Review Article Open Access
Microinflammation as a Candidate for Diabetic Nephropathy
Amal Abd El hafez*Pathology Department, Faculty of Medicine, Mansoura University, Egypt
- *Corresponding Author:
- Dr. Amal Abd El hafez
Sultan Bin Abdulaziz Humanitarian City
Riyadh, 11536, P.O. box 64399, Kingdom of Saudi Arabia
Tel: +966557662665
E-mail: amalabdelhafez@gmail.com
Received date: May 30, 2014; Accepted date: June 05, 2014; Published date: June 07, 2014
Citation: El hafez AA (2014) Microinflammation as a Candidate for Diabetic Nephropathy. Microinflammation 1:101. doi: 10.4172/2381-8727.1000101
Copyright: © 2014 El hafez AA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
Diabetic Nephropathy (DN) is a major cause of mortality in patients with Type 1 and 2 diabetes throughout the world. This review draws attention to the important role of microinflammation and the complex pathways implicated in the development and progression of DN. These pathways include the collaboration of metabolic, hemodynamic and hormonal factors with oxidative stress in patients with genetic susceptibility to create an inflammatory milieu. The key role of inflammatory cells in the kidney, particularly infiltrating macrophages and T-lymphocytes is highlighted. The major inflammatory cytokines and chemokines, receptors, adhesion molecules as well as transcription factors and transduction pathways involved in the pathogenesis of DN are also discussed. Understanding of these inflammatory pathways guides important therapeutic appliances and improves the discovery of new therapeutic targets that can be translated into clinical treatments for DN.
Keywords
Diabetic nephropathy; Microinflammation; Pathways; Cytokines; Macrophage; Lymphocyte; Therapeutic
Abbreviations:
AGE: Advanced Glycation End-Product; ACE: Angiotensin-Converting Enzyme; ADIPORs: Adiponectin Receptors; AP1: Activator Protein 1; cAMP/PKA: cAMP-dependent Protein Kinase; CCL2: CC Chemokine Ligand 2 also known as MCP-1 (monocyte chemoattractant protein-1); CCL5: RANTES; CCR chemokine receptor 2; CD: Cluster Differentiation; CREB: cAMP-response-element-binding Protein; CSF: Colony Stimulating Factor; CXCL: CXC Chemokine Ligand; CX3CL1: CX3C Chemokine Ligand 1; CX3CR1: CX3C Chemokine Receptor 1; DN: Diabetic Nephropathy; DM: Diabetus Mellitus; ECM: Extracellular Matrix; eNOS: Endothelial Nitric Oxide Synthetase; ESRD: End-Stage Renal Disease; GLUT: Glucose Transporter; ICAM1: Intercellular Adhesion Molecule 1; IFNγ : Interferon γ ; IL: Interleukin; ILR: Interleukin Receptor; JNK: c-Jun N-terminal Kinase; JAK2: Janus Kinase 2; LDL: Low-Density Lipoproteins; LFA-1: Lymphocyte Function Associated Antigen-1; MAPK: Mitogen Activated Protein Kinase; MCP-1: Macrophage Chemoattractant Protein-1; MIP-1α/CCL3: Macrophage Inflammatory Protein-1α; MMP: Matrix Metalloproteinase; MTHFR: Methylenetetrahydrofolate Reductase; MyD88: Myeloid Differentiation Factor 88; NFAT: Nuclear Factor Of Activated T-cells; NF-κB: Nuclear Factor κB; PAI-1: Plasminogen Activator Inhibitor-1; PDGF: Platelet Derived Growth Factor; PI3K: Phosphoinositide 3-Kinase; PKC: Protein Kinase C; RAGE: Receptor for Age; ROS: Reactive Oxygen Species; S100A8: S100 calcium binding protein A8; SAPK-2: Stress-Activated Protein Kinase-2; Sp1: Stimulating Protein 1; Sp-1:Specificity Protein-1; STAT: Signal Transducer and Activator of Transcription; TGF: Transforming Growth Factor; Th: T helper; TIR: Toll/IL-1 receptor; TLR: Toll-like receptor; TNF: Tumor Necrosis Factor; VCAM1: Vascular Cell Adhesion Molecule 1; VEGF: Vascular Endothelial Growth Factor
Introduction
Diabetes mellitus (DM) and its complications have become a public health problem [1]. Diabetic nephropathy (DN) is a major cause of mortality in patients with Type 1 and Type 2 diabetes throughout the world [2] and between 20% and 40% of diabetic patients ultimately develop nephropathy [3]. In human glomeruli, glomerular hypertrophy, expansion of diffuse mesangial matrices, exudative lesions, segmental nodular sclerosis (thickening of the glomerular basement membrane) and/or podocyte loss with compensatory expansion of the remaining podocyte foot processes are the main pathological features of diabetic nephropathy which direct to the ultimate development of glomerulosclerosis, tubulointerstitial fibrosis, impairment of renal function and progression to end-stage renal disease (ESRD) [3,4].
