Micropropagation of Fruit Crops: A Review
Received: 01-Mar-2023 / Manuscript No. ACST-23-90500 / Editor assigned: 03-Mar-2023 / PreQC No. ACST-23-90500 (PQ) / Reviewed: 17-Mar-2023 / QC No. ACST-23-90500 / Revised: 28-Apr-2023 / Manuscript No. ACST-23-90500 (R) / Published Date: 05-May-2023
Abstract
Micropropagation is one from tissue culture which allows the production of large number of plants from small pieces of the mother plant in relatively short period of time and limited space. Micropropagation as recent technique is mainly used for bulk rapid propagation of several commercial plant species included date palm. Tissue culture technique may offer a possible method to produce a large number of genetically uniform palms identical to other plants and normal fruit after 4 years from planting and production of date palm plants free from diseased, almost 100% survival rate compared with vegetative of shoots due to the presence of a strong root system on them. Surface sterilization is the most important step in preparation of explants for in vitro because controlling bacteria and fungi contamination of most fruit plants from field sources is very different. The successful applications on micropropagation of date palm during in vitro acclimatized depending on the appearance of number of leaves before transplanting in the greenhouse. Thus, the ultimate goal of this review was to find out the factors controlling micropropagation of fruit trees. India is homeland to diverse of significant but minor fruit crops such as Indian gooseberry, (Emblica officinalis Gaertn.), Karonda (Carissa carandas L.), Bael (Aegle marmelos Corr.), Jamun (Syzygium cuminii L.), jackfruit (Artocarpus heterophyllus L.) which bear high nutritional, medicinal, therapeutic values and of great commercial importance (medicinal, food and cosmetics). Due to a paucity of desirable planting materials, the commercial production process for these crops is restricted. Micropropagation has the potential to significantly increase the number of new cultivars or genotypes of such fruit crops. The objective of this review study is to compile existing research work done on the micropropagation of these underutilized fruit crops.
Keywords: Tissue culture; In vitro; Somaclonal variation; Embryo rescue; Molecular marker
Introduction
Micropropagation is one of the important contributions of plant tissue culture to commercial plant propagation and has vast significance. The technique provides a rapid reliable system for a production of large number of genetically uniform disease free plantlets. Micropropagation is the technique of developing plants from very small portion of plants such as shoots tip root tip, embryo, stem, pollen grain, callus or single cell. Plant tissue culture owes its origin to the revolutionary concept of totipotency of plant cell propounded by the famous German plant physiologist, Haberlandt in 1902. This technique has opened a vast scope for improve of fruits and plantation crops though micropropagation, creating genetic diversity, germplasm conservation virus elimination, development of somatic hybrids and gene transfer [1]. Micropropagation holds a great promise in fast multiplication of fruit and nut crops, which are invariably propagated asexually to meet the ever increasing demand for adequate and timely supply of clean planting material [2]. It is possible through micropropagation to produce millions of identical plants under controlled and aseptic conditions, economy of time and space, affording greater output and augmentation of elite, disease free propagules, safer and quarantined movements of germplasm across nations. It also induces precocity in flowering, precision timing uniformity and often increases yield. In fruit and plantation crops, comparatively difficult to micropropagate, protocol have been developed for Citrus, apple, banana, papaya, pineapple, grape, peach, plum, almond, walnut, strawberry, oil palm and date palm. In India, commercial exploitation of micropropagation is limited to oil palm, strawberry and banana. It is primarily because of the highly heterozygous nature of the material which requires independent protocol for the different genotypes, problems of clonal fidelity, involvement of rootstock and overall costs. However, attempts are on to standardize protocol for crops like mango, cashew, walnut, oil palm, coconut, litchi, sapota and cocoa [3].
India with second largest producer of fruits in world is also considered as homeland to a wide range of potentially valuable yet underutilised fruit crops. Which bear high nutritional, medicinal and therapeutic values and can be grown even on neglected marginal conditions [4]. Underutilized fruit crops include aonla, bael, karonda, jamun, jackfruit etc. These crops can be cultivated successfully with minimal inputs under distressing extreme temperature conditions. Minor fruits contain a range of vital nutrients, vitamins and minerals, as well as bioactive compounds that have been attributed to antioxidant effects against various free radicals. Besides these advantages these minor fruit crops are not extensively cultivated and also geographically as well as quantitatively their consumption and commercial trade are limited as compare to major fruits. Underutilized fruits are essential part of traditional foods that is among rural as well as tribal communities and have requisite potential to ensure food security and also to cope up with poverty [5]. ‘Biodiversity international’ recommended that underutilized fruits can be used as alternative sources to fight against hidden hunger. Due to a scarcity of suitable planting material, commercial production of these crops is limited. Micropropagation has the potential to significantly increase the number of important cultivars of these fruit crops. Breeding and selection experiments are extremely difficult method due to the long life cycle. Numerous biotic and abiotic stress factors substantially reduce fruit tree productivity [6]. Conventional methods are insufficient to overcome these issues, necessitating the urgent integration of plant biotechnology approaches for fruit tree improvement. Micropropagation refers to an artificial technique of producing genetically identical or clonal plantlets in vitro under aseptic condition with defined nutrient medium using tissue culture methods. This technique is independent of seasonal constraints. It is an important component of nursery, propagation as well as orchard industries as it provides uniform, stable, disease free, true to type, elite propagules and qualitative plantlets with rapid multiplication. Plant tissue culture technology has been commercially applied for microbe free plants [7].
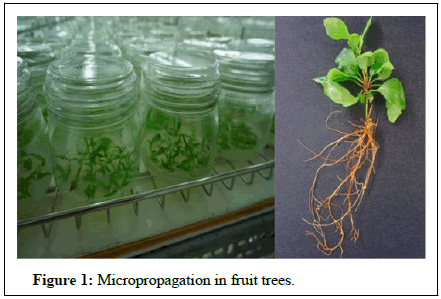
The propagation of plants is a fundamental occupation of human beings. Civilization may have started when ancient man learned to plant and grow kinds of plants which fulfilled nutritional needs for himself and his animals. Our cultivated plants originated mainly by three general methods. Some kinds of plants were selected directly from wild species but, under the selective hand of man, evolved into types that differ radically from their wild forms. Other kinds of plants arose as hybrids between species, accompanied by changes in chromosome number, e.g. pear, strawberry and prunes. Another group of plants occur naturally as rare monstrosities. Although un-adapted to a native environment, they may be useful to man [8]. Fruit plants do not come true, when raised from seed and therefore fruit plants are generally raised through vegetables methods of propagation, either on their own roots by cutting or layering techniques or on the roots of other plants (rootstocks) by budding and grafting techniques. The most of fruit plants are raised on rootstocks rather than on their own roots because growing of fruit trees on rootstocks has many advantages. The propagation of plants is a fundamental occupation of human beings. Civilization may have started when ancient man learned to plant and grow kinds of plants which fulfilled nutritional needs for himself and his animals [9]. Our cultivated plants originated mainly by three general methods. Some kinds of plants were selected directly from wild species but, under the selective hand of man, evolved into types that differ radically from their wild forms. Other kinds of plants arose as hybrids between species, accompanied by changes in chromosome number, e.g. pear, strawberry and prunes. Another group of plants occur naturally as rare monstrosities. Although un-adapted to a native environment, they may be useful to man. Fruit plants do not come true, when raised from seed and therefore fruit plants are generally raised through vegetables methods of propagation, either on their own roots by cutting or layering techniques or on the roots of other plants (rootstocks) by budding and grafting techniques [10]. The most of fruit plants are raised on rootstocks rather than on their own roots because growing of fruit trees on rootstocks has many advantages (Figure 1).
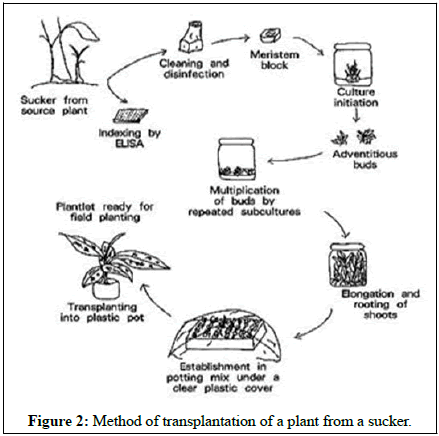
Micro-propagation types
• Meristem culture e.g. orchid, carnation.
• Tissue culture e.g. banana, date palm.
• Ermbryosis e.g. tobacco.
• Embryo tube use e.g. orchid (Figure 2).
Literature Review
Importance of tissue culture techniques
All the cells in an organism carry the same genetic information, yet show variations in expression. Our knowledge of cell and tissue cultures has been developing with full swing, especially in bio transformation, forestry, genetic engineering, morphogenesis, somatic hybridization, secondary metabolite production, hybridization, variety development and their conservation, maintaining pathogen free plants and rapid clonal propagation, totipotency, differentiation, cell division, cell nutrition, metabolism radio biology, cell preservation, etc. It is now possible to cultivate cells in quantity or as clones from single cells; to grow whole plant from isolated meristems and to induce callus or even single cell to develop into complete plant either by organogenesis or directly by embryogenesis in vitro. The production of haploid through tissue culture form anthers or isolated microspores and of protoplasts from higher plant cells has served as the basic tools for genetic engineering and somatic hybridization [11]. Tissue culture technique helps to propagate plants of economic importance such as orchids and other ornamental plants in large numbers by their meristem culture or by other in vitro methods. This provides them virus free plantlets, propagation of valuable economic plants through tissue culture based on the principle of totipotency (every cell within the plant has potential to regenerate into a whole plant). In plant breeding, embryo, ovary and ovule culture as well as in vitro pollination have been employed to overcome morphological and physiological sterility and incompatibility. In recent years, plant tissue culture technique is in increasing use for producing haploids from anthers or isolated microspores and of protoplasts from higher plant cells and the recognition of the potential of these materials in genetics and plant breeding [12]. One of the most significant developments in the field of plant tissues culture during recent years are the isolation, culture and fusion techniques which have their special importance in studies of plant improvement by cell modification and somatic hybridization. Plant tissue culture technique is a boon in the studies of the biosynthesis of secondary metabolites and provides an efficient means of producing economically important plant products (fine chemicals). Plant tissue culture also provides raw material to pharmaceutical, cosmetic and confectionary industries examples are berberine, ginseng, shikonin and vanilla [13].
The applications of micro propagation in fruit crops are as follows:
• Ex situ conservation of minor fruits can be done using in vitro culture.
• Using micropropagation, a small amount of plant tissues is enough to produce millions of clones of fruit crops annually. Producing an equal number of plants using traditional methods would take a long time.
• Disease free plants can be generated using tissue culture particularly ‘meristem tip culture’.
• Yield attributes can be increased.
• Qualitative attributes can be improved using tissue culture.
• Management of large number of plants in limited laboratory area.
• Ensuring favourable growing condition in limited laboratory area.
• In vitro stock proliferation (rapid replication of a cell, component or organism) can be performed any time in a year.
• It is possible to interchange plant material quickly across international borders without the risk of disease transmission through ‘Introduction’ process. This strategy reduces the amount of time required for quarantine.
• Economy of time and seed rate.
Propagation of temperate fruits
Many methods of vegetative propagation have been standardized for different temperate fruit crops. However, one method may be highly suitable for a particular crop but it may not be suitable for the others. Similarly, one crop may be propagated on large scale with different methods of propagation, whereas, the other may have only one method. The success of different methods is influenced by the environment also and as a result, one method for a particular crop may be highly successful in a particular locality, but cannot be of any value in the other [14]. The different methods of vegetative propagation include:
Propagation by seeds for rootstock production: Propagation by apomictic seedlings (Malus toringoides and M. sikkimensis).
• Propagation by cuttings and layering (propagation on its own root system).
• Propagation by grafting and budding (propagation on the root system of other plants).
• Propagation by specialized vegetative structures (runners in strawberry).
• Micropropagation.
• Propagation by seed.
The great majority of rootstocks for many temperate and nut species, including peaches, nectarines (Prunus persica L.), apricots (P. armeniaca L.), Asian pears (Pyrus pyrifolia (Nakai) species are raised by seed. Propagation by seed has significant advantages for the nurseryman; in particular, it is both simpler and cheaper to achieve than propagation by vegetative methods [15]. Whether trees propagated on seedling rootstocks have any advantages to the fruit grower, however, is much less clear and in many instances trees on seedling rootstocks are greatly inferior to those on clonal rootstocks. In species such as apple, where viruses are thought not to be transmitted through seed and where nurseries in some parts of the world find it difficult to maintain the health status of virus free clonal rootstocks, seedling rootstocks may have clear benefits. Seedling propagation also offers the potential for avoiding transmission of root borne diseases such as crown gall (Agrobacterium tumefaciens). Rootstock liners from infected stool or layer beds frequently transfer this troublesome disease to the new scion tree and the new site [16]. Seedling stocks raised on soils free of crown gall avoid this problem. Unless clonal rootstocks have been selected which confer other benefits to the scion, such as reducing vigour of growth, inducing early and abundant cropping or conferring resistance to soil borne pests and/or diseases, then there are less benefits gained by changing from seedling to clonal rootstocks. Nevertheless, seedlings of many heterozygous, out crossing fruit species are extremely variable in performance when used as rootstocks and one clear benefit of changing to a clonal rootstock is the improved uniformity of growth and cropping in the scion [17]. It is unfortunate that the change from seedling to clonal rootstocks is invariably resisted by nurserymen for both economic and logistic reasons. The uniformity of performance of seedling rootstocks may, however, be improved to some extent by using seed of a single clonal variety (e.g. ‘red delicious’ apple or ‘Bartlett’ pear) or seed from a self-fertile cultivar grown in a monoculture; using seed collected from virus free mother orchards planted in isolation (e.g. Pontavium and Pontaris Prunus avium lines of mazzard rootstocks available in France) (Figure 3).
