MLH1, MSH2, PMS2 and MSH6) as in the autosomal dominant disorder Hereditary non-polyposis syndrome (HNPCC), also known as Lynch syndrome (LS) or due to hypermethylation of the MLH1 gene. LS is associated with Colorectal carcinoma (CRC) and extra-colonic malignancies including tumors of the endometrium, ovary, pancreas, urinary bladder, stomach, skin, biliary tract and the central nervous system. Early identification and management of such patients and their family members can significantly reduce morbidity and mortality associated with such tumors. Current literature suggests that MSI testing is important not only in the genetic context, but it also has prognostic and predictive value, as has been shown in treatment of CRC. Initial screening mechanisms such as the Amsterdam criteria and Bethesda guidelines were based on personal and family history. Since then various clinical prediction models have been developed that utilize clinical and pathologic features in the risk assessment of patients with MMR deficiency. However, due to the limitations in these screening methods, many institutions are moving towards universal screening of patients with CRC and endometrial carcinomas.In this review, we outline the rationale for and current methods of testing for MSI, along with their relative merits, and discuss the thorny question of screening criteria and who should be screened
Keywords
Microsatellite instability testing; Lynch syndrome; DNA mismatch repair
Introduction
Faulty DNA repair due to defects in the mismatch repair genes results in microsatellite instability (MSI). Microsatellites are short tandem repeats that are present throughout the genome and are sensitive to errors during the cell cycle. Malfunctioning of the DNA repair mechanisms many occur as a result of germ line mutations in the MMR genes (MLH1, MSH2, PMS2 and MSH6) as in the autosomal dominant disorder hereditary non-polyposis syndrome (HNPCC), also known as lynch syndrome (LS) or due to hypermethylation of the MLH1 gene. LS is associated with colorectal carcinoma (CRC) and extra-colonic malignancies including tumors of the endometrium, ovary, pancreas, urinary bladder, stomach, skin, biliary tract and the central nervous system [1]. Early identification and management of such patients and their family members can significantly reduce morbidity and mortality associated with such tumors [2]. Current literature suggests that MSI testing is important not only in the genetic context, but it also has prognostic and predictive value, as has been shown in treatment of CRC. Initial screening mechanisms such as the Amsterdam criteria and Bethesda guidelines were based on personal and family history. Since then various clinical prediction models have been developed that utilize clinical and pathologic features in the risk assessment of patients with MMR deficiency. However, due to the limitations in these screening methods, many institutions are moving towards universal screening of patients with CRC and endometrial carcinomas (EC) [3-6].
In this review, we outline the rationale for and current methods of testing for MSI, along with their relative merits, and discuss the thorny question of screening criteria and who should be screened.
Importance of Identification of MSI Patients
Patients with LS have a significant lifetime risk of developing colonic and extra-colonic malignancies that may occur in a synchronous or metachronous fashion. Much of what we understand about the pathogenesis of MSI is based on research performed on CRC. Endometrial carcinoma has captured considerable attention lately, because of its higher prevalence in the group compared to other extra-colonic malignancies, and also due to the fact that it often is the sentinel cancer in women with MSI [7]. All other neoplasms are much less rigorously studied. Current literature states that MSI-associated tumors have an overall improved survival with lower risk of regional and systemic metastasis, indicating that the patients may be managed with a more conservative approach. For example, CRC tumors that are positive for MSI have been shown to be less sensitive to standard chemotherapy with 5-FU and more responsive to irinotecan. Whether extra-colonic MSI tumors have unique responses to conventional treatment is unknown at this point in time. Work to delineate the mechanism of these sorts of chemo-resistance in MSI tumors is also needed to better predict which alternative therapies may be preferred.