The recent thought of microinflammation as a candidate for DN
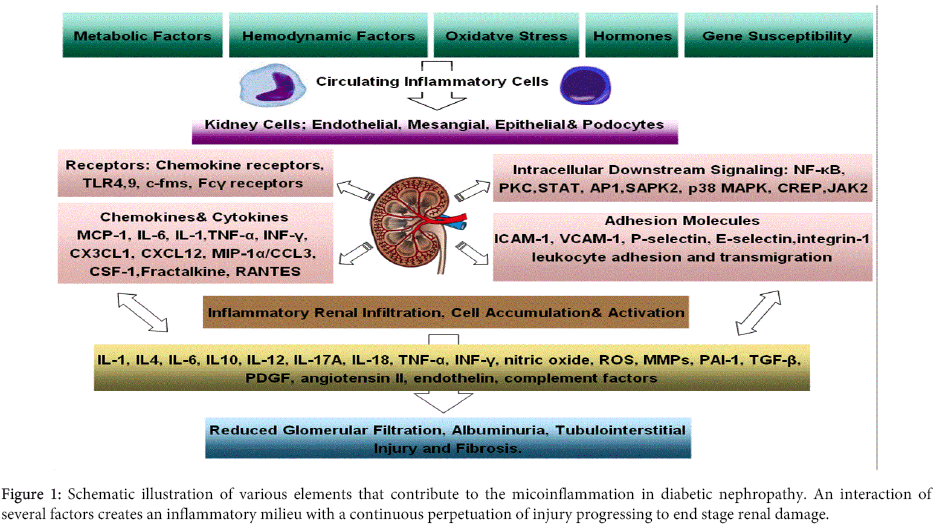
Traditionally, multiple mechanisms were accredited to contribute to the development and outcomes of DN, such as an interaction between metabolic and hemodynamic factors, oxidative stress, hormonal factors, adipokines and genetic susceptibility which sets a continuous perpetuation for kidney injury [5-7]. However, new perspectives in activated innate immunity and inflammation appear to be important factors in the pathogenesis of DM and its associated complications. Hence, recent studies disclosed that these conventional mechanisms are only partially responsible for the development and/or progression of DN [8], and that low-grade or subclinical inflammation, termed ‘’microinflammation’’, plays a vital role in the pathogenesis of this diabetic complication [2,8-10]. The relationships between microinflammation and the pre-existing traditional factors during the development and progression of DN involve complex pathways with hyperglycaemia laying at upstream and microinflammation and subsequent evolution of DN representing downstream of these pathways addressed here in brief (Figure 1).
Metabolic Factors
Chronic hyperglycemia has long been implicated as a major contributor to several diabetic complications through three major mechanisms: non-enzymatic glycosylation that generates Advanced Glycosylation End (AGE) products, activation of Protein Kinase C (PKC), and acceleration of the aldose reductase (polyol) pathway. Oxidative stress seems to be a theme common to all three mechanisms [11]. Mesangial cell expansion, increased mesangial cell matrix production and mesangial cell apoptosis seem to be mediated in part by an increase in the mesangial cell glucose concentration, since similar effects can be induced in a normal glucose environment by overexpression of glucose transporters, such as GLUT1 and GLUT4, thereby increasing glucose entry into the cells [12,13]. Besides, hyperglycemia induces mesangial fibrosis that requires activation of interleukin (IL)-8. As a contributor in renal inflammation, high glucose promotes mesangial production of macrophage chemoattractant protein-1 (MCP-1 also known as CCL2, CC chemokine ligand 2), IL-6, and tumor necrosis factor (TNF)-α, which, together with adhesion molecules, favor leukocyte recruitment and adhesion to endothelial cells [14,15].
Furthermore, AGE products and AGE-modified proteins may bind to leukocytes, stimulating the synthesis and release of proinflammatory cytokines in DN [16]. Hyperglycemia might also upregulate Vascular Endothelial Growth Factor (VEGF) expression in podocytes [17], which could markedly increase vascular permeability [18].
The mechanism by which hyperglycemia leads to PKC activation involves de novo formation of diacylglycerol, oxidative stress and induction of the activity of Mitogen-Activated Protein Kinases (MAPK) in response to extracellular stimuli [19]. The subsequent events for this intracellular signaling in glomerular endothelial cells include endothelial dysfunction, inflammation and microvascular thrombosis [14,15].
Additionally, hyperlipidaemia represents another independent metabolic risk factor for the progression of DN. Its molecular mechanism involves toll-like receptor 4 (TLR4) interaction with its potent ligand S100 calcium binding protein A8 (calgranulin A; S100A8), in macrophages infiltrating the glomeruli of DN patients. Also, activation of S100A8/TLR4 signaling was elucidated in an animal model of diabetic glomerular injury accompanied with hyperlipidaemia, which may provide a novel therapeutic target in progressive diabetic nephropathy [20].
Hemodynamic Factors
It is highly probable that hemodynamic factors in DN may trigger the inflammatory responses and cytokine production [21]. Hemodynamic factors imply the activation of various vasoactive hormone systems, such as the renin-angiotensin-aldosterone and endothelin systems. In response, the secretion of profibrotic cytokines, such as transforming growth factor β1 (TGF-β1) is increased and additional hemodynamic alterations ensue, such as increased systemic and intraglomerular pressure. The increased intraglomerular pressure entails decreased resistance in the afferent and-to a lesser extent-in the efferent arterioles of the glomerului predisposing to glomerular hyperperfusion. Many other factors have been reported to be involved in this defective autoregulation, including prostanoids, nitric oxide and VEGF. These early hemodynamic changes facilitate albumin leakage from the glomerular capillaries and overproduction of mesangial cell matrix, as well as thickening of the glomerular basement membrane and podocyte injury [5,17].
Oxidative Stress
Accumulating evidence suggests that oxidative stress plays a central part in the pathogenesis of DN [22]. For the source of oxidative stress, vascular NADPH oxidase, uncoupled endothelial Nitric Oxide Synthetase (eNOS) and mitochondria were the major candidates [23]. High glucose induces intracellular Reactive Oxygen Species (ROS) directly via glucose metabolism and auto-oxidation and indirectly through the formation of AGEs and their receptor binding [24]. ROS mediate many negative biological effects in DM, including peroxidation of cell membrane lipids, oxidation of proteins, renal vasoconstriction and damage to DNA. The metabolism of glucose through harmful alternate pathways, such as via PKC activation and AGE formation, in the setting of hyperglycemia also seems partly dependent on ROS [6]. In addition, ROS upregulates TGF-β1, PAI-1(plasminogen activator inhibitor-1) and extracellular matrix proteins (ECM) by glomerular mesangial cells, thus leading to mesangial expansion. Also, ROS activate other signaling molecules, such as PKC and MAPKs, and transcription factors, such as nuclear factor (NF)-κB, AP-1 (Activator Protein-1), and Sp-1 (Specificity Protein-1), leading to transcription of genes encoding cytokines, growth factors and ECM proteins [24].