Using seed of apomictic rootstock selections: Apomictic seedlings, with their intrinsic uniformity, are quite widely used in Citrus tree propagation. Some success was also achieved in selecting apomictic types of mains rootstock for apple, but these lines proved unpopular with both nurserymen and fruit growers. One problem was that the apple apomicts were of the facultative type, producing zygotic as well as apomictic seeds in their fruits. Culling these out at the nursery stage proved difficult. Other reasons for their poor acceptance were their strong vigour when used as rootstocks, incompatibility with some scions and sensitivity to virus infection. In comparison with vegetative propagation relatively little research is now conducted into the techniques of seedling rootstock propagation for temperate fruits. The techniques of after ripening and dormancy breaking, essential with seed of many Rosaceae, are now well elucidated. Aids to dormancy breaking, such as scarification and stratification are widely adopted by commercial nurserymen. Treatment with hormones, which may also aid dormancy breaking are less frequently adopted, however. Treatment with Gibberellic Acid (GA3) and Benzyl Adenine (BA), (both at 20 mg/litre), of peach seeds which had previously been stratified at 10°C and 15°C enhanced germination, whereas treatment with thiourea was ineffective in Thai trials [18]. Similar results were recorded by Shatat and Sawwan, who demonstrated that germination of Prunus mahaleb seed was improved significantly by treatment with promalin (a mixture of GA4+7 and BA) at 3000 mg/litre. Research in Poland on germination of plum rootstock seeds showed that soaking seed in a solution of 500 ppm ethephon just before sowing improved germination. This work also demonstrated that autumn sowing of partially stratified seeds produced better results than spring sowing of fully stratified seeds [19]. Little recent work has focused on the biochemistry of dormancy in temperate fruit tree seeds although research by Kar and Bhartiya indicated that the quantities of ortho diphenols present in apple seeds were associated with their depth of dormancy. Many innovative changes in seedling rootstock propagation now focus on the development of improved machinery for undertaking the various operations associated with land preparation, seed sowing, seedling management and lifting operations [20].
MS medium for micro propagation: It shows in Table 1.
| Macronutrients | ||
|---|---|---|
| Components | Chemical formula | Weight (mg.l-1) |
| Ammonium nitrate | NH4NO3 | 1650 |
| Potassium nitrate | KNO3 | 1900 |
| Calcium chloride anhydrate | CaCl2H2O | 440 |
| Magnesium sulphate anhydrate | MgSO4.7H2O | 370 |
| Potassium phosphate monobasic | KH2PO4 | 170 |
| Micronutrients | ||
| Boric acid | H3BO3 | 6.20 |
| Potassium iodide | KI | 0.83 |
| Manganese sulphate.4H2O | MnSO4.4H2O | 22.30 |
| Zinc sulphate.7H2O | ZnSO4.7H2O | 8.60 |
| Molybdic acid (sodium salt).2H2O | Na,MoO4.2H2O | 0.25 |
| Cupric sulphate.5H2O | CuSO4.5H2O | 0.025 |
| Cobalt chloride.6H2O | CoCl2.6H2O | 0.025 |
| Chelated iron | ||
| Sodium ethylene diamine tetraacetate | Na2-EDTA | 33.6 |
| Ferrous sulfate. 7H2O | FeSO4.7H2O | 27.8 |
| Vitamins | ||
| Thiamine. HCl | C12H17ClN4OS.HCl | 0.1 |
| Nicotinic acid (free acid) | C8H11NO3HCl | 0.5 |
| Pyrodoxine. HCl | C6H5NO2 | 0.5 |
Table 1: MS medium for micropropagation.
Mother plant (explants type, genotype and explants age): The highest shoot number and optimal shoot regeneration were obtained when cultured on MS medium supplemented with 6.0 mg L-1 BA and 0.1 mg L-1 NAA on leaf sections of Pyrus communis. A protocol for in micropropagation of mature plants of Citrus limon using nodal shoot segments. The problem of phenolic exudation and contamination of guava c.v. “Banaras” was not observed when somatic embryo derived young and aseptic plantlets were used as explant source. Using shoot tips of three fig cultivars (Aboudi, Gizy and Sultany) plantlets from the establishment stage were cultured individually on Murashige and Skoog (MS) medium supplemented with 0.5 mg L-1 6-benzylaminopurine were increasing explants development and reducing necrosis and browning. In the same time, Emam found that shoot tips of pear rootstock was successfully developed when cultured on MS medium as compared with one nodal cutting. Strosse, et al., concluded that shoot tips from young suckers in banana was successfully developed when cultured on MS medium during the establishment stage. Also, Sutherland, et al., found that shoot tips explants in banana were superior increasing proliferation of homogenous and diseases free plantlets. Whereas, Baiea MH, found that leaf discs explants of two peach rootstocks were successfully developed when cultured on MS medium. The best number of shoots (5.35 shoots/responding explant) of Jatropha curcas explants occurred from axillary buds. Similarity shoot tip, stem and axillary bud explants were successfully used for micropropagation of Jatropha curcas. In the same, Sumalatha A, found that shoot tip as the explant was successfully used for micropropagation of banana plants. The best shoot proliferation of Jojoba distinguished clone from shoot tip and nodal segments (Figures 4-6).
Stock solution: It shows in Table 2.
| S. No. | Ingredients | W/V concentration (mg) | Volume of stock solution prepared (ml) | Volume of solution taken per litre of medium (ml) |
|---|---|---|---|---|
| 1 | Macro elements | |||
| NH4NO3 | 16500 | |||
| KNO3 | 19000 | |||
| MgSO4.7H2O | 3700 | 500 | 50 | |
| CaCl2.2H2O | 4400 | |||
| KH2PO4 | 1700 | |||
| 2 | Micro elements | |||
| MnSO4.4H2O | 2230 | |||
| ZnSO4.7H2O | 860 | |||
| H3BO3 | 620 | 500 | 5 | |
| Na2MoO4.2H2O | 25 | |||
| CuSO4.5H2O | 2.5 | |||
| 3 | KI | 166 | 200 | 1 |
| 4 | Iron sources | |||
| Na2 EDTA | 3725 | |||
| FeSO4.7H2O | 2785 | 100 | 1 | |
| 5 | Organic supplements | |||
| Myoinositol | 1000 | 100 | 10 | |
| Thiamine HCL | 10 | |||
| Nicotinic acid | 50 | |||
| Pyridoxine HCL | 50 | 250 | 2.5 | |
| Glycine | 200 | 100 | 1 | |
Table 2: Stock solution.
The nodal segments as the explants were successfully used for callus induction as compared to leaf and root segments of Citrus plants. In the same time, somatic embryo derived young and aseptic plantlets were used as explants source were successfully used for micropropagation of guava c.v. "Banarasi". The same time, the longest roots were obtained when the c.v. golden delicious control and by genotype 4566 were cultured on WPM medium as compared with the genotypes 4608, Gi47 7/4 and C/907.
Surface sterilization of explants: Banana plants were surface disinfected by 0.1% HgCl2 solution containing tween 20 for 5 min and washed thoroughly with sterile, deionized water. Meanwhile, sterilize shoot tip and nodal bud using Sodium hypochlorite Clorox (NaOCl) for 20 min which gave good explants survival in pomegranate. Using a combination of NaOCl and Na methylate for 20 min are the two most successfully sterilize axially bud and segments in pomegranate which gave good explant survival (65%). In the same time that using NaOCl (0.75%) gave the maximum survival percentage (46.66%) followed by NaOCl (1.0%) with 26.66% survival percentage as compared with NaOCl (1.25%) with the minimum survival (6.6%) of Jack fruit. In another study during trials on in vitro propagation of mangoes, using combination of 10% sodium hypochlorite "Clorox" (sodium hypochlorite 5.25%) NaOCl and 0.05% Mercuric Chloride (HgCl2) for dipping durations 7 min and 10 min give the highest survival percentages and lower contamination percentage. Also, using 10% sodium hypochlorite was successful in surface sterilization and gave good explant in very low visual contamination of rootstocks Mariana (Prunus mariana). In addition, surface sterilization gave good explanation for grape tissue (Vitis vinifera) successfully by using combination of 10% sodium hypochlorite and surfactant drops for 10 minutes. Three doses, 0.05%, 0.1% and 0.2% of aqueous sodium hypochlorite or mercuric chloride solution separately for 10 minutes were used as surface sterilize. It was found that using 0.1%-0.2% mercuric chloride increased aseptic culture establishment but inhibited by break due to toxicity to explant of Bambusa tulda. In the same time dipping explants in 15% of sodium hypochlorite for 15 minutes was effective in sterilizing grape root stocks.
Phenol exudation and its control: It was mentioned that in Figure, using activated charcoal in the cultural medium induced initiation proliferation of woody plant but the negative effect of activated charcoal by adsorbing growth regulators and lowering pH of the medium. In the same time dark treatment for few days of initiated shoots reduces the release of phenolic compounds from the shoots of apple root stocks. Similarly attempted in vitro culture of pomegranate c.v. Mridula, nodal segments with sterile wax decreased the phenol exudation and lead to good explant percentage of cultural establishment. Using ascorbic acid was the most successfully method to reduce oxidation during the establishment stage of apple. Different methods such as keeping explants in dark after culture, adding antioxidants such as citric acid (100 mg L-1) and ascorbic acid (150 mg L-1) for 30 minutes to the medium as well as adding activated charcoal (3 g L-1) to the medium. These treatments reduce phenolic compound production and browning of olive explants.
Medium (type, strength and state): It was reported that full strength of Murashige and Skoog medium is more suitable for establishment of coffee plantlets. Number of leaves and shoot length of fig plants was induced on double strength Murashige and Skoog medium. Meanwhile, the highest value of shoot number was obtained on half MS strength medium. Mukherjee PN, Husain SC, Misra and VS Rao, mentioned that shoot proliferation from nodal explants of grape root stock was induced on half strength Murashige and Skoog medium. On the highest proliferation rates and shoot growth of apple as shown in Figure 7 were also obtained with MS medium replacing Fe-EDTA by Fe-EDDHA as iron source. In the same case, Woody Plant Medium (WPM) is the most important to avoid contamination by fungi and bacteria as well as phenolic oxidation during the establishment stage. Similarly the highest shoot proliferation on Citrus limon was obtained with MS medium with lower concentrations of ammonium and potassium nitrates.
On the other hand, used 3/4 medium strength was the most suitable medium for development of Jojoba clones. Acclimatization of plantlets derived from tissue culture confirmed the efficiency and importance of this method. The subculture of rooted shoots into MS salts solution and increasing light intensity enhanced the plantlet photosynthesis and then changing it from heterotrophic to autotrophic status of date palm. The highest survival percentage (70%-86%) of date palm was obtained on half medium strength after 1 month pre acclimatization stage as compared with the direct transfer to the greenhouse ranging from 12%-28%. Mazri MA, also, one-half strength MS medium was the most suitable medium for the maximum number of roots and root length of almond plantlets, c.v. Nonpareil. The highest rooting with higher number of roots (10.33 roots/shoot) per microshoot found on half strength MS basal medium in pomegranate was obtained found that the best significant improvement of greening and explants development per micro flower bud on full and one-half medium strength compared with one-quarter medium strength of pear “Le Conte’’ flower bud. In respect that several factors affecting on in vitro root formation of date palm c.v. Boufeggous plantlets such as with solid or liquid medium just before acclimatization. While, the used of liquid medium is more suitable for medium state (solid or semisolid media) before the transfer of plantlets to the greenhouse of Barhee date palm. Also, use of a liquid medium before the transfer of plantlets to the greenhouse of date palm c.v. Najda was very effective in improve shoot quality. The highest survival percentage of in vitro acclimatized date palm c.v. Barhee was recorded with plantlets growing on liquid Ms medium compared with the other medium status. The highest rooting percentages on apple was observed when the plantlets were cultured in liquid organized medium supplemented with IBA at 25 μM on all genotypes including M. domestic c.v Golden delicious.
