Microplastic In Cultured Oysters from Different Coastal Areas of Japan
Received: 01-Sep-2022 / Manuscript No. jmsrd-22-73475 / Editor assigned: 05-Sep-2022 / PreQC No. jmsrd-22-73475 (PQ) / Reviewed: 19-Sep-2022 / QC No. jmsrd-22-73475 / Revised: 26-Sep-2022 / Manuscript No. jmsrd-22-73475 (R) / Published Date: 03-Oct-2022 DOI: 10.4172/2155-9910.1000360
Abstract
Marine pollution caused by microplastics has become a serious social issue nowadays. Studies in Europe comparing the amount of microplastics contained in seafood by type and place of origin show that mollusks from Asia are the most heavily contaminated. This study was aimed to investigate the presence of microplastics in the coastal waters of Japan. For this, we quantitatively analyzed the presence of microplastics in oysters using Raman scattering, optical, and digital microscopic observations. Nylon, polypropylene, and high-density polyethylene were confirmed by Raman spectra in oysters. Fibrous microplastics are the most commonly found. Our results will be used further to assess the potential impact of microplastics on marine biodiversity and possible threats to human food safety.
Highlights
• Microplastic uptake was identified in cultured oysters from twelve sites in Japan.
• It is first study on microplastics from Japanese oysters.
• Microplastic types were found including nylon, polypropylene, and high-density polyethylene.
• Fibrous microplastics were the most commonly found.
Keywords
Microplastic fibers; Farmed oysters; Crassostrea gigas; Coastal areas
Introduction
Studies dealing with the current extent of human impacts on marine ecosystems show that the oceans are becoming highly affected by human activities [1]. The accumulation of mismanaged plastic waste is now a global concern [2]. Since the 1970s, research on the presence of plastic debris in the ocean shows the increase of the average concentration of plastic particles. For example, a study from the western Sargasso Sea indicated an average of 3500 pieces and 290 grams per square kilometer [3]. Not being biodegradable in most cases, plastics widespread use constitutes a persistent environmental burden [4,5]. Plastic is a cheap and light weight material which can be easily molded into various shapes. This has driven its annual world production to increase from 1.7 million tons in the 1950s, to 288 million tons in 2012 [6]. Our lives now seem to be inseparable from plastic. However, its negative effects, including many adverse effects on life and the environment, are starting to be of the utmost concern [6]. Data show that most plastic waste in the oceans comes from terrestrial sources and is carried mostly by winds, wastewater treatment systems and road runoff [7,8], with the remainder coming from artifacts such as fish nets and ropes, aquaculture cages, etc. [9]. Studies show that among macroplastic garbage, plastic bags seem to be the most harmful to marine life [10]. Modern plastics are usually complex mixtures of polymers with chemical additives [11]. Plastic waste is usually colonized by bacteria [12] and often leaches chemical pollutants [13] into the waters. As mentioned, plastics are widely distributed on the surface and coastal waters of the global oceans and their impact on marine life is enormous. For example, 44% of all seabird species are known to ingest plastic, as do sea turtles, cetaceans, and fish [14,15,16]. In recent years, more attention has been paid to microplastics [17], particles less than 5 mm that result from the breakdown of larger items [3]. Among the possible sources of these particles are waters from washing synthetic textiles discharged into the ocean. From the perspective of its composition, polypropylene (PP), polyethylene (PE), polystyrene (PS), polyvinyl chloride (PVC) and polyethylene terephthalate (PET) are the main components with higher probabilities of ending up in the ocean environment [18]. Indeed, several studies have found that both food and drinking water [19] [20]. may contain different types of microplastics. They have also been found in human feces [21]. leading to an increase in the awareness of its possible effects in human health. Compared with large plastics, microplastics are much more abundant and more easily absorbed by organisms, thus increasing its toxicity potential. A study from the Beijiang River surface sediment found that microplastics were loaded with metals such as Ni, Cd, Pb, Cu, Zn and Ti mostly derived from inherent load [22]. Strong sorption of PCBs (polychlorinated biphenyls) to nanoplastics and microplastics was also observed [23], as well as the presence of contaminants, such as polyfluoroalkyl substances (PFAS) [24]. Microplastics can also transport pollutants into marine food webs [25]. Moreover, in China, researchers established a model of microplastic accumulation in Baiyangdian Lake. The results showed that they spread and accumulate very quickly throughout the food web, eventually reaching high trophic level aquatic organisms [21]. Studies from six locations along the French–Belgian–Dutch coastline looked at the absorption of microplastics under field conditions detecting them in mussels (Mytilus edulis) and lugworms (Arenicola marina). In France, microplastics were detected throughout the Bay of Brest, with polyethylene (PE, 53-67%), polypropylene (PP, 16-30%), and polystyrene (PS, 16-17%) fragments dominating both the surface water and the sediments [26]. Microplastic contamination of bivalves from the French Atlantic coast showed a high ratio of grey color particles with size ranging from 50 to 100 μm in blue mussels (Mytilus edulis) and Pacific oysters (Crassostrea gigas) [27].
