MicroRNAs: New Roles in Cancer Treatment and Diagnosis
Received: 01-May-2023 / Manuscript No. jcd-23-92187 / Editor assigned: 04-May-2023 / PreQC No. jcd-23-92187 / Reviewed: 18-May-2023 / QC No. jcd-23-92187 / Revised: 22-May-2023 / Manuscript No. jcd-23-92187 / Published Date: 29-May-2023 DOI: 10.4172/2476-2253.1000177
Abstract
Small no protein-coding RNAs called microRNAs control the expression of a wide range of genes by basepairing on the untranslated region (UTR) of their target mRNAs, which either causes the target mRNA to degrade or inhibits its translation. In human breast cancer, aberrant miRNA expression has been associated with tumour formation, metastasis, diagnosis, prognosis, and therapeutic response. Certain miRNAs have been thought to have potential clinical uses as a diagnostic and therapeutic tool for breast cancer. Here, we outline and discuss the several lines of evidence that point to the critical connection between miRNAs and breast cancer, as well as its treatment options.
Keywords
Diagnosis; MicroRNAs; Cancer treatment; Therapeutic tool; Breast cancer; Diagnostic/prognostic biomarkers
Introduction
Significant advancements in cancer biology have been made possible by the identification of a class of tiny non-protein-coding RNAs known as microRNAs (miRNAs). MiRNAs are 19–25 nucleotide regulatory, non–protein–coding RNA molecules that control the expression of many different genes by base-pairing on the 3UTRs of the target mRNA, which causes mRNA degradation or inhibits translation. MiRNA expression patterns are carefully regulated and have significant effects on ontogenesis. In the last ten years, more and more human genes have been discovered to be regulated by miRNAs. Several studies have shown a correlation between miRNA expression and different malignancies, with miRNAs being both tumour suppressors and oncogenes, MicroRNAs [1-3] (Figure 1).
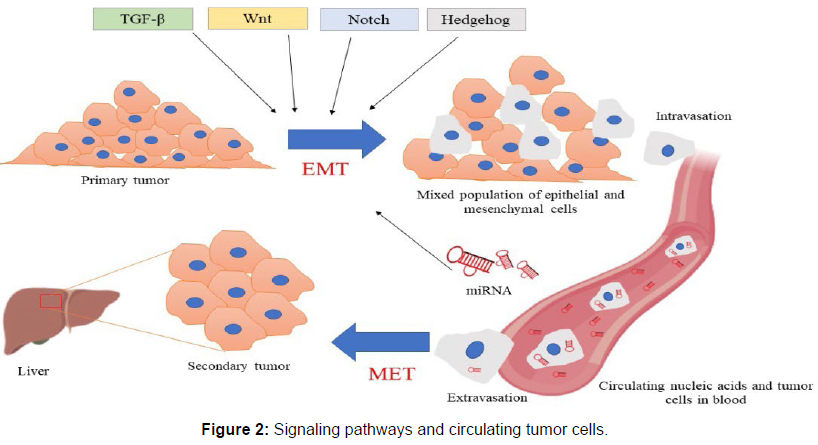
Subsets of miRNAs that are down-regulated or accumulate suggest a tumour suppressor or oncogenic function, respectively. Examples include let-7, which is down-regulated in lung cancer, miR-15 and miR-16, which are deleted or down-regulated in chronic lymphocytic leukaemia, and miR-17-5p and miR-20a, which regulate the ratio of cell death to proliferation. More than 700 human miRNAs have been annotated as of this writing in the miRBase registry (miRBase version 12.0), yet the majority of the genes that these miRNAs regulate are poorly understood. Moreover, these miRNAs are anticipated to control 30% of the human genome’s protein-coding genes, underscoring their significance as universal regulators of gene expression. We will examine the potential utility of miRNAs for the diagnosis, prognosis, and prospective therapeutic targets of breast cancer in this review, with a particular emphasis on recent findings of miRNAs associated to the development of breast cancer, An interplay of microRNAs, signaling pathways and circulating tumor cells [4-6] (Figure 2).