Supporting media: The best number of shoots and shoot length in pineapple plantlets were induced to form shoots on MS medium containing 2 g L-1 gelrite. Meanwhile, an agar at the rate of 7 g L-1 was better than gelrite during multiplication stage. Similar, increased response with the decrease in agar from 0.8%-0.4% in the medium improve the in vitro root and shoot parameters as compared with the other supporting structures banana (Musa spp.) c.v. Grand naine plantlets. Also, added to the media with gelling specialist agar at 5 g L-1-8 g L-1 in the medium were successfully used for micropropagation of banana plants.
Carbon source and additive: It was revealed that medium supplementation with 30 g L-1 sucrose gave the best result of shoot tip explants of papaya. Similarly, sucrose at the rate of 30 g L-1 concentration as carbon source was proved to be best resolute on in vitro rooting of banana (Musa spp.) c.v grand naine plantlets. In the same time using of MS medium supplemented with 20 g L-1 fructose induced shoot proliferation of the two fig cultivars (Sultany and Aboudi) as compared with the explants an media without sucrose. In the respect, addition of both fructose and glucose to Murashige and Skoog medium resulted in improving shoot length and leaf number of jojoba plants. While, sucrose proved to be the best carbon source for shoot number. On the same time, supplemented the culture medium with 30 g L-1 -60 g L-1 sucrose encouraged maximum explant development and shoot growth of date palm while fructose produced the highest values of dry weights as compared with other carbon sources treatments such as glucose, sucrose and maltose. The same addition also, one-half medium strength treatment enhanced the proliferation percentage and shoot number compared with the other treatments.
Whereas, addition of either adenine sulphate (80 mg L-1) or yeast extract (300 mg L-1) was effective in highest callus production and reduced necrosis and browning of both Nemagaurd and Okinawa root stock. The same, Olive Medium (OM) supplementation with 50 ml L-1 coconut water gave the best result of the highest proliferation rates with an average of 3.4 new explants on each 30 days in olive. While used sucrose concentrations at the rates of (30 g L-1-40 g L-1) as a carbon sources gave the best results for micropropagation of banana pants.
Light, temperature conditions and pH requirements: In vitro plantlets of date palm c.v. Barhi are generally grown under 2000 lux, through using different numbers of white cool fluorescent lamps for 16 h of light and 8 h of dark, improved most parameters under study such as root length, root number, shoot length and greening as compared with the other light intensities. Meanwhile, increasing shoot thickness was recorded when the cultures were subjected to light intensity of 3000 lux. In vitro plantlets of date palm are generally grown under high light intensity enhanced the plantlet photosynthesis. Similarly, tasted in vitro plantlets under high light intensity (4000 lux-12000 lux) and temperature (26°C-36°C) might cause charring of leaves and wilting of plantlets. Also, in vitro root formation of date palm C.V. Barhi, the best results for shoot thickness was recorded when the cultures were subjected to a light intensity of 3000 lux. However, number of root, root length, number of leaves and greening were recorded when the cultures were subjected to light intensity of 2000 lux.
The same result found that several factors affecting the in vitro root formation of most fruit trees, the best results for both root number and root length were recorded when the culture were subjected to light intensity of (4.000 lux-12000 lux) and temperature (26°C-36°C) might cause charring of leaves and increasing of plantlets. While, during acclimatization plantlets of date palm, increasing light intensity increased the plantlets photosynthesis.
On the other hand in vitro plantlets of banana were generally grown under temperature 28°C and presented of light to 12 h-16 h. Also, cold pretreatment of explant for 3 days at 5°C encouraged the best responses of peach root stocks. The best root and minimum time for root initiation with longest length at pH 5.5 during the preparation of the medium on in vitro rooting of Banana plantlets c.v. Grand naine were obtained by Ahmed SA, Sharma B, Bhushan AK Singh and VK Wali.
Plant growth regulator: The supplementation of the culture medium with BA (0.5 mg L-1) and IBA (0.25 mg L-1) induced the highest number of shoots after 30 days of culture on Jatropha curcas. The supplementation of the subculture medium with 0.5 mg L-1 BA and 0.5 mg L-1 IBA was necessary for differentiation of the shoot bud as shown in. While only 1.0 mg L-1 IBA induced the shoot elongation of Jatropha curcas. similarly, the supplementation of the culture medium with 6.00 mg L-1 IBA induced the best multiplication of coffee plantlets, in the some cases, number of roots and root length/plantlets were greatest with 3.0 g L-1 IBA. In another study the supplementation of the culture medium with 3 mg L-1 BA and 0.5 mg L-1 NAA induced the regeneration response (71.89%) from model segment in Citrus plants. In some cases, maximum rooting percentage (71%) was obtained with modal segment derived callus cultured on Ms medium supplemented with 0.5 mg L-1 NAA and 3 mg L-1 BA17. While the supplementation of the culture medium with 2.0 mg L-1 BA and 0.0 IBA mg L-1, 0.5 IBA mg L-1 or 1.0 IBA mg L-1 induced the best number of regenerated plantlets of either Nemagaurd or Okinawa rootstock.
The same supplementation of the culture MS medium with 1.0 mg L-1 BA induced the best multiplication of almond plantlets c.v. Nonpareil. In the same cases, number of roots and root length/plantlets was greatest with 8.0 mg L-1 indole acetic acid. Similarly, the supplementation of the culture medium with 1.0 mg L-1 BAP combined with 1.0 mg IBA was the best for in vitro culture initiation of apple plantlets. Also, the supplementation of the culture medium with 1.0 mg L-1 and 1.5 mg L-1 BAP combined with 1.0 mg L-1 IBA induced the survival percentage (96.7%) of MM 106 and (93.3%) Anna apple plantlets. In addition, supplementation of MS medium with 0.5 mg L-1 BAP induced the shoot proliferation (5.37 shoots/explant) of a dwarfing cherry root stock. Similar results recorded that the supplementation of the olive medium with 2.22 mg L-1 BAP induced the best proliferation rates with average of 3.4 new explants after 30 days. In the same cases, rooting percentage 85% was obtained by OM with 3 g L-1 IBA. In the same time, the highly cytokinin induced the best shoot multiplication of jojoba. Also, callus induction was greatest with auxins alone or combined with cytokinin. Cytokinin 6 Benzyl Aminopyrine (BAP) has been found essential for obtaining good shoot proliferation in pomegranate. In the same cases, Murashige and Skoog (MS) medium containing 2.0 mg L-1 IBA has been found essential for obtaining good rooting during in vitro rooting. Highest frequency of shoot proliferation was obtained in culture medium quorin and lepoivre medium supplemented with BA at the rate of 0.4 mg L-1 and IBA 0.05 mg L-1. Also, the highest rooting percentages (70.37) with dual auxin i.e. IBA and NAA supplemented to the rooting medium found that the highest rooting percentages (93.33%) and longer roots (3.29 cm) were obtained in culture medium added 0.5 mg L-1 IBA. In the same cases, high frequency shoot elongation was also obtained with MS medium supplemented with higher concentration of cherry laurel Prunus laurocerasus L. Moreover, high frequency shoot proliferation was obtained on the basal medium supplemented with lower concentrations of BAP of sweet cherry cultivar “Lapins”. On the other hand, good shoot elongation was also obtained with a higher concentration of BAP. Increased shoot proliferation of olive was also obtained with increasing BAP concentration up to 2.1 mg L-1 in the media. The effect of high concentrations of BAP may be prevented by supplemented GA3 to the culture media during proliferation stage. The highest shoots and number of shoots (4.66) per proliferated explant were obtained on MS culture medium supplemented with BAP at the rate of 1.5 mg L-1) of jack fruit.
Agricultural media: Data in Figure 8, the highest survival percentages of plantlets, plant height (cm), plant thickness (cm) and number of leaves/plant occurred when agricultural media during acclimatization consists of a mixture of sand and peat moss (1:1) was used of coffee plantlets. Also, date palm c.v. Barhee plantlets were successfully acclimatized when transferred to pots containing a mixture of (25% vermiculite +50% and +25% peat moss). While, rooted shoots (plantlets) were transplanted in small pots containing a mixture of peat moss and perlite (2:1) and placed in plastic tunnels or in a greenhouse. The survival percentage was 85% after 3 months when the plants were transferred to bigger pots of Maktom c.v. date palm.
In addition, the highest survival percentage (95%) of olive plantlets were obtained during acclimatized when transferred to pots containing a mixture of vermiculite: Perlite 3:1 (V/V) substrate. In the same cases, the highest survival percentage (85%) after 3 months of date palm c.v. Maktom plantlets during acclimatization stage when transplanted in a small pots containing a mixture of peat moss and perlite (2:1) and placed in plastic tunnels in a greenhouse.
Disinfection of propagule: Wash the pseudo stem collected from the field with running water to remove adhering soil.
Immerse the excised pseudostem in a container of undiluted household bleach (5.25% NaOCl) for 30-45 minutes. Decant the bleach solution and keep the surface sterilized pseudostem in the container.
Tissue culture medium for shoot growth: Based on Damasco and Barba’s recipe.
Inoculation: Mix the medium according to Table 3. Autoclave medium scalpels, forceps, cutting plates and Magenta boxes according to standard autoclaving procedure.
Work under surface sterilized laminar flow hood:
• Trim the surface sterilized banana pseudo stem, peeling off the outer leaf sheath that comes in contact with the bleach. Transfer to a clean cutting dish and continue cutting until the shoot measures 1 cm × 1 cm, with the corm tissue as thin as possible.
• Transfer the shoot tip to a fresh cutting dish and cut the shoot into quarters longitudinally, through the center. Transplant each quarter onto a solid culture medium.
• Maintenance of shoot cultures.
• Keep shoot cultures in an air conditioned room under a 16 hours photoperiod 40 μE/m2s (provided by two 40 watt fluorescent tubes).
• Observe the cultures for contamination. Discard contaminated cultures as soon as contamination is noted.
• Observe for browning and bulging of corm tissue, greening of leaf tissues and growth of new shoots during the first month of culture.
• When shoots coming out from the apex of the leaf axis are almost 2 cm tall, the shoot tips are ready for subculture.
• Proliferation of shoots (subculture).
• Transfer the shoot or sections of shoot to fresh culture medium whenever the propagules are about 2 cm tall. Overgrown shoots are less proliferative. If shoots are beyond 2 cm, make a longitudinal cut through the apex of the growing shoot.
• Subculture onto half strength MS medium supplemented with 5 mg/l BAP and 100 ml/l coconut water. This medium, without auxins, is used to avoid early forming of nubbins at high frequencies. All subculturing needs to be conducted in sterile conditions.
• Subculture about 3-4 weeks until desired number of shoots is obtained.
| Ingredients | Concentration of stock solution | Amount needed to prepare 1L of medium |
|---|---|---|
| MS macronutrients (0.5x) | 25x | 20 ml |
| MS micronutrients (0.5x) | 100x | 5 ml |
| MS vitamins (1x) | 100x | 10 ml |
| Fe-EDTA (1x) | 200x | 5 ml |
| Sugar | 30 g | |
| BAP | 100 mg/L | 50 ml (5 mg) |
| Coconut water (optional) | 100 ml | |
| Agar | 6 g | |
| Note: MS: Murashige and Skoog medium. | ||
Table 3: Ingredients for tissue culture medium for banana shoot proliferation.
Record number of proliferated shoots: Repeat the subculture for no more than 5 cycles. A higher number of sub culturing cycles will lead to off type banana mutations such as dwarfism, elongation or other abnormalities. When sufficient shoots have proliferated as nuclear stock, proceed with rooting.
• Prepare rooting medium in Table 4 (Damasco 2005) and use within a week of preparation for best results.
• Let the last cycle of the shoot subculture establish 3-4 weeks (proliferation period) so as to obtain small plantlets.
• Separate individual shoots from a cluster of shoots and transfer them onto rooting medium.
• Roots will form in 3-4 weeks.
• When the plantlets have 3-4 expanded leaves and are well rooted, they are ready to be planted into soil.
| Ingredient | Concentration of stock | Amount for 1L of medium |
|---|---|---|
| MS macronutrients (0.5x) | 25x | 20 ml |
| MS micronutrients (0.5x) | 100x | 5 ml |
| MS vitamins (1x) | 100x | 10 ml |
| Fe-EDTA (1x) | 200x | 5 ml |
| Sugar | 30 g | |
| Coconut water (optional) | 100 ml | |
| Agar | 6 g | |
| Note: MS: Murashige and Skoog medium. | ||
Table 4: Banana rooting medium.
Preparing tissue cultured banana plantlets for field planting prior to planting tissue cultured banana plantlets into soil, the seedlings need to be hardened or acclimatized to the external environment. This can be done by transferring them to a liquid medium (without agar) or exposing them to partial sunlight in the tissue culture vessel under greenhouse conditions for a few days. Any agar medium adhering to the tissue cultured plantlets should be gently washed off, after which they are ready to be planted into potting media in a nursery (Figure 9).
Choose a potting mix with good moisture holding and drainage characteristics, for example 2 parts sunshine pro mix, 1 part perlite and 3 parts medium to coarse grade vermiculite. Keeping the media moist to maintaining the health of the tissue cultured seedlings (Figure 10).