In Japan, microplastics were found in 31 out of 36 sites along 29 rivers where samples were collected in surface waters. Results show that the concentration of microplastics highly correlates with urbanization and population density and that the amount of particles in rivers seems to depend on the human activities along the river basins [28]. Furthermore, microplastics were found in the digestive tract of 64 anchovies (Engraulis japonicus) in Tokyo Bay, representing 77% of the samples studied [17]. The correlation of microplastics’ amount and human activities was also found in a study of coastal surface water done in the subtropical island of Okinawa, Japan [29].
Studies from the eastern coast of Australia seaports, report large amounts of microplastics in both sediments and oysters. Note that the density of particles in oysters was significantly higher than in sediments and black fibers between 0.1 mm–0.5 mm in size were the most abundant [30]. Experiments with oysters (Saccostrea glomerata) show that it can ingest and accumulate microplastics of 2 μm and 0.5 μm, that are then transferred through the membranes of the digestive glands to the haemolymph [31]. These studies emphasize the necessity to monitor microplastics in aquaculture and in the ocean, in order to enforce seafood safety.
Shellfish is an important component of marine ecosystems. Oysters are filter feeding organisms and as such they are especially easy to come into contact with microplastics [32]. Accumulating floating microparticles during the filtration process. Seafood products are important sources of proteins, polyunsaturated lipids and phospholipids. Economic losses (microplastic toxicity in marine organism, the cost of savage a huge amount of marine litter, etc.) due to microplastic pollution and marine debris in the marine environment are expected [33-35]. In Japan, oysters are very popular as food.
With the development of the aquaculture industry, oysters (mostly Crassostrea spp.) have become even more looked after. So far, Japanese researchers have predominantly studied viruses and their threat to oysters [36-38]. As the threat of microplastics to marine life becomes ever more serious, oyster aquaculture is expected to be highly affected. Japan is an important oyster producer. Its oysters are exported worldwide and considered of prime quality. In this context, we decided to study material from various aquaculture farms in Japan, in order to analyze the presence, quantity, size, and type of microplastics present to assess its the level in farmed oysters. Normally, farmed oysters are generally pre-washed, and UV irradiated before entering the market, so our research also aims to test the efficiency of these pretreatments on microplastics content. The possible sources of microplastics and its potential health risks are also discussed.
Materials and Methods
Sampling sites
We bought oysters (Crassostrea gigas) from twelve sites distributed in Japan (Table 2). The material was approximately two years old (personal communication from the seller). At least four oysters were sampled from each location. To avoid microplastic contamination, we kept oysters under frozen conditions wrapped in aluminum foil for further experiments. A total of 106 samples were studied. We also visually assessed the presence and quantity of fishing nets and lines, as well as other plastic garbage in beaches near the areas from where the studied material came.