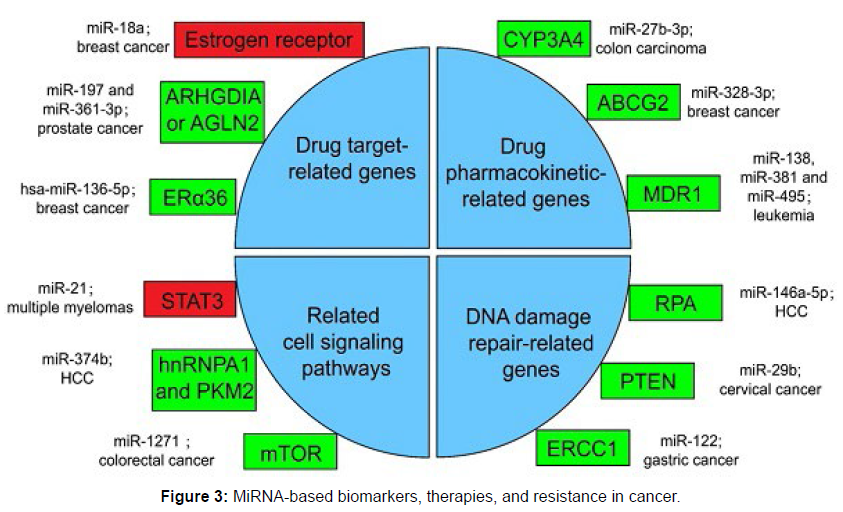
Other research aims to characterise the miRNA expression levels for certain cancer types, while other of the current miRNA research effort targets unique miRNA modifications and investigates the process involved. It is clear that miRNA levels can influence the severity and stage of cancer, as seen below. This is crucial because accurately determining the disease’s level of aggressively is essential to selecting the best treatment strategy. For instance, less aggressive treatment is required for low grade malignancies compared to high grade tumours. This great desire to use miRNA screening for diagnosis has two specific goals: it enables earlier diagnosis and it makes diagnostic more effective [7]. The discovery of circulating miRNAs as one of the highly promising biomarkers for early cancer diagnosis is very encouraging. Intracellular miRNAs are thought to be packaged into exosomes or micro vesicles and subsequently released from cells into the bloodstream. Also, it has been discovered that circulating miRNAs are highly stable, making them excellent candidates for use as earlier cancer detection biomarkers, miRNA-based biomarkers, therapies, and resistance in Cancer [8] (Figure 3).
For instance, the diagnosis of colorectal cancer now relies either on invasive technique, like a colonoscopy, or on much less accurate techniques, such faecal analysis. Patients eventually receive a diagnosis considerably later as a result of the frequently seen resistance to comply with such measures. Seven miRNAs were found to be altered when tissue and blood samples were analysed from individuals with various stages of colorectal cancer, according to Yong et al. Three of these miRNAs—mir-193a-3p, mir-23a, and mir-338-5p—had strong positive connections between blood and tissue samples. Importantly, the levels of each rose as the cancer’s stage advanced, pointing to a crucial function for this trio in both mechanism and diagnosis [9].
Prostate cancer has historically struggled with ambiguities and challenges in the diagnostic process, much like colorectal cancer. The Gleason scale is the most popular prostate cancer diagnosis tool. Tumors are classified according to their size and histological characteristics. When it comes to this strategy, there is some grey area, which makes picking the best course of action for therapy much more challenging [10]. The Gleason method may be replaced with a more precise and trustworthy one if miRNAs are used to classify prostate cancer subtypes. Two cohorts of men with prostate cancer were screened in order to address this. Among the two cohorts, they discovered that four specific miRNAs—miR-143, miR-145, miR-200c, and miR-375—had seen the most significant alteration. The three miRs with the strongest ability to discriminate between cancerous and non-cancerous tumours were miR-143, miR-145, and miR-375. Together, the three allowed them to correctly identify between cancerous and noncancerous samples 77.6% of the time [11].
It is conceivable for researchers to create a miRNA screen around the molecular markers used for diagnosis; with HER2 positive breast cancer lines and two patient cohorts. This is possible if a cancer type already has a standard way of subtype definition. The team discovered a wide range of miRNAs that suppressed HER2 and discovered a significant association between higher levels of miR-342-5p with survival time. This kind of characterisation enables the development of a miRNA signature for diverse cancer subtypes, providing therapeutic choices while also serving the extremely useful function of diagnosis [12].