Fertilize with slow release or liquid fertilizer:
• Place banana seedlings in a partially shaded area (50% shade) for 2 weeks before exposing them to full sunlight.
• Plants should be placed in a BBTV free and banana aphid free area. Other aphids, whiteflies, and spider mites are commonly found on banana plants in greenhouses and clustered nurseries and these should be managed by employing insecticide when populations are high. However, after the plants are transplanted into the field, these pests are typically not problematic.
• The full acclimatization process should take about 2 months or until seedlings reach about 8 inches or taller, depending on variety, before field planting.
• If using tissue cultured banana to replace plants in a BBTV infected field, an aggressive scouting program for BBTV should be in place. This includes inspecting young plants every 5 days, as new leaves unfold every 5 days.
• The length of time to harvest after transplanting tissue cultured banana depends on the cultivar. In general, ‘dwarf apple’ bananas may be harvestable within 9-10 months after transplanting into the field.
Biotechnological tool in fruit crop improvement
Tissue culture: It is one of the most widely used techniques for rapid asexual in vitro propagation. This technique is economical in time and space affords greater output and provides disease free and elite propagules.
It also facilitates safer and quarantined movements of germplasm across nations. When the traditional methods are unable to meet the demand for propagation material this technique can produce millions of uniformly flowering and yielding plants. Micropropagation technology ensures true to type, rapid and mass multiplication of plants that possesses special significance in vegetative propagated plant species. This technology has witnessed a huge expansion globally.
Some basic techniques of tissue culture, such as anther/microspore culture, somaclonal vanat10n, embryo culture and somatic hybridization are being exploited to generate useful genetic variability for obtaining incremental improvement m commercial cultivars (Figure 11).
Micropropagation: Micropropagation of almost all the fruit crops is possible now. Production of virus free planting material using meristem culture has been made possible in many horticultural crop strawberry is perhaps first fruit crop in which micropropagation technique has been standardized. In vitro plants are more uniform, produce higher number of runners, have better survival in the field and the fruit yield increase in 24% than plants propagated by the traditional method. Banana is commercially propagated through tissue culture. The future of banana industry in the country is totally dependent upon control of BBTV disease. The ultimate choice is productions of disease free banana plants through in vitro techniques to replace infected fields. There are number of fruit crop in which propagation method is sexual. Some of them are dioecious i.e. male and female flower borne on different flowers. So through commercial point of view it is desirable to produce more female plant which, are the ones which bear fruits, this is possible only through tissue culture techniques. The grafting a very small shoot tip excised from an elite mother tree grafted onto a decapited rootstock seedling grown under aseptic condition. Murashige, et al., made the first attempt in Citrus and subsequently it was refined by Navarro, et al.
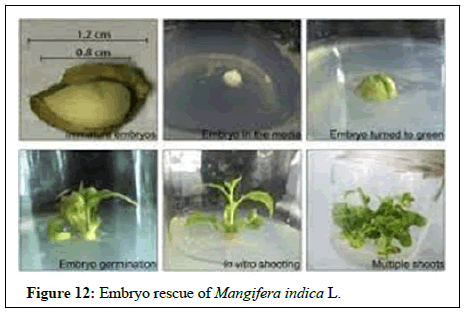
Embryo rescue: Embryo rescue is another area, where plant breeders are able to rescue their crosses, which would otherwise abort. Culture of excised embryos of suitable stages of development can circumvent problems encountered in post zygotic incompatibility. This technique is highly significant in intractable and long duration horticultural species. Ovule culture in grapes aids in developing hybrids. Seedless grapes. Embryo rescue has helped in accelerating the pace of stenospermocarpic grape improvement and ‘sweet scarlet’ and ‘Thomcord’ are two of the seedless table grapevines, which were released using this technique (Figure 12).
Anther/pollen culture: Application of anther culture is obtaining haploids and homozygous diploids. It provides a short cut for obtaining pure lines. As tetraploid plants are produced in greater abundance in breeding programme, it may be feasible to culture their anther to produced useful diploids.
Regeneration of androgenic embryos in apple (Malus × domestica Borkh.) via anther and microspore culture has been succeeded.
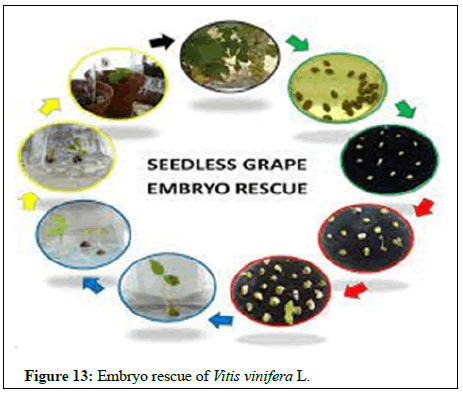
In vitro mutagenesis: Mutagens used physical (gamma rays, X-rays, UV rays) and chemical (EMS, MMS etc.). In fruit crops EMS and gamma rays are widely used. Notable example of in vitro mutagenesis in banana variety grand Nain explants used was shoot tips; mutagen source and dose were 80 Gy gamma rays developed improved cultivar Novaria-10 with improved trait early flowering and high keeping quality (Figure 13).
Somatic hybridization: It is crossing of crop plants through fusion of somatic cells. Protoplast of two cells is fused. It breaks the barriers of cross incompatibility among plant species and makes incompatible crosses possible. Somatic hybridization has been explored for the rootstock and scion improvement of Citrus. In scion improvement production of allotetraploids 2x × 4x=3x (seedless) and direct 3x production whereas, in rootstock improvement, production of tetraploid rootstock for greater dwarfing effect, which is used in HDP.
Gene transfer/wide somatic hybrids development of resistant rootstock through gene transfer from sexually incompatible or difficult to hybridized genera. Somatic hybridization research of almost two decades in Citrus is yielding fruit now.
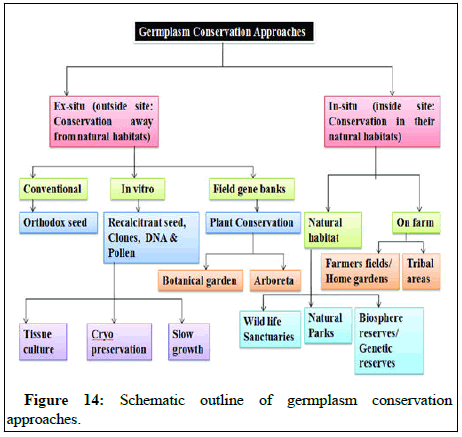
In vitro germplasm conservation: In vitro conservation is of great significance in providing solutions and alternative approaches to overcoming constrains in management of genetic resources. Complete plants have been successfully regenerated from tissues cryopreserved at -196°C in liquid N2 in several crops for several months to years. This method is now being practically used at several national and international germplasm banks (Figure 14).
In crops, which are propagated vegetatively and which produce recalcitrant seeds and perennial crops which are highly heterozygous seed storage is not suitable. In such crops especially, in vitro storage is of great practical importance. These techniques have successfully been demonstrated m a number of fruit crops and there are now various germplasm collection centers.
A portion of plants regenerated by tissue culture often exhibits phenotypic variation atypical of the original phenotype. Such variation, termed soma clonal variation may be heritable i.e. genetically stable and passed on to the next generation. Also, presently, synseed technology using encapsulation of non-embryogenic propagules derived under in vitro condition has become an important asset to micropropagation and in vitro conservation of planting material for long term.
Somaclonal variation: Somaclonal variation is considered a source of new plant genotypes for breeding, and advances at the tissue culture level have opened new possibilities for applications in viticulture. The advancements made in tissue culture techniques has made it possible to regenerate various horticultural species in vitro as micropropagation protocols for commercial scale multiplication are available for a wide range of crops. However, plant tissue culture may generate genetic variability i.e. somaclonal variations as a result of gene mutation or changes in epigenetic marks. The occurrence of subtle somaclonal variation is a drawback for both in vitro cloning as well as germplasm preservation. Somaclonal variation has provided a new and alternative tool to the breeders for obtaining genetic variability relatively rapidly and without sophisticated technology in horticultural crops, which are either difficult to breed or have narrow genetic base. Somaclonal variations are beneficial for creating variability and resistance to stresses (Table 1).
Molecular marker: A molecular marker is a DNA sequence that is readily detected and whose inheritance can easily be monitored. The phenotype is imperfect predictor of genotype. The phenotype is always expression of its genotype and environment interaction. Two plants having similar phenotype but genotype point of view they may be different. Application of molecular marker in fruit crops are genetic diversity assessment, estimation of genetic distance between cultivar and phylogenic analysis, detection of quantitative trait loci and marker assisted selection. Molecular marker is used in sex determination of dioecious plants at juvenile stage. Genetics of control mechanisms that underlies sex differentiation in date palm is not known. Sex of the plants becomes known only at the time of first flowering, which takes around 5 years. In comparison, molecular diagnosis (if available/feasible) promises quick and reliable identification of sex types very early when plantlets are growing in seedbeds. To develop such an assay, genomic DNA from 45 individual plants (25 female and 20 male) belonging to different varieties of date palm was subjected to PCR amplification using 100 RAPD and 104 ISSR. In papaya identification of female and hermaphrodite specific marker papaya sex determination by RAPD.
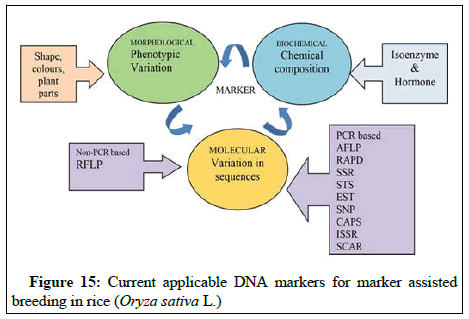
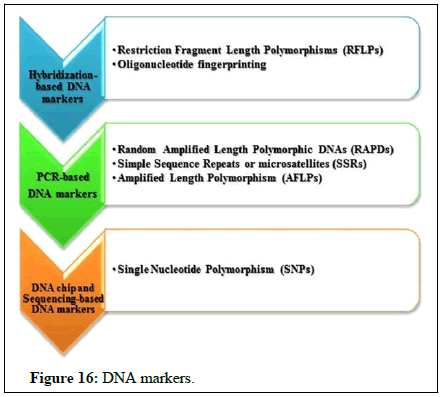
Genetic diversity assessment: Assessment of diversity has traditionally been made through morphological characters, chemical composition and cytological characters; however, they have several limitations especially in perennial crops. Morphological characters are often limited in number, have complex inheritance pattern and are vulnerable to environmental conditions, hence it is desirable to develop alternative methods, which are rapid, reliable and more or less not influenced by environment. Therefore, in the recent past, rapid use of molecular markers has complemented the classical strategies. The use of DNA markers has enabled the characterization of genotypes independent of the influences of environmental growth conditions, physiological age of the plant and the type of tissue being analyzed. Molecular markers have been looked upon as tools with a large number of applications including localization of a gene and improvement of plant varieties by marker assisted selection (Figures 15 and 16). Molecular markers thus, are excellent tools for analysis of genetic diversity and relationship (Table 5). Polymerase Chain Reaction (PCR) based Random Amplified Polymorphic DNA (RAPD) markers have been extensively used in DNA finger printing. These DNA markers are generated by PCR amplification of random genomic segments with single primers (usually 10 nucleotides long) of arbitrary sequence. These markers are mostly dominant and detect vanat1on in both coding and non-coding region of genome. RAPD analysis is technically simple and provides an approach to characterize different genotypes, thus to estimate genetic diversity which will further be useful in improvement of breeders.
| Fruit | Marker type | References |
|---|---|---|
| Apple | AFLP and RAPDs | Coart, et al.; Botez, et al.; Sestras, et al. |
| Avocado | Mini satellite DNA | Ashworth, et al. |
| Banana | RAPDs | Brown, et al. |
| Citrus | RFLP | Durham, et al. |
| Grapes | RFLP and SSRs | Bourquin, et al. |
| Mango | CpSSR and RAPDs | He, et al.; Marcela et al. |
| Pistachio | Mini satellite marker | Riaz Ahmad, et al. |
| Cashew | RAPD and ISSR | Thimmappaiah, et al. |
| Pear | SSRs and AFLP | Sisko, et al. |
Table 5: DNA markers for genetic diversity assessment in fruit crops.
Advantages of tissue culture over traditional propagation methods:
• Tissue culture allows exact replicas of plant matter to be produced. This means that plants with exceptional genes can be multiplied (excellent for food, flower, hemp and other profitable crops).
• Mature plants develop quicker than traditional propagation methods.
• Sometimes there are situations where seeds or pollinators are absent. In these cases, tissue culture can allow for the production of multiple uniform plants, without seeds or pollination.
• Tissue culture needs to be performed under sterile conditions, dramatically reducing the chance of disease, pathogens and pests.
• Some plant species, such as Nepenthes and orchids are difficult to grow from seed and tissue culture can be a way to grow with plants with greater ease.