| Sample location | Location | Number of specimens | Average weight per oyster without shell (g) |
|---|---|---|---|
| S1 | Northern island | 10 | 30.5 |
| S2 | Pacific Ocean | 20 | 35.19 |
| S3 | Northern island | 5 | 41.5 |
| S4 | Pacific Ocean | 5 | 24.39 |
| S5 | Pacific Ocean | 5 | 19.95 |
| S6 | Pacific Ocean | 4 | 49.68 |
| S7 | Sea of Japan | 12 | 31.33 |
| S8 | Pacific Ocean | 10 | 33.61 |
| S9 | Pacific Ocean | 10 | 21.44 |
| S10 | Inland island | 10 | 18.96 |
| S11 | Pacific Ocean | 5 | 23.87 |
| S12 | Southern island | 10 | 25.02 |
Table 2: Location and average weights of oysters (without the shell) from different coastal areas of Japan.
Methods
Microplastics were extracted from oysters’ following Chen’s procedure [33]. The shells were removed, and the body’s surface slightly washed. The body was then placed into a 10% KOH solution, and a water bath used to accelerate the digestion process. With the help of a vacuum pump the solution was filtered through filter paper (20 μm~25 μm) and this was fixed onto a flat, labelled plastic disk which was then placed into a labelled Petri dish. These were then sealed with sticky tape. A similar filtration procedure was applied to the dissected digestive tract of the oysters.
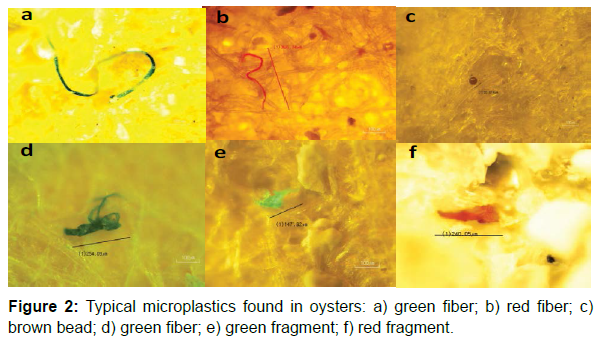
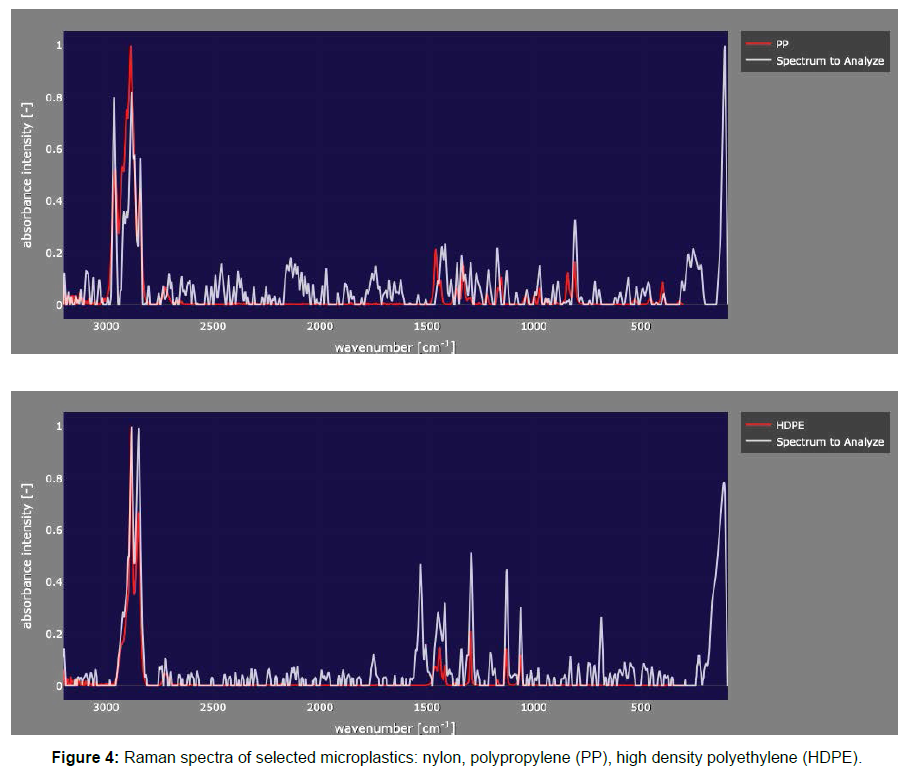
The dissected digestive and gill systems were observed through a digital microscope to preliminarily assess the presence of microplastics (Figure 1). This is a simple procedure that can be used to obtain images and evaluate the shapes and sizes of the microplastics. The color can also be used to determine the presence of microplastics. In addition, we used tweezers to assess if the microplastics fibers would break. Raman spectra (RENISHAW inVia) and optical microscopy (Nikon ECLIPSE 50i) were used to identify whether a particle was plastic or not. Raman microscopy combines Raman spectroscopy and optical microscopy. It is one of the most efficient and effective ways to identify plastics by focusing a laser beam onto a small spot to obtain a spectrum. Raman spectroscopy can specify different polymers by probing different vibrational modes in the molecule. Once a spectrum is acquired, the next critical step is to identify exactly which polymers make up the unknown sample. Having a complete, high quality spectral database is critical for carrying out accurate