Deciding Whom to Screen
While the practice of reflex MSI testing in CRC and EC appears to be well accepted at NCI Comprehensive Cancer Centers (NCI-CCCs), and is being increasingly adopted by other sizeable medical centers in the United States, it appears that this strategy faces many barriers when it comes to other tumors included in the spectrum of LS. First of all, the cumulative risk of the extra-colonic malignancies varies by gender and individual MMR gene mutations [8-11]. Secondly, given the low incidence of the tumors, the use of IHC and molecular testing may not be cost-efficient in routine clinical practice. However, on an individual case basis, the evaluation of MSI status maybe of considerable importance in the treatment of tumors and further testing of first-degree relatives. So the question arises when extracolonic malignancies, other than EC, should be tested for MSI. The initial screening mechanisms such as the Amsterdam criteria, which were first developed in 1990 relied on physicians to obtain adequate family history and were met with significant challenges [12]. The Bethesda guidelines were proposed by the NCI in 1997 and revised in 2004 for the selection of patients and families that would benefit from genetic testing (Table 1).
| Initial Screening Models | Sensitivity | Specificity |
|---|---|---|
| Amsterdam Criteria I (1990) | 47-91% | 62-84% |
| Amsterdam Criteria II (1998) | 77-81% | 46-68% |
| Bethesda Guidelines (1997, 2004) | 86-92% | 49-58% |
Table 1: Sensitivity and specificity of initial screening models.
At this point in time, the application of clinical prediction models including MMRpredict, the PREMM [1,2,6] model and MMR Pro is directed towards the detection of MSI-related CRC. These approaches have been shown to be superior to the revised Bethesda recommendations [13,14]. However, these systems are not very sensitive and their efficacy is largely dependent on the clinician’s index of suspicion and the ability to obtain a complete family history. Based on their scoring of predicted probability, the sensitivity ranges from 62% with MMRpro to 100% for the PREMM model (Table 2).
| Model | Predicted Probability (%) | Number of Individuals | Number of carriers Observed |
|---|---|---|---|
| MMRpredict | 75-100 | 26 | 22 (84.62%) |
| PREMM | 75-100 | 7 | 7 (100%) |
| MMRpro | 75-100 | 50 | 31 (62%) |
Table 2: Comparison of MMR predict, PREMM and MMRPro prediction models.
Testing Methods
MSI testing algorithms are primarily based on immunohistochemistry (IHC), polymerase chain reaction (PCR) based assays and exon sequencing.
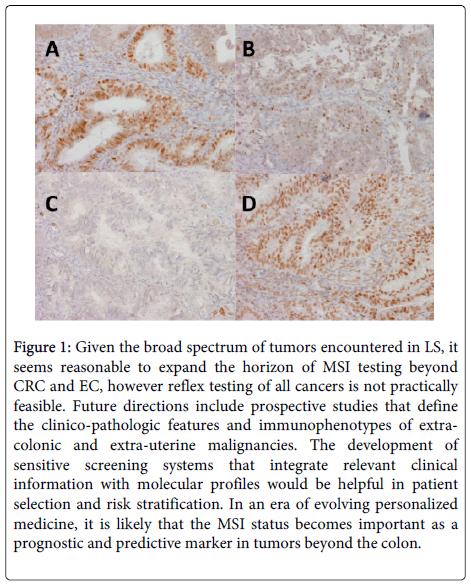
IHC is being increasingly employed to detect the presence or absence of the MMR proteins in tumors and is usually the first step of the MSI work up. Normal epithelium and lymphocytes serve as internal controls demonstrating positive nuclear staining. Any loss of staining in the tumor cells is indicative of the absence of the protein in the tumor. A positive screening IHC is followed up by molecular testing.
PCR-based assays are helpful in detecting the microsatellite repeats and represent another sensitive method in the scheme of MSI testing. PCR amplification can separate the defects due to hypermethylation from germline mutations, leading to identification of patients with HNPCC. Based on the results of the PCR assay, complete sequencing of the involved exon is recommended.