• Tissue culture can be used to preserve plant genetics, an essential technology with the threat of species extinction.
• Tissue culture can be used with any plant cells that have a characteristic known as totipotency; in other words, plant cells that can regenerate from a cell into complete, mature plants. Almost any piece of plant matter can be used parts of stem, roots or leaves but this will depend on the plant and related protocol.
Identification of QTLs
Many important heritable characters are a consequence of the joint action of several genes. Such characters are often referred to as polygenic or quantitative. Several characters of plant species, among which are traits of agronomic importance are inherited quantitatively. Yield, maturity date and drought tolerance are examples of such characters. The genetic loci for such characters have been referred to as Quantitative Trait Loci (QTLs). The essential feature which makes feasible the finding and characterization of a QTL is its linkage with a known marker locus segregating with Mendelian ratios. DNA markers provide this opportunity by making it feasible to identify, map and measure the effects of genes underlying quantitative trait. In grape QTLs were used for features such as like critical photoperiod, growth cessation or dormancy, Bud Break (BB) and winter hardiness. Approximate positions of 28 major genes were mapped in different populations of peach (orange background), almond (yellow background) and myrobalan plum (green background) on the framework of the Prunus reference map. Gene abbreviations correspond to: Y, peach flesh colour; sharka, Plum pox virus resistance; Mi, nematode resistance from peach; D, almond shell hardness; Br, broomy plant habit; DI, double flower; Cs, flesh color around the stone; Ag, anther color; Pep, polycarpel; Fe, flower color; Lb, blooming date; F, flesh adherence to stone; D, non-acid fruit in peach, Sk, bitter kernel; G, fruit skin pubescence; Nl, leaf shape; Dw, Dwarf plant; Ps, male sterility; Sc, fruit skin color; Gr, leaf colour; Ma, nematode resistance from myrobalan plum; E, leaf gland shape; Sf, resistance to powdery mildew. Genes DZ and Br are located on an unknown position of G2.
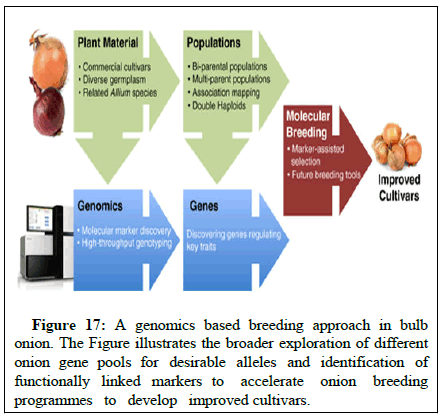
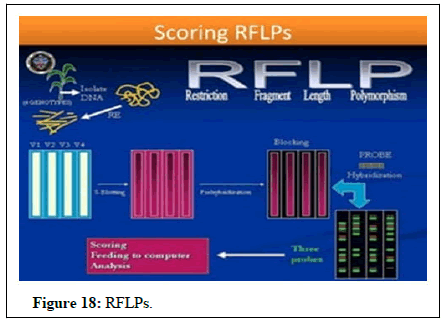
Marker assisted selection: The use of molecular markers can greatly accelerate the pace of selection in fruit crops with inherent long juvenile period and when trait under study is controlled by the recessive gene and incorporating more than one gene for disease resistance. Presently, the markers linked to resistance to dagger nematode (Xiphinema index) and pierce disease are being routinely used at university of California in the breeding programs aimed at improving grapevine rootstock. The resistance to most successful resistance gene (VJ) for scab has been overcome in apple by new race pathogen (Figures 17 and 18).
Genetic transformation: Process of transfer, integration and expression of transgenes in the host cells is known as genetic transformation. Resistant to biotic stress for example Citrus decline, CTV, greening, papaya ring spot virus resistance, plum pox virus etc. Higher tolerance to abiotic stress i.e. salinity, flood, drought etc. shortening the juvenile growth phase and quality improvement such as shelf life improvement and edible vaccine.
Shelf life improvement: The compositional similarity of the transgenic papayas with delayed ripening trait with the non-transgenic control papayas. Fruit characteristics such as days to maturity, fruit weight and total soluble sugars were similar for both transgenic and control papaya trees. Two ACC oxidase genes, CpACO1 and CpACO2 are reported and CpACO1 is associated with late stage fruit ripening and leaf senescence whereas CpACO2 is induced before colors break. Two of the ripening related genes MaMads-rin and MaExp2 are used for banana transformation in order to increase shelf life and fruit quality Liu and Juan, et al., have shown that PG plays a significant role in strawberry ripening. Two ripening related PG genes, FaPG1 and FaPG2 identified for regulation of fruit ripening. Using tobacco rattle virus induced gene silencing, the down regulation of FaSS1 transcripts significantly delayed fruit ripening, as evidenced by the changes of firmness and soluble sugar and anthocyanin contents, as well as the transcripts of several ripening related genes, including PE1, PLI, XYL2, CHS, CHI and DFR. Furthermore, the mRNA expression level of FaSS1 was inhibited by abscisic acid or sucrose, but not by glucose after fruit disc incubation in vitro. The two banana E class (SEPALLATA3 (SEP3) MADS box genes, MaMADS1 and MaMADS2, homologous to the tomato (Solanum lycopersicum) RIN-MADS ripening gene. Transgenic banana plants repressing either gene via antisense or RNA interference (RNAi) were created and exhibited specific ripening delay and extended shelf life phenotypes, including delayed color development and softening.
Disease resistance: A viral diseases papaya ring spot virus came to Hawaii in the 1940’s and had wiped out papaya production on Oahu by the 1950's. Transgenic papaya was developed through coat protein mediated gene transformation. The two transgenic varieties were developed i.e. sun up and rainbow, those are resistant to papaya ring spot virus, which revolutionized the papaya cultivation in Hawaii.
Plum pox virus causes fruit deformation and reduced quality, premature fruit drop, leaf chlorosis, tree decline and death in severe infections or coinfections with other viruses. Honey sweet an improved cultivar of plum was developed using mechanism of RNAi silencing, which was resistant to virus (Figure 19).
Discussion
Propagation temperate fruits crops
Apple: Different grafting and budding techniques are employed for the vegetative propagation of commercial apple cultivars, but tongue and cleft grafting are most widely used. Now a days, chip budding has come out as a good alternative and is being widely practiced in other countries. The apple plantation is also raised on seedling rootstock. Seeds of crab apple, white dotted red, yellow newton, golden delicious and northern spy have good seed viability, germinability and seedling growth. Srivastava observed grafting to give better results than budding with the best performance given by tongue grafting, while cleft grafting is very easy and can be done in late winter when the bark is not yet slipping. Although, these methods give good results but may be discouraged as it requires more bud wood and resulted in variable plants canopy due to varied maturity of bud wood. Also, there are more chances of transmission of pathogens through the grafted scion wood. Now a days, chip budding has come out as a good alternative and is being widely practiced in other countries. This method takes care of the economic use of bud wood and the cambium of scion and rootstock is placed opposite one another resulting into quick union in the weeks, following budding. The plants produced by this method are very vigorous as they produce excessive longer laterals that are an important attribute both for the sale of trees by nurseryman and by fruit growers. It has been reported by Howard, that in chip budded plants there is a lower incidence of bud loss due to union cankers from Nectria galligena, because bud wood are not included below the rind and moreover it is faster to perform over all other budding methods. Chip budding results in better growth, rapid union formation, higher bud take success and high budding ratio. Howard reported chip budding to be superior over other methods used in terms of height and number of laterals produced. Czarnecki found chip budding resulting in more vigorous growth of maiden trees of apple cv. Macspur on M26 as compared to T-budding, however, number of maiden trees were same from both methods. Ananda and Negi recommended chip budding over shield budding or tongue grafting for its ease of application, high success rate, rapid bud union formation, uniformity, high production of first grade trees and low labor demands. On evaluating chip budding in apple, Howard, et al., obtained high percentage success, strong graft union, rapid bud union etc. and found it, as an easy practice producing large scale of saleable plants.
The budding method is highly responsible for the propagation success but season/time of propagation also plays a very important role in this regard. Porebski, et al., observed chip budding to be best if performed on mid-August to early September in apple. Kumar and Ananda conducted an experiment on the effect of propagation method in spur type cultivars of apple viz.; ‘red spur’ and ‘well spur’ for comparing the performance of tongue grafting, chip budding, shield budding and annular budding and reported that tongue grafting gave the maximum linear growth (130.60 cm and 87.38 cm) and radial growth of scion (2.77 cm and 2.68 cm) and rootstock (3.12 cm and 2.88 cm) in Redspur and Wellspur cultivars, respectively. It was also found that tongue grafting also produced maximum number (1.33 and 1.77) and length of feather (28.54 cm and 17.67 cm) and height of feather emergence (30.36 cm and 14.42 cm) from the union, in both the cultivars, respectively.
Rootstocks for apple
Malling (M) series: Developed at East Malling research station in England in 1913.
Dwarfing: M-8, M-9, M-26, M-27.
Semi-dwarfing: M-2, M-4, M-7.
Semi-vigorous: M-13.
Vigorous: M-12, M-16, M-7 and M-13 are tolerant to excessive soil moisture. Most suitable for use as dwarfing inter stock is M-27. M-9 is sensitive to injury from very low winter temperature.
Malling-Merton (MM) series: Developed by crossing Malling rootstocks with northern spy to evolve wooly aphid resistant rootstocks.
Semi-dwarf: MM-106.
Semi-vigorous: MM-104, MM-111
Vigorous: MM-109, MM-111 is resistant to drought while MM-104 is tolerant to wet soils. MM-106 is the most widely used rootstock.
Merton series: Out of 4 Merton rootstocks (Merton 778, 779, 789 and 793), Merton 793 has proved a very useful rootstock. It produces trees little smaller than M-16, is resistant to wooly aphid and collar rot and is adaptable to a wide range of soil and induces early fruiting.
Polish (P) series: P-series of apple rootstocks have been developed in Poland by crossing common Antonovka with M-9. Five rootstocks (P1, P2, P16, P18 and P22) have been found promising. All these rootstocks are winter hardy and resistant to crown rot but susceptible to fire blight. P18 is semi-dwarfing while all others are dwarfing. The P2 and P22 show particular promise as dwarfing inters tock.
Budagovsky (Bud) series: Bud series of rootstocks introduced by the Michurin College of horticulture, Michurinsk, Russia. These rootstocks, viz., Bud-9, Bud-490 and Bud-491 are of promise. Bud-9 and Bud-491 are dwarfing, while Bud-490 is semi-dwarfing. Bud-490 and Bud-491 are very winter hardy and Bud-9 and B-490 are resistant to crown rot.
Ottawa series: There are two Ottawa series of apple rootstock, the Ottawa Hybrid Seedlings (OH) and the Ottawa clonal series (O). The six OH series rootstocks (OH1 to OH6) are still under test but most of these hybrids are resistant to latent viruses commonly found in commercial apple varieties. In the Ottawa clonal series out of the 14 rootstocks originally selected (O1 to O14) five rootstocks viz. O5, O6, O9, O10 and O11 were discarded in the course of testing because of poor performance or incompatibility. Among the others, two rootstocks namely O4 and O 8 have shown promise. These are more productive and were hardy than MM-106.
Michigan Apple Clone (MAC) series
MAC 1: Trees on this rootstock are approximately M-7 in size but do not sucker and are well anchored.
MAC 9 (Mark): Trees on this rootstock are similar in size to M-9 or slightly larger and equivalent in productivity.
MAC 24: Trees on this rootstock are semi-vigorous and in the MM-111 class. It is well anchored.
Miscellaneous rootstocks:
Bemali: First introduction from Balsgard, Sweden. Bemali produces trees about the size of M-26, is highly productive and resistant to wooly aphid.
Jork 9 (J9): This is an open pollinated M-9 seedling released by the Jork research station, West Germany, that has the advantages of more easy propagation with equal productivity over M-9.
Alnarp 2: Introduction from Alnarp fruit tree station, Sweden, is winter hardy, vigorous and induce early bearing and productive.
Robusta 5: Originated in Canada, is vigorous, winter hardy, resistant to fire blight.
Pear
In temperate areas the choice of rootstocks lies between pear and quince. Plants grown on quince rootstock can be planted in soils with higher water table and with the object of having dwarf pear trees. However, seedlings of commercial cultivars can also serve the purpose of a good rootstock. In subtropical areas plants are raised on wild pear (Pyrus pashia) stock locally known as Kainth and also quince seedling. Seedlings of kainth or quince are raised after stratification of seeds for 30-50 days at (1°C-5°C). The stratification can also be met by sowing the seeds in open nursery in November. Seedlings are tongue or splice grafted before the bud break. In temperate areas ‘T’ budding is commonly practiced in July-August.