spectral matching. In our research, we used the software Open Specy. In this typical database, spectra of reference materials are recorded and stored with their chemical and physical properties in the sample record metadata. We uploaded the spectra and matching with the spectra from database. The software automatically displays the highest matching polymer spectra. The spectra obtained through Raman were loaded into the gallery software, Open Specy for analysis and identification of the plastic polymer type.
Results
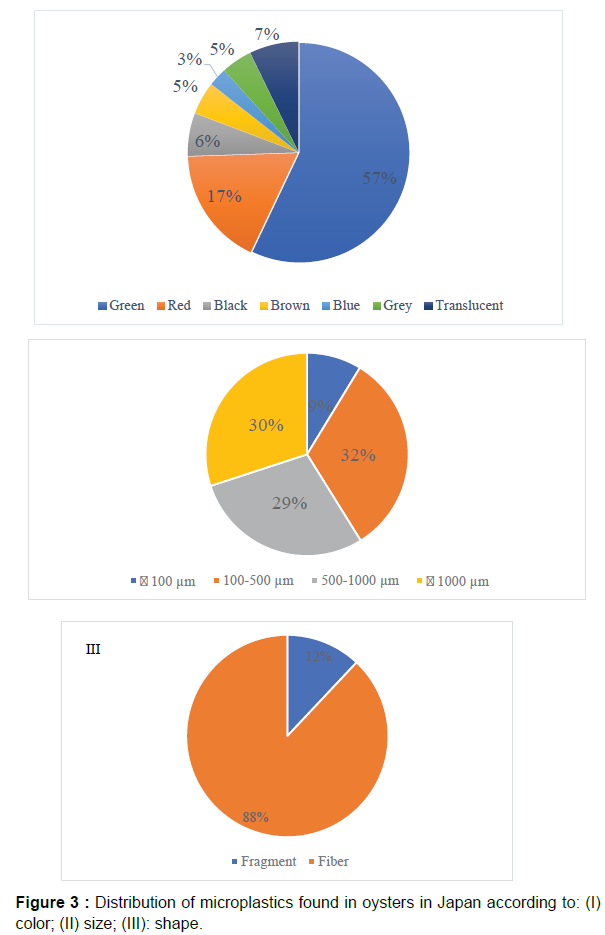
Microplastics were found in almost all oysters studied. Our results indicate that microplastics were widely detected in cultured oysters from Japan. Microplastics (fibers and fragments mainly) were found in almost all oysters, and showed differences in length, size, and color. A total of 333 microplastics pieces were collected from 106 oysters. The average number of microplastics per individual amounted to 3.14 ± 4.00 particles Although the oysters came from different places in Japan, in terms of average number of microplastics per oyster S4 material contains the highest value (up to 11.8 ± 8.5 items), followed by S5 (up to 7.8 ± 10.2 microplastics/oyster). The least microplastics per individual oyster come from for S2, S3 and S10 (1.5 ± 1.5, 1.4 ± 1.3, and 1.5 ± 1.0 respectively) (Table 1). The most common colors of typical fibers were red and green, whereas plastic fragments were mainly red and blue. Indeed, green particles constituted 57% of the total microplastics found, with red accounting for 17%. Much less microplastics were blue (3%) grey or brown (about 5% in both cases). From the microscopic images, we can see that some fibers formed clusters (Figure 2, d). Sometimes, the edges of the fragment are damaged (the degrade phenomenon) (Figure 2, e). Fiber was the most common type forming 88% of the total microplastics found. The rest were mostly fragments. The most abundant size (32%) found was 100 μ to 500 μ, followed by particles over 1000 μ (30%). Microplastics smaller than 100 μ was only 9% (Figure 3). The observation of dissected oyster gills and digestive systems confirmed the presence of microplastics in the gills of some oysters. The filtered material after the digestion of these organs also showed the presence of microplastic fibers (Figure 2). The analyses of the Raman spectra showed that the microplastics found in oyster samples are nylon, high density polyethylene and polypropylene