Hormonal Factors and Adipokines
Formerly, increased plasma pro-renin activity was noted as a risk factor for the development of DN. Pro-renin binds to a specific tissue receptor that promotes activation of MAPK [7]. Also, activated renin-angiotensin-aldosterone system has been proven to be a crucial determinant of leukocyte activation and cytokine expression in generating proinflammatory and proliferative effects [25].
Currently, various adipocyte-secreted factors and hormones termed adipokines including adiponectin, leptin, resistin, visfatin, chemerin and vaspin have been identified and they may link the metabolic abnormalities and microinflammation in Type 2 DM. An increase in leptin and resistin or a reduction in adiponectin activity would be potential participants in diabetes pathology [1].
The most well-known member of this family is ‘’leptin’’ which exert several pro-inflammatory effects. It also impairs endothelial cell functions, stimulates the proliferation of glomerular endothelial cells, increases TGF-β1 synthesis and collagen type IV production and up-regulates surface TGF-β type II receptors through signal transduction pathways involving PI3K (phosphoinositide 3-kinase) [17]. In human settings, several reports demonstrated that serum leptin levels correlated with proteinuria in Type 2 DM [26]; however, others reported no association of serum leptin levels and the presence of DN [27]. Leptin also stimulates hypertrophy, but not proliferation of cultured rat mesangial cells, and infusion of leptin for 3 weeks into normal rats promotes the development of glomerulosclerosis and proteinuria [17].
On the contrary, adiponectin has shown differential roles in the various stages of diabetic nephropathy. At early stages of DN, adiponectin suppresses the activation of NF-κB, TNF-induced monocyte adhesion to aortic endothelial cells and the expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM-1) and selectins in animal models. In addition, ADIPORs (adiponectin receptors) coupled with intracellular signaling pathways involving AMPK and cAMP/PKA (cAMP-dependent protein kinase) have been implicated in the function of endothelial cells and inflammatory cells. Moreover, adiponectin is implicated in the biology of podocytes and adiponectin-knockout mice exhibit increased albuminuria and fusion of podocyte foot processes, and therapeutic infusion of adiponectin increases the activity of MAPK, reduces oxidative stress and reverses the albuminuria in these mice. Nonetheless, in overt DN with macroalbuminuria or renal insufficiency, serum adiponectin level was found to be increased and positively correlated with the degree of insulin resistance in Type 2 DM [28].
Genetic Susceptibility
Familial clustering of diabetic nephropathy was also reported in both Type 1 and Type 2 DM, strongly suggesting the association of genetic factors with DN. Several candidate genes, such as those for the renin-angiotensin system, genes for glucose and lipid metabolism, genes for the PCK system and inflammatory cytokine genes (IL-1, IL-6, IL-18, TNF-α and MCP-1) might be candidate genes for conferring susceptibility to DN [8,29]. In addition there is an evidence for the association of angiotensin-converting enzyme (ACE) gene I/D polymorphism and the methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism with development of DN in Type 2 DM [30].
Macrophage/Lymphocyte sharing & interaction
The recruitment of leukocytes is a key event in DN. Increased infiltration of immune cells including monocytes/macrophages, T-cells, B cells and masT-cells into the kidney, as well as augmented expressions of inflammatory cytokines, chemokines, adhesion molecules, receptors and transcription factors which modulate the local inflammatory response in the kidneys, have been reported in patients with DN [1,31-35].
It is generally accepted that chronic hyperglycemia results in endothelial dysfunction and microvascular thrombosis in the glomerular vessels and leads to mesangial expansion and glomerulosclerosis. Concurrently, hyperglycemia hastens renal inflammatory monocyte/macrophage infiltration and adhesion provoked by the vascular pathological environment and by increasing proinflammatory cytokine secretion from renal cells [14,15].
Accumulation and activation of monocytes/macrophages has been demonstrated in renal biopsies in both experimental diabetes and patients with diabetic nephropathy being aggravated with the duration of diabetes and the severity of renal injury [36-38]. Disturbance in proinflammatory CD14(+)CD16(+) monocytes was noted in Type 2DM and DN ureamic patients. Such immunological dysfunction may be related to the activation of TLR4/NF-κB and STAT5 (signal transducer and activator of transcription) signaling pathways [2].
The interaction of monocytes/macrophages with mesangial cells drives monocytes/macrophages to migrate from the circulation to the kidney in the early stages of DN. Increased renal expression of MCP-1 is considered to be important in the initiation of this process. In addition to MCP-1, IL-6, TNF-α and a variety of other chemokines produced by mesangial cells promote leukocyte recruitment and adhesion to endothelial cells [15]. This adhesion is mediated through mesangial activation of ICAM-1, VCAM-1, E-selectin, P-selectin and integrin-1, with the resulting attachment of inflammatory cells to vascular endothelial cells and their infiltration into both the glomeruli and the interstitium [38]. Consequently, the infiltrating monocytes/macrophages may induce or accelerate the mesangial cell proliferation, glomerulosclerosis and injury in diabetic kidneys [14,15].