There is growing evidence that manipulating miRNA levels can improve on-the-shelf cancer therapies in addition to using miRNA screenings for diagnosis. More specialised research that concentrates on these highly dysregulated miRNAs and what they may be targeting has emerged thanks to the miRNA screening procedure. Reintroducing or blocking the dysregulated miRNA in addition to conventional cancer medications may result in a more effective therapeutic strategy [13]. Each cancer type has produced a large number of miRNAs as a result of this methodology’s natural evolution, and these miRNAs themselves may be potential targets for various malignancies. Many miRNAs have been found to have therapeutic potential; however, to emphasise key and essential parts of the research, only a few chosen miRNAs and their target pathways are reviewed in the sections that follow [14].
Cancer cells, however, may stimulate mitochondrial bioenergetics as part of a prosurvival tactic when faced with glucose restriction. “Mitochondrial complex I inhibitors sensitise cancer cells to cell death under glucose shortage,” demonstrates how forced oxidative phosphorylation and mitochondrial complex I inhibitors work together to induce cancer cell death. Surprisingly, cancer cells that have been immortalised or grown under high glucose circumstances do not respond to combined treatments that impact glycolysis and cause mitochondrial dysfunction. These findings imply that an alternate therapeutic strategy to raise the sensitivity of cancer cells to death may involve forcing the switch from glycolysis to oxidative phosphorylation along with the use of mitochondrial inhibitors [15].
Discussion
Many families of miRNAs have also been found to be markedly down regulated in the majority of malignancies, according to research. A large body of recent data strongly implies that the let-7 family can considerably reduce proliferation, inhibit invasion, and diminish cell growth in cancer cells, as well as make them more sensitive to therapy. According to Chen et al., overexpression of let-7a in vitro or in vivo dramatically desensitized acute myeloid leukaemia (AML) to the therapy with cytarabine (commonly known as Ara-C) [16], and let-7a levels in patients with AML corresponded significantly with a better prognosis. Their team discovered that CXCR4 controls let-7a in acute myeloid leukaemia. In SK-BR-3 cells, it was discovered that lin28 control of let-7a also affected chemoresistance [17]. High levels of lin28 were linked with metastasis and/or relapse, and their downregulation reduced paclitaxel resistance. demonstrated that pancreatic cancer cell lines’ processing of let-7a was controlled by lin28 and SET, Also, they discovered a correlation between resistance to gemcitabine and an increase in RRM2, a putative target of let-7a that is involved in the conversion of rib nucleotides to deoxyribonucleotides, as well as a build-up of unprocessed pre-let-7. In light of this, let-7 mimics and lin28 may both be significant therapeutic targets [18].
The miR-200 family is a new class of metastasis suppressors and therapeutic sensitizers. Although the miR-200 family is best known for its function in EMT suppression, it is now being shown to also play a factor in modulating the response of cancer cells to conventional treatment regimens. MiR-141 and miR-429 have received significantly less attention in recent miR-200 family research, which has largely concentrated on miR-200a, -200b, and -200c [19]. Difluorinated curcumin (CDF), a curcumin analogue, was shown to be able to upregulated miR-200a, -200b, and -200c in pancreatic cancer lines. Moreover, it was revealed that the expression of CDF elevated the crucial tumour suppressor PTEN. Found that CDF-mediated downregulation of miR-21 and upregulation of miR-200b and miR-200c together with higher levels of PTEN and decreased NF-B DNA binding activity. Pancreatic cancer cells were dramatically made more sensitive to gemcitabine by the modulations of these microRNAs [20].
Conclusions
A patient with a macular hole, a variety of retinal pathologies that have a poor prognosis, may experience visual impairment. Even though the development of macular surgery techniques has shown that they may generally increase visual acuity, macular holes occasionally provide a difficult surgical challenge and the possibility of multiple recurrences. Moreover, patients who have successful surgeries still have metamorphosis or tiny scotomas that cannot be fixed at all, frustrating both patients and surgeons. All macular hole subtypes can now be treated with a variety of methods, but there isn’t yet a universally accepted method that ensures the best surgical results. Regarding the available treatments, there is currently no clear consensus on the classification, which hinders uniform research and meta-analysis to better understand this illness. To standardise clinical research, recent published classifications on FTMH, LMH, and MMH based on OCT examination will be helpful. Upcoming randomised clinical studies will more thoroughly examine the results of treatment for a common subtype of macular holes.