Rootstocks for pear: In pears, there are not many clonal rootstocks as in apple and generally seedling rootstocks are the most widely used rootstocks. Although different Pyrus species such as P. communis, P. pyrifolia, P. pashia, P. calleryana, P. betufolia, P. ussuriensis, P. elaeagrifoliaare used as pear rootstocks, yet the species P. communis, P. betufolia and P. calleryana offer the most useful traits for a general use rootstock. Although less easy to propagate clonally than quince, they offer winter hardiness and hot climate tolerance, compatibility for all scion cultivars, tolerance to wet soils, reduced susceptibility to high pH and resistance to many diseases.
Clonal rootstocks for pears have been limited primarily to selections of Quince that were developed since 1930 at East Malling. Of the five selections made from East Malling research station, viz., Quince A, B, C, D and E only three, i.e. Quince A, B and C are important from commercial point of view. Rootstock breeding programme has been successful in the development of OH × F series of clonal rootstocks, some of which are identified to have disease resistance with high yield potential, besides having a range of size controlling effect.
Quince rootstocks:
Quince A: Vigorous and most important.
Quince B: Intermediate, produces smaller trees than Quince A.
Quince C: Most dwarfing, precocious and susceptible to winter injury.
C.132 selected from HRI-East Malling and Cts 212 selected from Pisa, Italy are more dwarfing than Quince C pyro dwarf, new rootstock selected from Germany is more dwarf than Quince A. Pyrus communis rootstocks: Old Home (OH) × Farmingdale (F) series.
Dwarf: OH × F 51
Semi-dwarf: OH × F 34, 69, 87, 230, 233.
Semi-vigorous: OH × F 217, 267, 361.
Vigorous: OH × F 18, 97, 112, 198.
These rootstocks are highly tolerant to winter cold and bacterial canker and moderately tolerant to crown gall and collar rot but difficult to propagate except by micropropagation.
Pyrus betufolia rootstocks: Oregon 260, 261 and 264: Vigorous, very tolerant to wet and dry soil and highly tolerant to bacterial canker, crown gall, collar rot and root lesion nematodes.
Pyrus calleryana rootstocks: Oregon 211 and 249: Very dwarfing, highly tolerant to wet soil and crown gall and collar rot diseases and root lesion nematodes.
Peach
Rootstock in sub-tropical areas are generally grown from desi peach tree or some commercial cultivars like Sharbati and Khurmani. Seed should be extracted from ripe fruit only which ensures better germination. Peach seed needs stratification for germination which can be done by placing them under moist condition at about 1°C-5°C for 60 days-70 days; however, this period varies with cultivars. Seeds can also be directly sown in the open ground in the month of November for stratification under natural conditions. Pre-soaking of peach seed in GA3 at 100 ppm for 24 hours before placing them under stratification improves germination and reduces time for germination. Peach seeds become buddable by June to July when they attain pencil thickness. Peaches are propagated commercially by tongue when stock and scion are of same size or cleft grafting when stock is thicker than scion and T-budding or ring budding. In hills, tongue grafting during January-February and T-budding during June to September are performed. However, in plains grafting is performed during November-January and budding during April-June and September. Saw-kerf or notch grafting is used to top work established off-type or undesirable variety trees. Sawkerf grafting can be done over a long period, usually February and March. This grafting method requires considerable skill, but it is the accepted method of top working knotty peach stock that very seldom splits properly for other grafting methods such as cleft grafting. Saw-kerf grafts limit the risk of infection inherent in reworking mature trees.
Seedling rootstock: Wild peach, Sharbati and Flordaguard.
Dwarf: Prunus besseyi.
Semi dwarf: St. Julian Hybrid No. 2, Damas GF.1869, (resistant to high pH and water logging) GF-677 and GF-557, Rubira.
Cherry
Modern stone fruit varieties are propagated vegetative to maintain desirable fruiting traits. Propagation by seed is not recommended because seed produced by cross fertilization may yield undesirable trait combinations. Because seedlings grown from crosses between commercial fruiting varieties contain a mix of parental genetic backgrounds they will not be identical to either parent and will differ in growth and fruiting habit. This variability is desirable for plant breeding and development of new varieties, not for uniform growth in an orchard establishment. Seed used for seedling rootstock is generated by self-fertilization in a tree that is genetically homozygous, or by controlled cross pollination between two parental varieties. Cherry plants are propagated mainly through grafting. Tongue grafting during February-March is recommended, which gives a bud take of more than 90%. For grafting, the scion wood is collected. During winter when the buds are dormant. Scion wood is packed in moss grass and then wrapped in moist gunny bags. These packed bundles of scion wood are stored at 2°C-4°C till these are used for grafting. It has been reported that micropropagation is a feasible method for the vegetative propagation of selected elite trees of wild cherry (Prunus avium) on a large scale and this method was used to produce plants for field testing. Roots from 74% of elite cherry trees are capable of producing shoots which are a good source of buds to initiate vigorous shoot cultures. Micropropagated cherry displayed juvenile characteristics and could be handled like trees produced from conventional seed.
Rootstock influences: Locally known ‘paja’ (Prunus cerasoides), is commonly used as a rootstock for sweet cherry in Himachal Pradesh, Kashmir and hills of Uttar Pradesh. But it has completely failed due to delayed incompatibility. The plants raised on this rootstock grow well for 8-9 years. After that their growth declines and plants completely die after 14-15 years. In some areas of Himachal Pradesh wild bird cherry (Prunus padus) is also used as a rootstock. Seedling of Mahaleb (P. mahaleb) is commonly used as a rootstock in Jammu and Kashmir. Clonal rootstocks Colt and mazzard F12/1 have been found promising. These are recommended for raising dwarf sweet cherry plants in Himachal Pradesh.
Seedling rootstock: Seedlings of paja, Mahaleb and mazzard are used for raising sweet cherry plants in India. Seeds of paja do not require chilling treatment to break dormancy but seeds of Mahaleb and mazzard require stratification before sowing. Seeds are extracted from fully ripe fruits. They are dried and stored in a cool place. Seeds are soaked in 500 ppm Go\for about 24 hr. then they are stratified by placing between the layers of sand in a cool place at 2°C-4°C for 80 days-120 days for Mahaleb and 120 days-150 days for mazzard to break seed dormancy. During stratification, the medium is kept moist. As the embryonic root comes out from seed coat, these are transplanted 6 cm deep and 10 cm-15 cm apart in rows spaced at 20 cm-25 cm in nursery beds. The nursery beds are mulched with 10 cm-15 cm thick hay and irrigated lightly. Mulch material is removed when seedlings attain 56 cm height. The nursery should be watered twice a week and kept weed free.
Clonal rootstock: In Himachal Pradesh, colt and mazzard F 12/1 clonal rootstocks are commercially recommended for raising its plants as trees on paja show symptoms of delayed incompatibility. Colt is semi dwarf, compatible with almost all varieties of sweet cherry, has good anchorage and is resistant to gummosis, crown rot moderately resistant to stem pitting virus and bacterial canker but susceptible to oak root fungus. mazzard F 12/1 is semi-vigorous and difficult to root. Mound or stool layering is the common method of clonal rootstock multiplication. The stool beds are established during December by planting healthy mother plants 30 cm-45 cm apart in rows spaced at 60 cm-70 cm. Before new growth starts, the mother plants are cut back to 2.5 cm above the ground level. New shoots develop on the stub in the spring. When the shoots are 25 cm-30 cm long, their bases are covered with a mound of soil or saw dust, building the mound to a height of 20 cm-25 cm as the shoots grow during spring. The suckers are ringed at the base and then covered with soil to encourage rooting. In difficult to root mazzard F 12/1 rootstock, IBA (7,500 ppm) is applied to the ringed portion of the shoots during summer. The shoots are separated in winter and then lined out in the nursery beds. If the suckers are well-rooted and more than cm is diameter, they are grated in the spring; otherwise they are kept in the bed for a year to produce strong plants for grafting. Colt rootstock is easy to root and can be multiplied through cuttings. Hardwood cuttings of 30 cm-45 cm length and of pencil thickness are taken in February. Cuttings are treated with IBA (2,550 ppm) for 10 seconds and planted in nursery beds for rooting. Rooted cuttings are lined out in December and grafted with scion variety in March. Gulen, et al., reported highest rooting percentage (80%) in cherry clonal rootstock cuttings which were banded for six weeks and treated with 5 mM IBA.
Plum and almond
Seeds require 2-3 months cold stratification and are best sown in a cold frame as soon as it is ripe. The stored seed are sown in a cold frame as early in the year as possible. The seeds should be protecting the seed from mice, etc. The seeds can bear dormancy (slow to germinate) and sometime takes 18 months to germinate. Prick out the seedling into individual pots when they are large enough to handle. Grow them on in a greenhouse or cold frame for the first winter and plant them out in late spring or early summer of the following year. Plums are usually propagated by budding onto either seedlings or rooted cuttings of rootstocks. Budding onto a rootstock provides the advantages of a root system that is resistant to certain pathogens or adverse environmental conditions while also growing the desired fruit producing cultivar on top. The most commonly used budding techniques for plums are T-budding and chip budding either early in the spring or late summer. Because plums are a relatively easy to root species, they can also be propagated by hardwood or sometimes Softwood cuttings under mist or in hothouses and then transplanted directly into their final location.
This method is cheaper and less labour intensive than budding because there is no rootstock or budding process involved. However, the benefits of having a resistant rootstock are lost. For example, it might be necessary to fumigate the soil to prevent susceptible varieties from nematode attack. Other options for plum propagation include splice grafts onto newly planted rootstock seedlings in the spring and top working. The former provides the benefits of a resistant rootstock and offers an alternative to budding, while the latter allows a grower to switch the variety grown in an existing orchard without the costly and time consuming process of removing and replacing all of the old trees. Grafting, however, is usually more expensive than budding and is a less efficient use of scion wood. Therefore, budding is usually preferred to grafting. Top working is done when the benefits of such a process outweighs the costs. For example, it is probably best to completely replace an orchard if the producing trees are greater than 10 or 12 years old. The most commonly used rootstocks for almond are seedlings raised from sweet almond, bitter almond and wild peach. However, keeping in view the shorter life of peach, it is preferable to use sweet or bitter almond seedling rootstocks. When the seedlings are of pencil thickness and have attained a height of 30 cm, they are T-budded in the month of July/August. The seedlings which do not make sufficient growth so as to become fit for T-budding are tongue or cleft grated in January/Feb. Tongue grafting is employed when stock and scion are of equal thickness.
Plum rootstocks: Seedling root stock: Wild peach and apricot, Kabul green gauze and Sloh (almond × peach); dwarf: pixy; semi dwarf: St. Julian hybrid-2, Damas-1869, Mirabi; vigorous: Marriana GF8/1, Myrobalan 27.
Almond rootstocks: Seedling root stocks: Bitter almond and wild peach; Kabul green gauze and Sloh; Semi dwarf: Ishtara; vigorous: Hansen 2168, hybrid GF-677 (replant area) and GF. 557.
Apricot
Apricots are generally propagated on wild apricot (Zaradalu), apricot and plum seedlings. For raising the seedling on which the budding is done, the seeds are stratified for a period of 45 days-50 days at 1°C-5°C. The seeds should be sown 30 cm apart in rows. To make better growth of seedling, small quantity of urea may be applied. The seedlings which attain pencil thickness in the month of May-June, should be budded at a height of 20 cm-25 cm above the ground by shield budding. It can also be tongue grafted in February. In Apricot, Dwivedi, et al., reported tongue grafting giving the highest mean sprouting (98.7%), bud take (94.7%), maximum linear (167 cm) and radial (0.85 cm) growth under Ladakh conditions. Apricot has been found to respond to in vitro condition in different ways depending upon the genotype, the culture conditions, the physiological and developmental stage of the explants, the orientation of the tissue on the medium and many other define or undefined factors. Partial success in in vitro propagation of growing shoot tips was reported by Kataeva who used growing shoot tips with 2-4 pairs of growing primordial leaves from actively growing axillary shoot as the explants. These ex plants were isolated from 6 fruiting trees of apricot, 7-16 years of age. The explants were cultured in various culture media. In this study, marked differences were noted in the rate of micropropagation which differed from tree to tree. Out of 6, 3 trees had low capacity to from axillary shoots. While rooting of the in vitro shoots was fairly successful, adaptation of rooted plant to ex vivo condition was the most critical aspect. After several combinations it was reported that the rooted plants survived with a success of 70%-80% in a soil-mix containing perlite, peat and sand (1:1:1). The scope of micro propagation is only limited to initial prolific of the scion wood which may have specific attributes as resistant to diseases, freedom from the viruses obtained through meristem culture in the case of newly released cultivars which are in short supply. However, clonal rootstocks need to be multiplied under in vitro environment for keeping them under strict phyto sanitary conditions. Cupidi described techniques of in vitro micrografting to obtain virus free propagating material, including virus free in vitro rootstock preparation, apex isolation and grafting, in vitro growth and hardening of obtained plantlet in apricot and other stone fruits.