| Sample location | Number of specimens | Total number of microplastics per sample | Average number of microplastics per oyster |
|---|---|---|---|
| S1 | 10 | 24 | 2.4 ± 1.7 |
| S2 | 20 | 30 | 1.5 ± 1.5 |
| S3 | 5 | 7 | 1.4 ± 1.3 |
| S4 | 5 | 59 | 11.8 ± 8.5 |
| S5 | 5 | 39 | 7.8 ± 10.2 |
| S6 | 4 | 30 | 7.5 ± 5.9 |
| S7 | 12 | 33 | 2.8 ± 1.3 |
| S8 | 10 | 29 | 2.9 ± 1.7 |
| S9 | 10 | 19 | 1.9 ± 1.2 |
| S10 | 10 | 15 | 1.5 ± 1.0 |
| S11 | 5 | 12 | 2.4 ± 1.1 |
| S12 | 10 | 36 | 3.6 ± 2.2 |
| Totals | 106 | 333 | 3.14 ± 4.00 |
Table 1: Microplastics in oysters from various locations in Japan.
Discussion and conclusions
The most common type of microplastics found in this study were fibers (88%) which agrees with data from 17 coastal sites in China where fibers constitute 60.67% of all microplastics found [22]. It should be noted that our material showed less diversity in the shape of plastics found. Microplastics found in this study can be divided into fragments and fibers (12% and 88% respectively). Similarly, a study using oysters along the Pearl River Estuary in China indicated the dominance of fibers with a ratio of 69.4% and microplastic size in oysters (Saccostrea cucullata) shows 83.9% of which were less than 100 μm. On the other hand, our research results show only 9% smaller than 100 μm, whereas the fraction from 100 to 500 μm has the highest proportion, up to 32%. In another study from the French Atlantic coast, the largest fraction of the particles found in the blue mussel (Mytilus edulis) and the Pacific oyster (Crassostrea gigas) ranged from 50 to 100 μm, [39]. In Korea, four popular bivalve species, oyster (Crassostrea gigas), mussel (Mytilus edulis), Manila clam (Tapes philippinarum) and scallop (Patinopecten yessoensis) were evaluated finding mainly fragments and particles smaller than 300 μm (76% and 65% respectively) of the total microplastics found [40]. In our study, fibers were the main shape of found.
In this study, the average of microplastics per oyster was 3.14 items. This is less than what was found in Perna viridis (3.28 items/individual) in India (see Table 3 for a reference), but most abundant type found was fiber, same as in China. In both European and Asian literature, the size of microplastics found was smaller than that from Japanese oysters.