As an organ-specific autoimmune disease both activation of the T-cell mediated immune system leading to insulitis and humoral B cell response producing immunoglobulins against beta cell autoantigens participate in the pathogenesis of Type 1 DM [39]. Developing a more aggressive T-cell phenotype and changing the balance between CD4+Th (T-helper) 1 cells and Th2 cells to confer a more proinflammatory milieu (Th1 dominant) may be associated with the progression towards overt diabetes. Furthermore, evidence demonstrating the association of the CD4+Th17 and Tregs subsets with pathogenesis of Type 1 DM is rapidly accumulating [40,41]. By contrast, adipose tissue inflammation is now recognized as a crucial process leading to the metabolic syndrome, insulin resistance and Type 2 DM. Akin to macrophage, T-cell infiltration of adipose tissue has been described in Type 2 DM [42,43], and the interaction between T lymphocytes with macrophages can regulate the inflammatory cascade in this disease [44]. Moreover, CD8+T-cells play essential roles in the initiation and maintenance of adipose tissue inflammation and systemic insulin resistance [45].
As T-cells express LFA-1 (lymphocyte function associated antigen-1; the receptor for ICAM-1), and as ICAM-1 expression is found on renal endothelial, epithelial, and mesangial cells, it is likely that this interaction plays a significant role in T-cell migration into the diabetic kidney [46]. Within the kidney, activated T-cells can cause injury directly through cytotoxic effects and indirectly by recruiting and activating macrophages. Proinflammatory cytokines secreted by T (CD4+,CD8+) cells could activate neighboring macrophages directly or indirectly by stimulating mesangial cell production of Colony Stimulating Factor-1 (CSF) and MCP-1. Once macrophages have activated, they can release nitric oxide, ROS, IL-1, TNF-α, complement factors, and Metalloproteinases (MMPs), all of which promote renal injury [47]. Besides, T-cells express the receptor for AGEs and the activation of CD4+ and CD8+ T-cells by AGE can initiate Interferon (IFN)-γ secretion by T-cells, which could induce further inflammation and oxidative stress within the diabetic kidney [48]. Although, CD8+ cells may perform a cytolytic function in the diabetic kidney [49], the function of CD8+ T-cells, however, becomes more significant at later stages of the disease when tissue loss is evident [50].
The infiltration of Th1 cells in the glomeruli in patients with Type 1 DM was closely related with elevated levels of ICAM1, P-selectin and IFNγ [46]. However, in Type 2 DM, little is known about the mechanism of Th1 activation, although increased serum levels of IFNγ and IL-2R (IL-2 receptor) have been reported in this disease [35]. Meanwhile, Th2 cells producing IL-4 and IL-10, can contribute to suppress Th1 cell activation as IL-10 exerts anti-inflammatory and immunosuppressive effects. Th17 is a distinct subset of helper T-cells which is critically involved in the pathogenesis of autoimmune diseases such as rheumatoid arthritis. Therefore, some studies have suggested that Th17 cells promote inflammation through elevated IFNγ and IL-17A in human Type 1 and 2 DM [51,52].
The Intrinsic Renal Cells
The intrinsic renal cells (endothelial, mesangial, podocyes, glomerular, and tubular epithelial cells) are able to synthesize many proinflammatory cytokines [53]. At high glucose levels, podocytes are considered the major sources of IL-1α and IL-1β, and they may also produce MCP-1 [54]. Elements of the diabetic milieu such as high glucose and advanced glycation end products (AGEs) act as potent stimulators of renal cells to elaborate chemokines. In addition, proinflammatory cytokines produced by leukocytes such as IL-1, TNF-α and INF-γ can induce the intrinsic renal cells to produce a spectrum of chemokines. These chemokines include: IL-8 (CXCL8), MCP-1, INF-γ inducible protein (CXCL10), macrophage inflammatory protein-1α (MIP-1α/CCL3), and RANTES (CCL5). The elaborated chemokines then further direct the migration of additional leukocytes into the kidney and set up an inflammatory cycle [48].
Cytokines and Chemokines
A described earlier, activated macrophages, lymphocyes as well as the intrinsic renal cells elaborate a host of proinflammatory, profibrotic, chemotactic and antiangiogenic factors which contribute to the progression of renal injury either directly or indirectly [32]. In the context of hyperglycemia, NF-κB is activated through PKC and ROS to rapidly stimulate the expression of several cytokines [55]. Furthermore, AGE products and AGE-modified proteins can bind to the receptor for AGE on macrophages and T-cells, stimulating synthesis and release of proinflammatory cytokines in DM [16]. The increase in the systemic and/or renal tissue expressions of these cytokines was reported to correlate with the severity of DN or with urinary albumin excretion [8].
Inflammatory cytokines including-but not limited to-TGF-β, TNF-α, IL-1, IL-6, IL10, IL12, IL-18, PAI-1, MMPs, platelet-derived growth factor (PDGF), angiotensin II and endothelin are critically involved in pathogenesis of DN [32,49]. For example, increased secretion of TGF-β by peripheral blood mononuclear cells has been reported in patients with DN and seems to be responsible for fibrogenic and proliferative effects on renal fibroblasts [56,57]. It is also a crucial pleiotropic cytokine associated with the development of Tregs and Th17 cells [58].
Monocytes and macrophages are the primary source of TNF-α, although intrinsic renal cells are also able to synthesize this cytokine. Moreover, it has been shown that increased urinary TNF-α excretion, as well as increased TNF-α levels in renal interstitial fluid, precede the significant increase in albuminuria [59]. TNF-α significantly contributes to sodium retention and renal hypertrophy, characteristic alterations during the early stages of DN, whereas exposure of tubular epithelial cells to TNF-α significantly increases the synthesis and secretion of lymphocyte and neutrophil chemoattractant factors, as well as the cell-surface expression of ICAM-1 [59]. Finally, TNF-α, independent from haemodynamic factors or effects of recruited inflammatory cells, promotes the local generation of ROS, with alterations in the barrier function of the glomerular capillary wall resulting in enhanced albumin permeability [1]. Similarly, it has been demonstrated that IL-1 increases vascular endothelial permeability, and it is involved in the proliferation of mesangial cells and matrix synthesis, as well as in the development of intraglomerular hemodynamic abnormalities related to prostaglandin synthesis [60].