Prunus rootstocks
Apricot can be grown successfully on rootstocks of other species within the Prunus genus, including peach, plum and various interspecific Prunus hybrids. Specifically, apricot has been successfully grown on several rootstocks including Nemaguard, Nemared, Lovell, Marianna 2624 (plum) and citation hybrid. Nemaguard (seedling peach rootstock) and citation are the most common rootstocks used in commercial apricot production in California. Nemaguard confers vigor, good anchorage and is resistant to root knot nematode. Mariana 2624 is occasionally used in commercial plantings in Northern California because it tolerates wet, heavy soils, although it is very susceptible to bacterial canker. Citation has been used in the southern edge of California apricot production in the central valley and is increasing in popularity. Apricot can also be grown on myrobalan plum rootstocks, although weakness and breaking at the graft union has been reported after high winds. As a result of this problem myrobalan rootstocks should only be used in very heavy or wet soils.
Apricot seedling rootstocks
Apricots can be grown successfully on apricot seedling rootstock. Within California, both Royal and Blenheim apricot varieties work well as rootstocks because they are immune to the root knot nematode and are somewhat resistant to the root lesion nematode and to crown gall. However, apricot seedlings are susceptible to both oak root fungus and Verticillium wilt and are not commonly used in commercial plantings.
New alternative rootstocks
Several new experimental rootstocks have been evaluated by the NC-140 rootstock trials over the past ten years, conducted by a group of North American scientists and the USDA. One goal of the NC-140 rootstock trials has been to identify new dwarfing rootstocks that limit tree height and size, without altering scion production or fruit characteristics. Ideally, the shorter, more compact trees will require less pruning and reduce labor costs by eliminating the need for ladders to harvest fruit. Although they may help reduce costs and increase competitiveness, dwarfing rootstocks developed by the USDA and evaluated in NC140 trials including HBOK32, HBOK10, HBOK50, Controller 5 and Controller 9 are not yet widely used in commercial plantings.
Walnut
Walnut seedlings are generally used as rootstock. Walnuts procured from current season’s crop should be sown directly in well prepared seed beds during November. If land is not ready for sowing and inclement weather prevails, nuts can be stratified in moist sand till soil becomes workable. In both cases, direct sowing or after stratification, i.e. the nuts should be sown maintaining 25 cm distance from nut to nut and 75 cm from row to row. Vegetative propagation can be used for production of clonal paradox rootstocks and for developing a finished tree with an English walnut cultivar (scion) grafted or budded onto the rootstock. Clonal paradox rootstock can be propagated by hardwood cutting or micropropagation techniques although micropropagation is the preferred technique.
Cuttings: Although hardwood and semi hardwood cuttings can also be used for rootstock propagation, they are not commonly used because success rates are often low. Such cuttings have rooting percentages from 30% to 80% and often have poor initial survival. Use cuttings from only vigorously growing shoots for propagation and root them in individual liners to promote uniform and deeper root branching. The bases of cuttings are treated with KIBA at 8 PPM-12,000 PPM before sticking. Semi hardwood cuttings are rooted in greenhouses on bottom heated mist benches in mid to late summer and hardwood cuttings on bottom heated mist benches in late fall and winter outdoors. The use of broadcast flats yields lower rooting percentages, shallower roots, and poor root development. Plant liner sized rooted cuttings in the field in late February or early March. Direct field rooting is not commercially successful.
Budding: Once the rootstock is established, growing well in the nursery and has a diameter matching scion wood, the English scion variety can be grafted or budded on the rootstock. Trees are typically propagated in the nursery by fall budding the rootstock between late August and mid-September (patch or T-bud, see below). A finished tree will be grown by the next fall if the buds heal over, remain dormant until the spring, and grow out in the following summer. The rootstocks that were initially too small or had failed fall buds, are whip and tongue grafted in the spring and the scion is encouraged to grow immediately following grafting. Both techniques create a finished tree in two years. A more recent technique, June budding, creates a tree in only one year. Rootstocks to be used in June budding are grown in very fertile conditions and typically reach budding size by June, coinciding with the time current season scion buds develop to a condition suitable for use as bud wood. The budded trees are then managed intensively for the rest of the summer to create a tree large enough to sell. Some growers choose to do much of what is done at the nursery in their own field. In field budding or grafting has the advantage of establishing an orchard with less up-front cost if a grower has the necessary propagation skills.
Patch budding is the most common budding method used for walnuts. However, T-budding can also be employed successfully. Collect budsticks when bark is slipping on both the rootstock and scion budstick. To ensure the best take, the budwood can be prepared by removing the leaves while still on the tree a few weeks before use. Patch budding is done using a double bladed knife to cut a square piece of bark from the rootstock which is replaced with the same size patch from the budstick containing a well-developed bud. T-budding uses a single bladed knife to slit the rootstock bark in the shape of a T and a shield shaped piece of stem including a bud from the budstick is cut and placed into the opening. After placement, the bud should be covered with budding tape to prevent desiccation. The tape is removed when the bud has healed, usually after a few weeks. Gautam in walnut got only 20% success with chip budding against 15% with tongue grafting. Moreover, the highest success (90%) was recorded using veneer grafting, while patch budding, splice grafting, bark grafting and saddle grafting gave 35.0%, 8.5%, 5.5% and 5.0% success, respectively.
Grafting: In California grafting is done in the late spring after the rootstock has produced leaves and the risk of graft failure due to bleeding has somewhat diminished. Bleeding (sap flowing from the rootstock) is the most difficult step to manage when grafting walnuts. To reduce the likelihood of bleeding, avoid grafting during times of the year with strong temperature fluctuations or two weeks before or after heavy rains and irrigation. Prior to grafting, the tree should be “bled”. To bleed a tree precut the rootstock two to three inches above the grafting site and make several cuts below the graft site without girdling the tree, 7-10 days before grafting. If bleeding occurs after grafting, restrict watering or make cuts on the stock below the graft. Whip and tongue grafting is the most common graft used to propagate walnuts. Collect scion wood from dormant trees between December and February from the basal portions of the previous season’s growth with well-developed buds. Place collected scion wood in moist wood shavings or a plastic bag and refrigerate. Choose a piece of scion wood that closely matches the diameter of the rootstock. The scion piece should only be long enough to contain two buds. Make a diagonal cut on both the scion and the rootstock and cut a small slit or tongue directly in the center. Place the two matching cuts together with the tongues locking them in place. Then, wrap the graft with grafting tape and seal the top of the scion with grafting sealer. Paint the whole graft and rootstock with white paint to prevent sunburn. Because grafting tends to produce multiple leaders, select the strongest shoot and stake it while keeping the other shoots trimmed low so that they do not compete with the chosen leader. Grafting can also be used to top work a mature walnut tree, though this is an unusual practice and only used when there is a need to change the cultivar or add pollinizer cultivars in an orchard. Omega grafting was conducted using one year old rootstocks and scions with a grafting machine. Graft unions were plunged into hot paraffin (70°C-80°C) and then cooled in cold water. The graft unions were forced in woody boxes, filled with wet sawdust in a room under controlled conditions at 27°C and 80% relative humidity. This method is also applied in walnut propagation. Percentage of grafting success in walnut cultivar ‘Izvor 10’ propagated by omega bench grafting was 40.8%, hot callus was 74.2% and epicotyl grafting was 75.5%, respectively.
Micropropagation: Micropropagation has increased in popularity over the past ten years because walnut is difficult to propagate clonally from cuttings. To micropropagate walnut, surface sterilizes individual stem segments containing one bud (no leaves) to remove surface bacteria and fungus (Figure 20). Next, place the basal portion of the stem segments into an agar medium containing a mixture of plant hormones, nutrients and sugars to promote bud growth. Many clonal microshoots can be produced from a single bud. These microshoots can be used for rooting (clonal rootstock or own root trees). For rooting, the bases of microshoots are treated with Potassium Indolebutyric Acid (KIBA) in vitro for 5 to 7 days and then stuck in a peat: Perlite medium on a fog bench in a greenhouse. Micropropagated plants need to be hardened off after planting in liner sized containers by gradually reducing humidity (from 100%) over a period of weeks in a greenhouse. In the spring or fall, hardened plantlets can be transferred to a nursery field and then budded or grafted when they reach an appropriate size.
Strawberry: Strawberry is commercially propagated by runner plants. Generally one plant produces 7-10 runners but under proper management, it can go up to 15 runners/plant. It can also be propagated through crowns (3-5 plants/crown), but division of crowns of older plants is too tedious and expensive for cultivars producing runner plants readily. Runner formation can be stimulated with the application of IBA (100 ppm) 10 days before flowering, and also with morphectin (50 ppm).
Propagation by seen is not suitable as the seedlings do not come true to type. Due to this, old strawberry buds may have many untrue seedlings undesirable for propagation. Where viruses and nematodes are present, primarily in commercial plantations, the growth and production of plants may be reduced by half or more, it is desirable to procure virus free plants for commercial plantations. In addition, these plants are raised in fumigated soils to control nematodes. Planting virus and nematode free stock, and keeping it clean provides protection against serious diseases. For large scale propagation of virus free plants, tissue culture is widely used. Under favorable conditions, one strawberry meristem can be multiplied to yield more than one million plants in a year. Plants can be regenerated from meristematic callus, anthers and immature embryos.
Peanut: Propagation by seed is typically only used to produce seedling rootstocks for grafting cultivar clones. Collect well-filled nuts during harvest season to produce a seedling. Remove husks and dry nuts to about 5% moisture. Pecan nuts have a protective shell which delays germination. To break dormancy, stratify nuts between 36°F and 41°F for at 30 to 90 days before moving them to room temperature. Allow seeds to acclimate to ambient room temperature at least one week before planting. Seeds can also be soaked in water the day before planting, causing the suture to split and breaking dormancy. Seeds should be planted in late winter, before March 1st. After two years a seedling should be 4 feet-5 feet tall and ready for grafting. Pecan trees are usually grafted in the spring of the third growing season or patch budded in late summer of the second growing season.
Indian gooseberry (Emblica officinalis Gaertn.): Aonla is becoming more popular in India due to its hardiness, adaptability for cultivation in a variety of agroclimatic conditions and its wide use in the pharmaceutical, nutraceuticals, cosmetics as well as post-harvest processing industries. The greatest constraint to the establishment of Indian gooseberry orchards is the lack of uniform planting material. The rate of success of vegetative propagation varies between 25% and 80% depending on the genotype. Aonla can be grown through seed materials as well or through ‘Inarching’ (grafting). However, because of cross pollination, seed does not produce a true to type plant. Conventional method of vegetative propagation of aonla is at slow rate and also season dependent. Furthermore, such plants take longer time to bear fruit. Also due to erect branching habit a large number of branches are not available close to ground surface ground level for approach grafting purpose. Another constraint in field multiplication and field survival is unavailability of the desired number of shoots for vegetative propagation such as grafting or budding. Aonla is an important indigenous hardy minor fruit with commercial importance originated in tropical South-eastern Asia, particularly in central and southern India and also grown at foot hills of Himalayas as well as in peninsular India. Various active tannins composition principles (Emblicanin A, Emblicanin B, Punigluconin and Pedunculagin) have been identified in addition to strong antioxidants, which contribute for numerous health benefits. Fruits of aonla are richest source of vitamin C (600 mg/100 g) after Barbados cherry and source of valuable minerals, riboflavin, calcium, iron, phosphorus, nicotinic acid, tryptophan, lysine and methionine and lysine as well as bearing herbicidal properties due to presence of high anti-ascorbic value. However, standardized micropropagation technology can be used for mass replication of true to type plantlets. The two most critical parameters that influence the performance of any micropropagation system are in vitro bud induction and shoot proliferation. Mishra and Pathak conducted an experiment in vitro shoot proliferation in aonla (Emblica officinalis Gaertn.) cv. Narendra aonla-7 by selecting axillary shoots having one node as explant. Before preparing the explant for in vitro, the shoots were clipped to 1 cm-2 cm length and determinate shoots attached with nodes were removed, leaving 0.5 cm from the base. The cut end of the explant was sealed with melted paraffin wax to prevent oxidative browning and contamination in vitro and this technique has been termed as ‘explant waxing’. This method has been reported to have an 80% success rate in establishing cultures. MS medium (Murashige and Skoog) which was supplemented with 0.8% agar, 3% sucrose, 0.4 mg/l kinetin and 1.0 mg/l GA3 were used for inoculation of shoot. The pH of media was maintained at 5.7 and cultures were kept at 25°C ± 2°C temperature, 50%-55% RH and 2000 lux of florescent tube light illumination with 16/8 hour of light and dark cycling.
The observation of the experiment reveals that aonla shoots collected between August-November had better culture establishment, bud induction and microshoot growth than shoots collected between April-July. Moderately hard (10 nodes-15 nodes) and slightly green shoot of aonla and exhibited highest in vitro bud induction and proliferated much more than the soft (1 nodes-10 nodes) or extremely hard, brown (20-30) nodal segments. Treatment of the explant with Bavistin (1.0%) and chloramphenicol (0.1%) for 240-260 minutes, followed by treatment with HgCl2 for 8 minutes under aseptic conditions, was used to control in vitro contamination. A determinate stem with leaves arises early after in vitro bud induction, bearing solely flowers. Indeterminate shoots, on the other hand are required for future multiplication. Therefore, the role of GA3 in the elongation of indeterminate shoots is significant. Under the presence of 4.33 μM GA3+13.9 μM Kinetin+342.11 μM glutamine high proliferation of shoot (13.33 shoots/culture) were obtained. Several other reports on aonla micropropagation involving a callus phase and seedling explant are present. However, this mechanism cannot be employed for true to type plant multiplication, but it can be useful for investigation of genetic transformation. E. officinalis callus cultures were used to produce high frequency plantlet regeneration.