On the other hand, the quantity of microplastics in Japan oysters was higher, and their size larger than reported in other studies (Table 3). In terms of the type of microplastics identified using optical microscopy, most researchers use FT-IR spectroscopy and Raman spectroscopy, which gives confidence to our findings. Therefore, our data can be compared to that in other studies with high confidence.
| Species | Location | Digestion | Identification | Quantiity | Most common types |
|---|---|---|---|---|---|
| Mytilus Edulis | Germany | HNO3 69% VanCauwenberghe et al.,2014 |
Raman Spectroscopy | 0.36 ±0.07 items/g (Without depuration) | Fiber and particle |
| Crassostrea gigas | France | HNO3 69 % Van Cauwenberghe et al.,2014 |
Raman Spectroscopy | 0.47 ±0.16 items/g (Without depuration) | Fiber and particle |
| Perna Viridis Meretrix meretrix | India | 10% KOH Dowarah et.al., 2020 |
Flourescense Microscope Raman Spectroscopy | 3.28 ±0.87 items / individual 3.28 ±0.87 items / individual |
Fragment Fragment |
| Mytilusgalloprovincialis Scrobicularia Plana Marphysa sanguinea Trachurus trachurus Scomber colias | Portugal | 10% KOH Pequeno et al., 2021 |
FT- IR Spectroscopy | 0.45 ±0.67 items / individual 0.30 ±0.63 items / individual 0.40 ±0.88 items / individual 2.24 ±2.05 items / individual 2.46 ±4.12 items / individual |
Fiber Fiber Fragment Fiber Fiber |
| Crassostrea gigas Crassostrea angulate Crassostrea Hongkongensis Crassostrea Sikamea | China | 10% (m/v)KOH+30% H2O2 Teng et al.,2019 |
FT- IR Spectroscopy | 2.93 items / individual |
Fiber |
Table 3: Studies showing microplastic contamination of marine life: location, type of digestion, identification method of microplastics, quantity, abundant type with the corresponding references.
During our collecting trips, we observed that farmed oysters were cleaned and irradiated with ultraviolet rays before being sold. This procedure aims to reduce or eliminate possible microbial contamination of food. Although we also cleaned them (using Milli-Q water to rinse both the shell and oysters` body) before the digestion procedure, we still found microplastics. We can conclude that all the cleaning methods done before the oysters are put on the market cannot remove the microplastics completely.
One of the problems we face in this type of studies concerns the source of the found microplastics. During our collecting trips we observed a large amount of domestic garbage stranded on beaches (Figure 5). Note that the dates in some plastic packages were relatively recent. In addition, many fishing gills and nets, as well as cages used in aquaculture lay on the beaches. These are all made up of nylon. From table 1, we see that the average number of microplastics in S4, S5, and S6 is higher than in other sites. These sites are located in the eastern Pacific coast, so it is possible that the pollutants’ origin is both domestic garbage and that from neighboring countries from where it is carried with ocean currents. Regarding S2, it is located in a relatively closed bay. This is an aquaculture area as well, and local fishermen are more likely to pay attention to keeping the water-body healthy. Indeed, in our interviews, we learned that the fisheries salvage garbage regularly.
One research characterized microplastics in the heavily urbanized, brackish water of Vembanad Lake (India), focusing on some commercially important bottom-feeding fishes and shellfish (Arius maculatus, Etroplus suratensis, E. maculatus and Villorita sp.). Its results also showed the presence of polyethylene in the samples [41]. Polypropylene and nylon 6 were also reported in south India [42]. Similarly, the main polymers in shellfish from Dongshan Bay, southeastern China, were polyester and Polyethylene terephthalate [43]. The abundance and types of microplastics found exhibited great variation among species. In fact, a wide variety of microplastics is present in marine organisms due to their durability and wide range of applications in fishing activities as well as in packing and textile industries.
Shellfish consumed as food may carry microplastics that will affect human health. As they are often consumed whole, the accumulation process in the consumers is a real possibility. Furthermore, plastics may have heavy metals and other toxic coatings that will also carry on to the final consumer. These concerns lead us to recommend more studies of the possible pathologic effects of microplastics ingested with shellfish, to better understand their impacts in public health.