It is likely that IL-6 affects ECM dynamics at the mesangial cell and podocyte levels, contributing to both mesangial expansion and glomerular basement membrane thickening. Renal IL-6 expression has been related to mesangial proliferation, tubular atrophy and the intensity of interstitial infiltrates in animal models of renal disease [61]. In addition, elevated IL-10 levels were observed in the sera of the patients with diabetic nephropathy, and a positive correlation of IL-10 and albuminuria was found [16,32].
High-glucose concentrations and AGE may induce macrophage production of IL-12, which can stimulate CD4+ cell production of IFN-γ and augments natural killer cell activity [62,63]. Likewise, IFN-γ secretion by T-cells can initiate and further accelerates inflammation by the activation of macrophages and vascular cells and exacerbation of oxidative stress within renal tissues [16,32]. Besides, IL-18 levels increase in diabetic patients with the development of urinary albumin excretion [64]. So, elevated urinary excretion levels of IL-18 reported in patients with diabetic nephropathy seems to be closely related to the progression of diabetic nephropathy [63].
Chemotactic cytokines are also major factors that induce the recruitment of inflammatory cells into the kidney, subsequently amplifying the immune-mediated damage [65]. Studies suggest that renal MCP-1 is involved in the direction of macrophage migration into the diabetic kidney through interaction with its chemokine receptor (CCR)-2 on macrophages [66]. MCP-1 is upregulated in patients with DN and its expression levels correlate with the number of infiltrating interstitial macrophages [67]. In addition, up-regulation of kidney MCP-1 has been shown as a feature of human diabetic renal injury associated urinary albumin excretion, tubulointerstitial injury and disease progression; meanwhile proteinuria itself may contribute to the upregulation of MCP-1 in DN [66].
Moreover, constitutive chemotactic RANTES expression directs subset-specific homing of CD4+ T-cells in the kidney [68]. CXCL12 (CXC chemokine ligand12) is produced by podocytes, contributing to podocyte loss [69]. CX3CL1 (CX3C chemokine ligand 1; also known as fractalkine) exists in two forms as a membrane-anchored or as a shed 95 kDa glycoprotein. The soluble CX3CL1 has potent chemoattractant activity for T-cells and monocytes and induces adhesion between activated endothelial cells, which express its receptor CX3CR1 (CX3C chemokine receptor 1) [63].
Adhesion Molecules
In the patients with DN, soluble forms of VCAM1 and ICAM, P and E-selectin are elevated during the progression from microalbuminuria to overt nephropathy [9,70].
Increased expression of ICAM-1, which serves as a ligand for LFA-1 on monocytes and lymphocytes, was detected in animal models of in Type 1 and 2 DM [71]. It has been shown that patients with both Type 1 and 2 DM complicated with DN have elevated concentrations of ICAM-1 compared with subjects without renal injury, suggesting that this molecule can be of pathogenic importance for the development of renal damage [70]. ICAM-1 expression found on renal endothelial, epithelial, and mesangial cells, plays a significant role in facilitating leukocyte adhesion, transmigration and activation within the kidney [71,72]. Previous studies demonstrated that mice deficient in ICAM-1 have defects in macrophages and leukocytes homing into renal tissues, resulting in substantial reduction of renal injury [73].
In addition, cross-sectional clinical studies have shown an elevation of circulating VCAM-1, P and E-selectin levels in patients with DN, which may result from underlying systemic endothelial dysfunction, increased production in damaged renal tubular or glomerular epithelial cells and/or decreased renal clearance of this molecule, depending on the stage of nephropathy [74]. More importantly, clinical prospective investigations in individuals with Type 2DM have shown that patients with increased albuminuria and high plasma concentrations of soluble VCAM-1 had an increased risk of death [73].
Receptors
In addition to the formerly addressed chemokine receptors, increased expression of TLR4 but not of TLR2 was noticed in the renal tubules of human kidneys with DN. In addition, TLR9 is expressed on infiltrating antigen-presenting cells during immune injury. TLR-mediated immune activation may occur during any type of renal injury by exposure to an increasing number of exogenous or endogenous molecules [75]. Interaction of the TIR (Toll/IL-1 receptor) domain of TLR4 and the adapter protein MyD88 (myeloid differentiation factor 88) triggers a downstream signaling cascade, leading to activation of the NF-κB pathway, which then activates the transcription of many pro-inflammatory genes that encode inflammatory molecules, including cytokines, chemokines and other effectors of the innate immune response [76]. The intensity of tubular TLR4 expression correlates directly with interstitial macrophage infiltration and hemoglobin A1c level and inversely with estimated glomerular filtration rate. The renal tubules also upregulate the endogenous TLR4 ligand high-mobility group box 1 in DN. In vitro, high glucose induces TLR4 expression via PKC activation, resulting in upregulation of IL-6 and chemokine ligands. Taken together, these data suggest that a TLR4-mediated pathway may promote tubulointerstitial inflammation in DN [77].
c-fms is the receptor for CSF-1, a major cytokine promoting macrophage accumulation, activation, and survival. Administration of a neutralizing anti-c-fms monoclonal antibody to diabetic mice with established albuminuria suppressed inflammation in the diabetic kidney, as evidenced by the reduction in macrophage accumulation, activation and proliferation [48].