Consensus approaches to optimize patient identification therefore now tend to apply a combination of one or another clinical prediction models with one or more of these testing methods in an algorithmic approach. Data accumulating from various studies seems to support this kind of approach [15]. For example, in our institution, we test all CRC patients under age 70 using IHC. Those showing loss of function of MLH1 get BRAF mutational testing, while those with defects in expression of the other proteins get germline testing for MSI. Patients with negative IHC stains but high clinical predictive model scores may still be referred for MSI molecular germline testing. Patients over age 70 are screened using IHC if their predictive model score is above a defined threshold, or if their tumor has histologic features in Table 3 above (Figure 1).
Figure 1: Given the broad spectrum of tumors encountered in LS, it seems reasonable to expand the horizon of MSI testing beyond CRC and EC, however reflex testing of all cancers is not practically feasible. Future directions include prospective studies that define the clinico-pathologic features and immunophenotypes of extracolonic and extra-uterine malignancies. The development of sensitive screening systems that integrate relevant clinical information with molecular profiles would be helpful in patient selection and risk stratification. In an era of evolving personalized medicine, it is likely that the MSI status becomes important as a prognostic and predictive marker in tumors beyond the colon.
| Morphologic Characteristics of MSI-high tumors assessed in Clinical Prediction Models |
|---|
| Tumor infiltrating lymphocytes |
| Crohn’s like inflammatory pattern |
| High grade histology |
| Medullary, signet ring, or mucinous phenotype |
Table 3: Morphologic characteristics of MSI-high tumors assessed in clinical prediction models.
References
- Lynch HT, Smyrk T (1996) Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer 78: 1149-1167.
- Lynch HT, Boland CR, Gong G, Shaw TG, Lynch PM, et al. (2006) Phenotypic and genotypic heterogeneity in the Lynch syndrome: diagnostic, surveillance and management implications. Eur J Hum Genet 14: 390-402.
- Umar A, Risinger JI, Hawk ET, Barrett JC (2004) Testing guidelines for hereditary non-polyposis colorectal cancer. Nat Rev Cancer 4: 153-158.
- Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, et al. (2005) Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 352: 1851-1860.
- Lynch HT, Boland CR, Rodriguez-Bigas MA, Amos C, Lynch JF, et al. (2007) Who should be sent for genetic testing in hereditary colorectal cancer syndromes? J ClinOncol 25: 3534-3542.
- Lynch HT, Lynch PM (2005) Molecular screening for the Lynch syndrome--better than family history? N Engl J Med 352: 1920-1922.
- Lu KH, Dinh M, Kohlmann W, Watson P, Green J, et al. (2005) Gynecologic cancer as a "sentinel cancer" for women with hereditary nonpolyposis colorectal cancer syndrome. ObstetGynecol 105: 569-574.
- Hampel H, de la Chapelle A (2011) The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev Res (Phila) 4: 1-5.
- Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, et al. (1997) Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet 6: 105-110.
- Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, et al. (1999) Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81: 214-218.
- Beamer LC, Grant ML, Espenschied CR, Blazer KR, Hampel HL, et al. (2012) Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow up of abnormal results. J ClinOncol 30: 1058-1063.
- Resnick KE, Hampel H, Fishel R, Cohn DE (2009) Current and emerging trends in Lynch syndrome identification in women with endometrial cancer. GynecolOncol 114: 128-134.
- Monzon JG, Cremin C, Armstrong L, Nuk J, Young S, et al. (2010) Validation of predictive models for germline mutations in DNA mismatch repair genes in colorectal cancer. Int J Cancer 126: 930-939.
- Pouchet CJ, Wong N, Chong G, Sheehan MJ, Schneider G, et al. (2009) A comparison of models used to predict MLH, MSH2 and MSH6 mutation carriers. Ann Oncol 20: 681-688.
- Kastrinos F, Steyerberg EW, Balmaña J, Mercado R, Gallinger S, et al. (2013) Comparison of the clinical prediction model PREMM(,,6) and molecular testing for the systematic identification of Lynch syndrome in colorectal cancer. Gut 62: 272-279.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15191
- [From(publication date):
April-2015 - Dec 20, 2024] - Breakdown by view type
- HTML page views : 10696
- PDF downloads : 4495