Acknowledgement
This study was financed by Hokkaido University DX Doctoral Fellowship. The author thanks the local fishmen cooperation. Prof. T. Kawaguchi suggestions for the sample collection process were very important, as well as by providing instruments for analysis of microplastics.
References
- Benjamin SH, Shaun W, Kimberly AS, Carrie VK, Fiorenza M, et al. (2008) A global map of human impact on marine ecosystems. Science 319: 9478-952.
- Lebreton L, Anthony A (2019) Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 5: 1-11.
- Carpenter, EJ, Smith JKL (1972) Plastics on the Sargasso Sea surface. Sci 175: 1240-1241.
- Brems A, Baeyens J, Dewil R (2012) Recycling and recovery of post-consumer plastic solid waste in a European Context. Therm Sci 16: 669-685.
- Haribowo R, Yoshimura M, Sekine M, Imai T, Yamamoto K, et al. (2017) Behavior of toxicity in river basins dominated by residential areas. Contemp Eng Sci 10: 305-315.
- Plastics Europe (2013) Plastics – the facts 2013. An analysis of European latest plastics production, demand, and waste data.
- Riya P, Chatterjee DP, Dutta K (2016) Concerns regarding “plastic” pollution: Reasons, effects, and needs to generate public awareness. Int J Humanit Soc Sci Stud 3: 123-148.
- Boucher J, Friot D (2017) Primary microplastics in the oceans: a global evaluation of sources. Environ Sci 180.
- Sebille EV, Spathi C, Gilbert A (2016) The ocean plastic pollution challenge: towards solutions in the UK. Grant Brief Pap 19: 1-16.
- Hardesty BD, Good TP, Wilcox C (2015) Novel methods, new results, and science-based solutions to tackle marine debris impacts on wildlife. Ocean Coast. Manag. 115: 4-9.
- HahladakisJN, Costas AV, Roland W, Eleni I, Phil P (2017) An overview of chemical additives presents in plastics: Migration, release, fate and environmental impact during their use, disposal, and recycling. J Hazard Mater 344: 179-199.
- Zettler ER, Tracy JM, Linda AAZ (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47: 7137-7146.
- Koelmans AA, Bakir A, Burton GA, Janssen CR (2016) Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 50: 3315-3326.
- Moore CJ (2008) Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ Res 108: 131-139.
- Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44: 842-852.
- Sivan A (2011) New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 22: 422-426. .
- Lusher Tanaka K, Takada H (2016) Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci Rep 6: 34351.
- Andrady AL (2011) Microplastics in the marine environment. Mar. Pollut. Bull 62.8,(1596-1605).
- Barboza LGA, Vethaak AD, Lavorante B, Lundebye A, Guilhermino L (2018) Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 133, 336-348.
- Danopoulos E, Twiddy M, Rotchell JM (2020) Microplastic contamination of drinking water: A systematic review. PLoS One. 15: e0236838.
- Zhang N, Li YB, He HR, Zhang JF, Ma GS (2021) You are what you eat: Microplastics in the feces of young men living in Beijing. Sci Total Environ. 767: 144345.
- Teng J, Wang Q, Ran W, Wu D, Liu Y, et al. (2019) Microplastic in cultured oysters from different coastal areas of China. Sci Total Environ 653: 1282-1292.
- Velzeboer I, Kwadijk CJAF, Koelmans AA (2014) Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 48: 4869-4876.
- Scott JW, Gunderson KG, Green LA, Rediske RR, Steinman AD (2021) Perfluoroalkylated substances (PFAS) associated with microplastics in a lake environment. Toxics9: 106.
- Carbery M, Connor OW, Palanisami T (2018) Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Intern.115: 400-409.
- Frere L, Paul PI, Rinnert E, Petton S, Jaffre J, et al. (2017) Influence of environmental and anthropogenic. Factors on the composition, concentration, and spatial distribution of microplastics: a case study of the Bay of Brest (Brittany, France). Environ Pollut 225: 211-222.
- Phuong NN, Poirier L, Pham QT, Lagarde F, Zalouk V (2018) Factors influencing the microplastic contamination of. bivalves from the French Atlantic coast: location, season and/or mode of life?. Mar Pollut Bull 129: 664-674.