Transcription Factors and Transduction Pathways
Several transcription factors such as USF (upstream stimulatory factor) 1 and 2, AP1 (activator protein 1), NF-κB, CREB (cAMP-response-element-binding protein), NFAT (nuclear factor of activated T-cells) and Sp1 (stimulating protein 1) are activated in hyperglycaemic environments. These transcription factors regulate the genes related to inflammation and ECM turnover [78]. Among the transcription factors, NF-κB is the most important in the pathogenesis of diabetic nephropathy. NF-κB is activated by a wide variety of stimuli such as cytokines, oxygen radicals, inhaled particles, ultraviolet irradiation and bacterial or viral products. In diabetic kidney disease, proteinuria itself is the important activator for NF-κB [79]. NF-κB binds to the promoter regions of several genes that play a pivotal role in the pathogenesis of diabetic nephropathy, such as those encoding TGF-β1, MCP-1 and ICAM1 [80]. NF-κB is also integrated in various biological pathways that are functionally involved in the pathogenesis of diabetic nephropathy, such as PKC [81], renin-angiotesin system [82], AGE accumulation [83] and oxidative stress [84].
Furthermore, the JAK2 (Janus kinase 2), SAPK-2 (stress-activated protein kinase-2) and STAT-1, -3 and -5 pathways are enhanced by various stimuli within the diabetic milieu, such as high glucose concentration, AGEs and angiotensin II, and various chemokines, growth factors. It is worthy to note that, ECM proteins are STAT-dependent genes and are closely related to mesangial cell proliferation [85].
Other Factors
Immune complexes formed in response to abnormal proteins generated in DM such as oxidized low-density lipoproteins (LDL) have been shown in vitro to stimulate production of MCP-1 and CSF-1, and promote glomerular fibrosis by stimulating collagen production by mesangial cells. Oxidized LDL immune complexes are also capable of activating the classical pathway of complement and inducing proinflammatory cytokine production by human macrophages, including IL-1, IL-6, and TNF-α. These responses occur through the ligation of Fcγ receptors on mesangial cells and macrophages and may involve the activation of the p38 MAPK, JNK (c-Jun N-terminal kinase) and PKC pathways [86].
Therapeutic Appliances
Accordingly, a variety of therapeutic strategies involving modulation of the inflammatory response are currently being investigated in diabetic kidney disease [10,87]. Some authors have shown that blockade of the renin-angiotensin system in patients with Type 2 DM and DN is associated with a reduction in urinary MCP-1 levels as well as an improvement in renal function [88]. Combination therapy with eicosapentaenoic acid (EPA), i.e. anti-microinflammation effect, angiotensin converting inhibitors (ACE-I) and angiotensin II type 1 receptor blockers (ARB), and 1,25-dihydroxyvitamin D3, i.e. anti-hypertensive and anti-reactive oxygen species effects, have shown efficacy in the treatment of diabetic nephropathy in experimental animal models [4]. Injection with the anti-microinflammatory EPA improved Type 2 diabetic nephropathy in experimental animal models by decreasing hypertriglyceridemia and albuminuria and improving glucose tolerance [4]. Glomerular mesangial matrix expansion and segmental sclerosis, as well as interstitial fibrosis were markedly decreased by EPA treatment. Diabetes induced up-regulation of MCP-1 and TGF-β expressions were inhibited by EPA, together with a reduction of glomerular macrophage infiltration and oxidative stress. It appears that EPA might be an effective therapeutic agent for DN [89].
Neutralizing MCP-1 activity could be an important therapeutic goal in the treatment of DN. From this perspective, a recent experimental study has shown that blockade of the MCP-1/CCR2 pathway ameliorated glomerulosclerosis [90]. Also, the inhibition of JAK/STAT pathways by AG-490, a specific JAK2 inhibitor ameliorated the progression of diabetic neuropathy by improving inflammatory responses by suppressing CCL2 and TGF-β [91]. Thus, understanding of these inflammatory pathways guides important therapeutic appliances and improves the discovery of new therapeutic targets that can be translated into clinical treatments for DN.
References
- Rivero A, Mora C, Muros M, García J, Herrera H, et al. (2009) Pathogenic perspectives for the role of inflammation in diabetic nephropathy. ClinSci (Lond) 116: 479-492.
- Yang M, Gan H, Shen Q, Tang W, Du X, et al. (2012) Proinflammatory CD14+CD16+ monocytes are associated with microinflammation in patients with Type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation 35: 388-396.
- Dronavalli S, Duka I, Bakris GL (2008) The pathogenesis of diabetic nephropathy. Nat ClinPractEndocrinolMetab 4: 444-452.
- Tomino Y (2012) Lessons From the KK-Ay Mouse, a Spontaneous Animal Model for the Treatment of Human Type 2 Diabetic Nephropathy. Nephrourol Mon 4: 524-529.
- Ziyadeh FN (2004) Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am SocNephrol 15 Suppl 1: S55-57.
- Vasavada N, Agarwal R (2005) Role of oxidative stress in diabetic nephropathy. Adv Chronic Kidney Dis 12: 146-154.
- Nguyen G (2006) Renin/prorenin receptors. Kidney Int 69: 1503-1506.
- Maeda S (2008) Do inflammatory cytokine genes confer susceptibility to diabetic nephropathy? Kidney Int 74: 413-415.
- Kajitani N, Shikata K, Nakamura A, Nakatou T, Hiramatsu M, et al. (2010) Microinflammation is a common risk factor for progression of nephropathy and atherosclerosis in Japanese patients with Type 2 diabetes. Diabetes Res ClinPract 88: 171-176.
- Hickey FB, Martin F (2013) Diabetic kidney disease and immune modulation. CurrOpinPharmacol 13: 602-612.
- Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813-820.
- Heilig CW, Concepcion LA, Riser BL, Freytag SO, Zhu M, et al. (1995) Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J Clin Invest 96: 1802-1814.
- Mishra R, Emancipator SN, Kern T, Simonson MS (2005) High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int 67: 82-93.
- Shanmugam N, Gaw Gonzalo IT, Natarajan R (2004) Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes 53: 795-802.
- Min D, Lyons JG, Bonner J, Twigg SM, Yue DK, et al. (2009) Mesangial cell-derived factors alter monocyte activation and function through inflammatory pathways: possible pathogenic role in diabetic nephropathy. Am J Physiol Renal Physiol 297: F1229-F1237.