Conflict of Interest
None
Acknowledgement
None
References
- Langie SA, Koppen G, Desaulniers D (2015) Causes of genome instability: the effect of low dose chemical exposures in modern society. Carcinogenesis 36: 61-88.
- Genuis SJ. Nutritional transition: a determinant of global health. Journal of Epidemiology and Community Health 59: 615-617.
- Williams MA, Zingheim RW, King IB, Zebelman AM (1995) Omega-3 fatty acids in maternal erythrocytes and risk of preeclampsia. Epidemiology 6: 232-237.
- Kobayashi T, Tanaka N, Matsumoto T, Ueda K, Hoshii Y et al (2015) HRCT findings of small cell lung cancer measuring 30 mm or less located in the peripheral lung. Jpn J Radiol. 33: 67-75.
- Hashimoto M, Heianna J, Okane K, Hirano Y, Watarai J et al (1999) Small cell carcinoma of the lung: CT findings of parenchymal lesions. Radiat Med 17: 417-21.
- Kazawa N, Kitaichi M, Hiraoka M, Togashi K, Mio N et al (2006) Small cell lung carcinoma: Eight types of extension and spread on computed tomography. J Comput Assist Tomogr 30: 653-61.
- Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ et al (2003) Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4: 181-9.
- Riquet M (1993) Anatomic basis of lymphatic spread from carcinoma of the lung to the mediastinum: surgical and prognostic implications. Surg Radiol Anat 15: 271-7.
- Shin MK, Kim JW, Ju Y-S, (2011) CD56 and High Molecular Weight Cytokeratin as Diagnostic Markers of Papillary Thyroid Carcinoma. J Korean Med Sci 45.
- Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis, Modern pathology: an official journal of the United States and Canadian Academy of Pathology. Inc 11: 155-168.
- Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M et al (2008) BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer 15: 191-205.
- Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW et al (2003) RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab 88: 2318-2326.
- Huang M, Yan C, Xiao J, Wang T, Ling R et al (2019) Relevance and clinic pathologic relationship of BRAF V600E, TERT and NRAS mutations for papillary thyroid carcinoma patients in Northwest China. Diagn Pathol 14: 74.
- Kurtulmus N, Duren M, Ince U, Cengiz Yakicier M, Peker O et al (2012) BRAF(V600E) mutation in Turkish patients with papillary thyroid cancer: strong correlation with indicators of tumor aggressiveness. Endocrine 42: 404-410.
- Rodolico V, Cabibi D, Pizzolanti G, Richiusa P, Gebbia N et al (2007) BRAF V600E mutation and p27 kip1 expression in papillary carcinomas of the thyroid <or=1 cm and their paired lymph node metastases. Cancer 110: 1218-1226.
- Jang EK, Song DE, Sim SY, Kwon H, Choi YM et al (2014) NRAS codon 61 mutation is associated with distant metastasis in patients with follicular thyroid carcinoma. Thyroid 24: 1275-1281.
- Fakhruddin N, Jabbour M, Novy M, Tamim H, Bahmad H et al (2017) BRAF and NRAS Mutations in Papillary Thyroid Carcinoma and Concordance in BRAF Mutations Between Primary and Corresponding Lymph Node Metastases. Sci Rep 7: 46-66.
- Hashimoto M, Miyauchi T, Heianna J, Sugawara M, Ishiyama K et al (2009) Accurate diagnosis of peripheral small cell lung cancer with computed tomography. Tohoku J Exp Med 21: 217-221.
- Crnic I, Strittmatter K, Cavallaro U, Kopfstein L, Jussila L et al (2004) Loss of neural cell adhesion molecule induces tumor metastasis by up-regulating lymph angiogenesis. Cancer research 64: 8630-8638.
- Dunderovic D, Lipkovski JM, Boricic I, Soldatovic I, Bozic V et al (2015) Defining the value of CD56, CK19, Galectin 3 and HBME-1 in diagnosis of follicular cell derived lesions of thyroid with systematic review of literature. Diagn Pathol 10: 196.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, CrossRef , Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Jamal R (2023) MicroRNAs: New Roles in Cancer Treatment and Diagnosis. J Cancer Diagn 7: 177. DOI: 10.4172/2476-2253.1000177
Copyright: © 2023 Jamal R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1353
- [From(publication date): 0-2023 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1122
- PDF downloads: 231