- Kataoka T, Nihei Y, Kudou K, Hinata H (2019) Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 244: 958-965.
- Ripken C, Kotsifaki DG, Chormaic SN (2021) Analysis of small microplastics in coastal surface water samples of the subtropical island of Okinawa, Japan. Sci Total Environ 760: 143927.
- Jahan S, Strezov V, Weldekidan H, Kumar R, Kan T, et al. (2019) Interrelationship of microplastic pollution in sediments and oysters in a seaport environment of the eastern coast of Australia.Sci. Total Environ.695: 133924.
- Scanes E, Wood H, Ross P (2019) Microplastics detected in haemolymph of the Sydney rock oyster Saccostrea glomerata. Mar Pollut Bull 149: 110537.
- Thomas M, Jon B, Craig S, Edward R, Ruth H, et al. (2019) The world is your oyster: low-dose, long-term microplastic exposure of juvenile oysters. Heliyon 6: e03103.
- Chen JYS, Lee YC, Walther BA (2020) Microplastic contamination of three commonly consumed seafood species from Taiwan: A pilot study. Sustainability 12.22, 9543.
- Cho Y, Shim WJ, Jang M, Han GM, Hong SH (2019) Abundance and characteristics of microplastics in market bivalves from South Korea. nviron Pollut 245: 1107-1116.
- McIlgorm A, Campbell HF, Rule MJ (2011) The economic cost and control of marine debris damage in the Asia-Pacific region. Ocean Coast. Manag. 54: 643-651.
- Iritani N, Kaida A, Abe N, Kubo H, Sekiguchi JI, et al. (2014) Detection and genetic characterization of human enteric viruses in oyster‐associated gastroenteritis outbreaks between 2001 and 2012 in Osaka City, Japan. J Med Virol. 86.12, 2019-2025.
- Nishida T, Kimura H, Saitoh M, Shinohara M, Kato M, et al. (2003) Detection, quantitation, and phylogenetic analysis of noroviruses in Japanese oysters. Appl. Environ. Microbiol. 69: 5782-5786.
- Ueki Y, Amarasiri M, Kamio S, Sakagami A, Ito H, et al. (2021) Human norovirus disease burden of consuming Crassostrea gigas oysters: A case study from Japan. Food Control. 121, 107556.
- Phuong NN, Poirier L, Pham QT, Lagarde F, Zalouk V (2018) Factors influencing the microplastic contamination of. bivalves from the French Atlantic coast: location, season and/or mode of life?. Mar Pollut Bull 129: 664-674.
- Cho Y, Shim WJ, Jang M, Han GM, Hong SH (2019) Abundance and characteristics of microplastics in market bivalves from South Korea. nviron Pollut 245: 1107-1116.
- Nikki R, Jaleel K UA, Ragesh S, Shini S, Saha M, et al. (2021) Abundance and characteristics of microplastics in commercially important bottom dwelling finfishes and shellfish of the Vembanad Lake, India Mar Pollut Bull 172: 112803.
- Devi SS, Sreedevi AV, Kumar AB (2020) First report of microplastic ingestion by the alien fish Pirapitinga (Piaractus brachypomus) in the Ramsar site Vembanad Lake, south India. Mar Pollut Bull 160: 111637.
- Pan Z, Liu Q, Xu J, Li W, Lin H (2022) Microplastic contamination in seafood from Dongshan Bay in southeastern China and its health risk implication for human consumption. Environ Pollut 303: 119163.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Yang L, Nakaoka M, Matsuishi T, Kawaguchi T, Fortunato H (2022) Microplastic In Cultured Oysters from Different Coastal Areas of Japan. J Marine Sci Res Dev 12: 360. DOI: 10.4172/2155-9910.1000360
Copyright: © 2022 Yang L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3648
- [From(publication date): 0-2022 - Oct 29, 2025]
- Breakdown by view type
- HTML page views: 3195
- PDF downloads: 453