- Goh SY, Cooper ME (2008) Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J ClinEndocrinolMetab 93: 1143-1152.
- Wolf G, Ziyadeh FN (2006) Leptin and renal fibrosis. ContribNephrol 151: 175-183.
- Chen ZJ, Yang YB, Huang SM (2007) Expression of VEGF in kidney of diabetic rats. Sichuan Da XueXueBao Yi Xue Ban 38: 633-636.
- Haneda M, Kikkawa R, Koya D, Shikano T, Sugimoto T, et al. (1995) Endothelin-1 stimulates tyrosine phosphorylation of p125 focal adhesion kinase in mesangial cells. J Am SocNephrol 6: 1504-1510.
- Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, et al. (2012) Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia 55: 2256-2266.
- Wu CC, Sytwu HK, Lu KC, Lin YF (2011) Role of T-cells in Type 2 diabetic nephropathy. Exp Diabetes Res 2011: 514738.
- Singh DK, Winocour P, Farrington K (2008) Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat ClinPractNephrol 4: 216-226.
- Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, et al. (2005) NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol 288: F1144-1152.
- Ha H, Lee HB (2001) Oxidative stress in diabetic nephropathy: basic and clinical information. CurrDiab Rep 1: 282-287.
- Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, et al. (2007) Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol 170: 1473-1484.
- Chung FM, Tsai JC, Chang DM, Shin SJ, Lee YJ (2005) Peripheral total and differential leukocyte count in diabetic nephropathy: the relationship of plasma leptin to leukocytosis. Diabetes Care 28: 1710-1717.
- Sari R, Balci MK, Apaydin C (2010) The relationship between plasma leptin levels and chronic complication in patients with Type 2 diabetes mellitus. MetabSyndrRelatDisord 8: 499-503.
- Guo LL, Pan Y, Jin HM (2009) Adiponectin is positively associated with insulin resistance in subjects with Type 2 diabetic nephropathy and effects of angiotensin II type 1 receptor blocker losartan. Nephrol Dial Transplant 24: 1876-1883.
- Ng DP, Nurbaya S, Ye SH, Krolewski AS (2008) An IL-6 haplotype on human chromosome 7p21 confers risk for impaired renal function in Type 2 diabetic patients. Kidney Int 74: 521-527.
- Kumar R, Sharma RK, Agarwal S (2013) Genetic predisposition for development of nephropathy in Type 2 diabetes mellitus. Biochem Genet 51: 865-875.
- Festa A, D'Agostino R Jr, Howard G, Mykkänen L, Tracy RP, et al. (2000) Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102: 42-47.
- Galkina E, Ley K (2006) Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am SocNephrol 17: 368-377.
- Ichinose K, Kawasaki E, Eguchi K (2007) Recent advancement of understanding pathogenesis of type 1 diabetes and potential relevance to diabetic nephropathy. Am J Nephrol 27: 554-564.
- Chung AC, Lan HY (2011) Chemokines in renal injury. J Am SocNephrol 22: 802-809.
- Zhang C, Xiao C2, Wang P3, Xu W4, Zhang A1, et al. (2014) The alteration of Th1/Th2/Th17/Treg paradigm in patients with Type 2 diabetes mellitus: Relationship with diabetic nephropathy. Hum Immunol 75: 289-296.
- Fornoni A, Ijaz A, Tejada T, Lenz O (2008) Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4: 10-17.
- Sell H, Eckel J (2009) Chemotactic cytokines, obesity and Type 2 diabetes: in vivo and in vitro evidence for a possible causal correlation? ProcNutrSoc 68: 378-384.
- Kang MK, Li J, Kim JL, Gong JH, Kwak SN, et al. (2012) Purple corn anthocyanins inhibit diabetes-associated glomerular monocyte activation and macrophage infiltration. Am J Physiol Renal Physiol 303: F1060-1069.
- Van Belle TL, Coppieters KT, von Herrath MG (2011) Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 91: 79-118.
- Bending D, De la Peña H, Veldhoen M, Phillips JM, Uyttenhove C, et al. (2009) Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest 119: 565-572.
- Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, et al. (2010) IL-17 immunity in human type 1 diabetes. J Immunol 185: 1959-1967.
- Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444: 860-867.
- Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, et al. (2008) T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology 28(7):1304-1310.
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, et al. (2002) Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415: 536-541.
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, et al. (2009) CD8+ effector T-cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914-920.
- Odobasic D, Kitching AR, Tipping PG, Holdsworth SR (2005) CD80 and CD86 costimulatory molecules regulate crescentic glomerulonephritis by different mechanisms. Kidney Int 68: 584-594.
- Wang Y, Harris DC (2011) Macrophages in renal disease. J Am SocNephrol 22: 21-27.
- Lim AK, Tesch GH (2012) Inflammation in diabetic nephropathy. Mediators Inflamm 2012: 146154.
- Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J (2011) Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7: 327-340.
- Lim AK, Ma FY, Nikolic-Paterson DJ, Kitching AR, Thomas MC, et al. (2010) Lymphocytes promote albuminuria, but not renal dysfunction or histological damage in a mouse model of diabetic renal injury. Diabetologia 53: 1772-1782.
- Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, et al. (2009) Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 183: 4432-4439.
- Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, et al. (2011) Elevated proinflammatory cytokine production by a skewed T-cell compartment requires monocytes and promotes inflammation in Type 2 diabetes. J Immunol 186: 1162-1172.
- Mora C, Navarro JF (2006) Inflammation and diabetic nephropathy. CurrDiab Rep 6: 463-468.
- Han SY, So GA, Jee YH, Han KH, Kang YS, et al. (2004) Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol 82: 568-576.
- Ha H, Yu MR, Choi YJ, Kitamura M, Lee HB (2002) Role of high glucose-induced nuclear factor-kappaB activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am SocNephrol 13: 894-902.