Karonda (Carissa carandas L.): Dey, et al., also conducted an experiment on various cuttings of Carissa carandas which were treated with different concentrations of sucrose (2%, 4% and 6%) as well as with IBA (7500 ppm, 8000 ppm and 8500 ppm) and observed that in a short period of time, different levels of sucrose and IBA had a significant impact on the success, survival and rooting of Karonda (Carissa carandas L.) cuttings. The optimum treatments for commercial vegetative multiplication of Karonda by stem cuttings were recommended is application of IBA @8000 ppm followed by 4% sucrose. Karonda (Carissa carandas L.) is an indigenous hardy, evergreen, sprawling semi vine spiny shrub of Apocynaceae family. It thrives well throughout the tropical and subtropical climates. It is susceptible to heavy rainfall and waterlogged conditions. It produces berry sized fruits which are sour and astringent in taste making it unsuitable for fresh consumption and therefore can be processed into various excellent quality products such as nakal cherry, jam, jelly, jam, syrup, squash, sauce (ripe fruits) and chutneys and pickles (unripe fruits) which are of high demand in the national as well as international market. The fruits are rich in vitamin C (1.6 mg-17.9 mg/100 g), protein (1.1%-2.25%) as well as abundant minerals such as iron (39.1 mg/100 g), calcium (21 mg/100 g) and phosphorus (38 mg/100 g) and also dried fruits are one of the richest source of iron content (39.1 mg/100 g). The roots are used to treat intestinal worms, scabies and itch and as an anthelmintic, stomachic and antiscorbutic substance. Karonda plant’s wood is white, firm and smooth and can be used for making combs and spoons. The plants can be trained to grow into a strong hedge and can be used as live fencing around fruit orchards. Wide application of karonda required large number of plants per unit area. In order to increase the area under cultivation of this vital underutilised crop, an alternate approach for quick and large scale propagation is urgently needed. Rai and Misra reported that during different seasons, shoot tips from mature Carissa carandas cv. Pant Sudarshan plants were cultured on Murashige and Skoog’s (MS) basal media supplemented with Benzyl Adenine (BA) and Indole Butyric Acid (IBA). The 1.5 cm long explants taken in the spring season (February-March) had the highest sprouting rate, followed by those obtained in the summer season (April-June). On MS basal media supplemented with 3.0 mg/L BA, shoot proliferation was highest. The optimum microshoot rooting was found in 1/2 MS with 0.8 mg/L IBA and 0.2 mg/L Naphthalene Acetic Acid (NAA). The rooted seedlings were successfully acclimatised in a potting mixture of vermiculite, sand and soil (1:1:1).
Bael (Aegle marmelos Corr.): In vitro propagation of bael has been described by employing ‘axillary bud multiplication and by leaf explants and also by nucellar callus technique. Bael (Aegle marmelos Corr.) is deciduous and ancient medicinal indigenous fruit tree of India belonging to family Rutaceae with chromosomes number (2n=36). Ripe fruit of bael is rich in minerals, vitamins and fibres as well as act as tonic, restorative, laxative and good for heart and brain. Pulp of fruit is rich in ‘psoralen’ and ‘marmelosin’. Hard shell, muciliganeous texture and numerous seeds of bael fruit make it difficult to eat out of hand and therefore, can be processed into diversified value added products such as bael candy, preserve, sherbet and powder. Bael is traditionally propagated via seed, which is sown in June and matures into seedlings after 1 year and are not true to type. Also, bael seeds have short viability and highly prone to insect attack. Root sucker propagation is slow and cumbersome. Excessive exploitation and indiscriminate collection of wild populations of bael species has maximized the pressure on wild sources, resulting in the extinction of this plant, which is now listed as vulnerable in a few Indian states.
Jamun (Syzygium cuminii L.): Micropropagation of Jamun using juvenile tissue, using seedling explants has been developed to produce large scale true to type planting material. Jamun (Syzygium cuminii L.) can be propagated in vitro by employing single nodal explants from seedlings. Shoot proliferation was better in single nodal explants cultured on half strength MS with 2 mg/l BAP+3% sucrose+3% activated charcoal. On 1/4 strength MS with 3% sucrose and 2.5 mg/l IBA, the shoot lets was successfully rooted. After six weeks of hardening on vermiculite (32.5% survival), the rooted shoot lets were planted in polybags and transported to the field.
Jamun is an important hardy medicinal fruit crop of Indo-Malayan region which can be easily grown in neglected and marshy areas. In folk medicine and the pharmaceutical industry, the jamun has garnered significantly more attention than in any other discipline. Fruits contain a wide range of antioxidant chemicals, including polyphenolic compounds, flavonoids, carotenoids and vitamins, all of which are essential for human health by reducing oxidative stress and inhibiting macromolecular oxidation. It contains both nutritive as well as therapeutical value. It is a rich source of iron and is used to treat diabetes, heart disease and liver disease. The seed powder of jamun fruit quickly reduces the amount of sugar in urine.
Jackfruit (Artocarpus heterophyllus L.): A tissue culture approach using apical bud cultures, plants shoot tip or nodal explant has been devised for rapid vegetative multiplication of jackfruit. For the multiple shoot development of jackfruit, healthy (disease free) and juvenile shoot tips were employed as explant and cultured in Murashige-Skoog (MS) media supplemented with varied concentrations of (0 mg/L, 1 mg/L, 2 mg/L, 3 mg/L and 4 mg/L) of plant growth regulators i.e. BAP (6-Benzylea minopurine). Jackfruit (Artocarpus heterophyllus L.) belongs to family Moraceae with chromosome number 2n=56 of Indian origin. It is also called as poor man’s food and regarded as staple food in African countries such as Uganda. Ripe fruits are utilised as table fruits, whereas immature fruits are used as vegetables. Rind and perigones are high in pectin, making them ideal for making jelly. Mature but unripe fruits can also be used to make chips and papad. Jackfruit seeds are a good source of starch and are used in a variety of cuisines. Due to high heterozygosis, propagation of jackfruit is not widely accepted. Tissue culture technique could be utilised for jackfruit multiplication to retain true to type quality fruit throughout the year. Additionally, jackfruit cultivation is typically confined due to a scarcity of superior cultivars and desirable planting materials. When MS media was supplemented with 2 mg/L BAP, shoot regeneration was significantly improved. The percentage of shoot proliferation increased as the subculture was increased up to the tenth maximum. These shoots were subsequently cultured on half strength MS medium supplemented with 0 mg/L, 1 mg/L, 2 mg/L, 3 mg/L and 4 mg/L IBA (Indole-3-Butyric Acid), with the number of roots/explants, root length and early root induction being maximum in the 2 mg/L IBA containing medium.
Future Prospect
Plant biotechnology has the potential to play a key role in the sustainable production of fruit crops. However, there is enormous potential for genetic manipulation of some vegetative propagated fruit crops in order to improve their disease and pest resistance. The use of appropriate constructs may allow the production of nematode, fungal, bacterial and virus resistant plants in a significantly shorter period of time than using conventional breeding, especially if several traits can be introduced simultaneously. It may also be possible to incorporate other characteristics such as drought tolerance, thereby extending the geographic spread of some fruit crops for production, and thus contributing substantially to enhanced food security and poverty alleviation. However, high caution is required for biosafety experiments and potential risk assessment bearing in mind that these are consumed by most humans and mainly by children.
Conclusion
The plants raised through asexual process are identical to mother plants. Cutting, budding, grafting, division and layering are main techniques of asexual propagation. Asexual propagation also includes clone and apomixes. Micropropagation has increased in popularity over the past ten years because walnut is difficult to propagate clonally from cuttings. Treatment with Gibberellic acid (GA3) and Benzyl Adenine (BA), (both at 20 mg/litre) of peach seeds which had previously been stratified at 10°C and 15°C enhanced germination considering all the above things provided we can easily adopt given methodologies in nursery and orchard industry to produce annually millions of clones of minor fruit crops which are true to type, stable, elite, uniform and disease free.
Funding Statement
The authors did not receive specific funding for this study.
Conflicts of Interest
The authors declare that they have no conflict of interest to report in this paper.
Acknowledgements
We acknowledge horticulture department of school of agricultural sciences, IIMT University, Meerut-250001, UP, India.
Contributions
Ashok kumar is the first author of the review article, whereas the co-author has contributed equally for the literature collection, manuscript documentation and its revision. All authors read and approved the final manuscript.
Ethics Declarations
Ethics approval and consent to participate. It is not applicable.
Consent for Publication
The authors confirm that the content of the manuscript has not been published or submitted for publication elsewhere.
Competing Interests
There are no competing interests between authors.
References
- Amin MN, Jaiswal VS (1993) In vitro response of apical bud explants from mature trees of jackfruit (Artocarpus heterophyllus). Plant Cell Tissue Organ Cult 33: 59-65.
- Ajithkumar D, Seeni S (1998) Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Rep 17: 422-4 26.
[Crossref] [Google Scholar] [PubMed]
- Ashrafuzzaman M, Kar S, Khanam D, Prodhan SH (2012) In vitro regeneration and multiplication of jackfruit (Artocarpus heterophyllus L). Res J Biol 2. 59-65.
- Dey K, Ghosh A, Mani A, Bauri FK, Dey AN (2017) Root generation of Karonda (Carissa carandas L.) cuttings in response of sucrose and IBA. J Pharmacogn Phytochem 6: 803-806.
- Hossain M, Islam R, Karim MR, Joarder OI, Biswas BK (1994) Regeneration of plantlets from in vitro cultured cotyledons of Aegle marmelos Corr. (Rutaceae). Sci Hortic 57: 315-321.
- Hossain M, Islam R, Karim MR, Rahman SM, Joarder OI (1994) Production of plantlets from Aegle marmelos nucellar callus. Plant Cell Rep 13: 570-573.
[Crossref] [Google Scholar] [PubMed]
- Islam R, Hossain M, Joarder OI, Karim MR (1993) Adventitious shoot formation on excised leaf explants of in vitro grown seedlings of Aegle marmelos Corr. J Hortic Sci 68: 495-498.
- Jain N, Babbar SB (2000) Recurrent production of plants of black plum, Syzygium cuminii Skeel, a fruit tree from in vitro cultured seedling explants. Plant Cell Rep 19: 519-524.
[Crossref] [Google Scholar] [PubMed]
- Kubola J, Siriamornpun S, Meeso N (2011) Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chem 126: 972-981.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473-497.
- Mishra M, Pathak RK (2001) Effect of nodal position and season on in vitro shoot proliferation in aonla (Emblica officinalis Gaertn). J Appl Hortic 3: 103-104.
- Miro CB, Acedo VZ (2015) Development of micropropagation protocol supporting sustainable production of jackfruit (Artocarpus heterophyllus L.). Acta Hortic 505-508.
- Parmessur Y, Aljanabi S, Saumtally S, Dookun-Saumtally A (2002) Sugarcane yellow leaf virus and sugarcane yellows phytoplasma: Elimination by tissue culture. Plant Pathol J 51: 561-566.
- Roy SK, Rahman SL, Majuar R (1990) In vitro propagation of jackfruit (Artocarpus heterophyllus Lam.). J Hortic Sci 65: 355-358.
- Rai R, Misra KK (2005) Micropropagation of Karonda (Carissa carandas) through shoot multiplication. Sci Hortic 103: 227-232.
- Yadav U, Lal M, Jaiswal VS (1990) In vitro micropropagation of the tropical fruit tree Syzygium cuminii L. Plant Cell Tissue Organ Cult 21: 87-92.
- Dwivedi SK, Singh B, Paljor E (2000) Studies on vegetative propagation of apricot (Prunus armeniaca L.) through grafting in Ladakh. Ind J Hortic 57: 39-41.
- Gandev S (2007) Budding and grafting of the walnut (Juglans regia L.) and their effectiveness in Bulgaria. Bulg J Agric Sci 13: 683.
- Gautam DR (1990) Studies on the winter and summer vegetative propagation techniques of walnut (Juglans regia). Acta Hortic 284: 27-31
- Grzyb ZS, Czynczyk A (1990) Attempts to improve seed germination and seedling growth of Wangenheim prune generative rootstock. Fruit Sci Rep 17: 101-110.
Citation: Kumar A, Bhuj BD, Dhar S, Dixit KMJ, Singh SP (2023) Micropropagation of Fruit Crops: A Review. Adv Crop Sci Tech 11: 595.
Copyright: © 2023 Kumar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1156
- [From(publication date): 0-2023 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 1029
- PDF downloads: 127