- Vesey DA, Cheung C, Cuttle L, Endre Z, Gobe G, et al. (2002) Interleukin-1 ß stimulates human renal fibroblast proliferation and matrix protein production by means of a transforming growth factor-ß-dependent mechanism. Journal of Laboratory and Clinical Medicine140(5):342-350.
- Hohenstein B, Daniel C, Hausknecht B, Boehmer K, Riess R, et al. (2008) Correlation of enhanced thrombospondin-1 expression, TGF-beta signalling and proteinuria in human type-2 diabetic nephropathy. Nephrol Dial Transplant 23: 3880-3887.
- Miossec P, Korn T, Kuchroo VK (2009) Interleukin-17 and type 17 helper T-cells. N Engl J Med 361: 888-898.
- Kalantarinia K, Awad AS, Siragy HM (2003) Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int 64: 1208-1213.
- Pfeilschifter J, Pignat W, Vosbeck K, Märki F (1989) Interleukin 1 and tumor necrosis factor synergistically stimulate prostaglandin synthesis and phospholipase A2 release from rat renal mesangial cells. BiochemBiophys Res Commun 159: 385-394.
- DallaVestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, et al. (2005) Acute-phase markers of inflammation and glomerular structure in patients with Type 2 diabetes. J Am SocNephrol 16 Suppl 1: S78-82.
- Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, et al. (2006) Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 147: 2518-2525.
- Fujita T, Ogihara N, Kamura Y, Satomura A, Fuke Y, et al. (2012) Interleukin-18 contributes more closely to the progression of diabetic nephropathy than other diabetic complications. ActaDiabetol 49: 111-117.
- Nakamura A, Shikata K, Hiramatsu M, Nakatou T, Kitamura T, et al. (2005) Serum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with Type 2 diabetes. Diabetes Care 28: 2890-2895.
- Navarro-González JF, Mora-Fernández C (2008) The role of inflammatory cytokines in diabetic nephropathy. J Am SocNephrol 19: 433-442.
- Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, et al. (2003) Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications 17: 11-15.
- Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, et al. (2000) Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int 58: 684-690.
- Moore KJ, Wada T, Barbee SD, Kelley VR (1998) Gene transfer of RANTES elicits autoimmune renal injury in MRL-Fas(1pr) mice. Kidney Int 53: 1631-1641.
- Sayyed SG, Hagele H, Kulkarni OP, Endlich K, Segerer S, et al. (2009) Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of Type 2 diabetes. Diabetologia 52: 2445-2454.
- Clausen P, Jacobsen P, Rossing K, Jensen JS, Parving HH, et al. (2000) Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with Type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabet Med 17: 644-649.
- Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, et al. (2000) Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 57: 167-182.
- Wong CK, Ho AW, Tong PC, Yeung CY, Chan JC, et al. (2008) Aberrant expression of soluble co-stimulatory molecules and adhesion molecules in Type 2 diabetic patients with nephropathy. J ClinImmunol 28: 36-43.
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH (2005) Intercellular adhesion molecule-1 deficiency is protective against nephropathy in Type 2 diabetic db/db mice. J Am SocNephrol 16: 1711-1722.
- Stehouwer CDA, Gall MA, Twisk JWR, Knudsen E, Emeis JJ, et al. (2002) Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in Type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 51: 1157-1165.
- Anders HJ, Schlöndorff D (2007) Toll-like receptors: emerging concepts in kidney disease. CurrOpinNephrolHypertens 16: 177-183.
- Medzhitov R (2001) Toll-like receptors and innate immunity. Nat Rev Immunol 1: 135-145.
- Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, et al. (2012) Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am SocNephrol 23: 86-102.
- Sanchez AP, Sharma K (2009) Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev Mol Med11: e13.
- Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, et al. (2004) NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant 19: 2505-2512.
- Yang B, Hodgkinson A, Oates PJ, Millward BA, Demaine AG (2008) High glucose induction of DNA-binding activity of the transcription factor NFkappaB in patients with diabetic nephropathy. BiochimBiophysActa 1782: 295-302.
- Kumar A, Hawkins KS, Hannan MA, Ganz MB (2001) Activation of PKC-beta(I) in glomerular mesangial cells is associated with specific NF-kappaB subunit translocation. Am J Physiol Renal Physiol 281: F613-619.
- Lee FT, Cao Z, Long DM, Panagiotopoulos S, Jerums G, et al. (2004) Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am SocNephrol 15: 2139-2151.
- Liang YJ, Jian JH, Liu YC, Juang SJ, Shyu KG, et al. (2010) Advanced glycation end products-induced apoptosis attenuated by PPARd activation and epigallocatechingallate through NF-?B pathway in human embryonic kidney cells and human mesangial cells. Diabetes Metab Res Rev 26: 406-416.
- Pillarisetti S, Saxena U (2004) Role of oxidative stress and inflammation in the origin of Type 2 diabetes-a paradigm shift. Expert OpinTher Targets 8: 401-408.
- Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, et al. (2009) Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469-477.
- Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF (2006) OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. Journal of Lipid Research 47: 1975-1983.
- Wada J, Makino H (2013) Inflammation and the pathogenesis of diabetic nephropathy. ClinSci (Lond) 124: 139-152.
- Amann B, Tinzmann R, Angelkort B (2003) ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care 26: 2421-2425.
- Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, et al. (2006) Eicosapentaenoic acid ameliorates diabetic nephropathy of Type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant 21: 605-615.
- Kanamori H, Matsubara T, Mima A, Sumi E, Nagai K, et al. (2007) Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. BiochemBiophys Res Commun 360: 772-777.
- Wang X, Shaw S, Amiri F, Eaton DC, Marrero MB (2002) Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in tgf-beta and fibronectin synthesis in mesangial cells. Diabetes 51: 3505-3509.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 16131
- [From(publication date):
September-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 11617
- PDF downloads : 4514