Microbial Fuel Cell Bio-Remediation of Lambda Cyhalothrin, Malathion and Chlorpyrifos on Loam Soil Inoculated With Bio-Slurry
Received: 02-May-2022 / Manuscript No. JBRBD-22-63384 / Editor assigned: 04-May-2022 / PreQC No. JBRBD-22-63384 (PQ) / Reviewed: 18-May-2022 / QC No. JBRBD-22-63384 / Revised: 20-May-2022 / Manuscript No. JBRBD-22-63384 (R) / Published Date: 27-May-2022 DOI: 10.4172/2155-6199.1000508
Abstract
Introduction: In microbial fuel cell technology, the substrate is consumed by microbes in anaerobic conversion of substrate to electricity. Bio-remediation of pollutants involves microbial environmental cleanup using green approach.
Problem: The primary problems with pesticides are linked to the non-negligible proportion of the sprayed active ingredient that does not reach its intended target thereby contaminating environmental compartments persistently.
Objective: The primary objective of this study was to assess the potential of microbial fuel cell technology in bioremediation of lambda cyahlothrin, chlorpyrifos and malathion in Limuru loam soil.
Method: H-shaped double chamber microbial fuel cell was fabricated where the anodic chamber was loaded with 750 mL loam soil inoculated with 750 mL bio-slurry doped with 10 mL of 10 ppm lambda cyhalothrin, chlorpyrifos and malathion pesticide solutions. The cathodic chamber was loaded with 1500 mL distilled water. The setup was incubated for a 90 days retention time where voltage and current were recorded daily using a multi-meter. The degradation level was assessed using a GC-MS after sample extraction using standard QuEChERs method.
Results: The voltage generated from the pesticide doped loam soil showed an upward trend from day 0 to day 15 in lambda cyhalothrin and malathion and from day 0 to day 20 in chlorpyrifos and pesticide mixture after which constant readings were observed for three days with downward trends thereafter. The maximum generated voltage was 0.537 V, 0.571 V, 0.572 V and 0.509 V in chlorpyrifos, lambda cyhalothrin, malathion and pesticide mix (MCL) respectively. The bioremediation levels for chlorpyrifos and malathion were 65.80% and 71.32%, respectively while no detectable, lambda cyhalothrin was observed after day 60 of the study.
Conclusion: This study concludes that bioremediation of lambda cyhalothrin, chlorpyrifos and malathion in Limuru loam soil can be achieved using microbial fuel cells.
Keywords
Bioremediation; Bio-slurry; Loam soil; Microbial Fuel Cells; Pesticides
Methodology
The procedure used to carry out loam soil analysis and bioremediation of the pesticide residues is explained in this section.
Loam Soil Analysis
Available nutrient elements (P, K, Na, Ca, Mg and Mn): Mehlich Double Acid Method
The oven-dry soil samples were extracted in 1:5 ratios (w/v) with a mixture of 0.1 N HCl from Kobian distributors and 0.025 N H2SO4. A flame photometer was used in determination of K, Ca and Na while calorimetrically was used in determination of P, Mg and Mn [1,2].
Total organic carbon: Calorimetric method
All organic C in the soil sample is oxidized by acidified dichromate at 150ºC for 30 minutes to ensure complete oxidation. Barium chloride was added to the cool digests. After mixing thoroughly, digests are allowed to stand overnight. The concentration is read on the spectrophotometer at 600 nm [3].
Total nitrogen: Kjeldahl method
Soil samples were digested with concentrated sulphuric acid containing potassium sulfate, selenium and copper sulfate hydrated at approximately 350ºC. Total N is determined by distillation, followed by titration with H2SO4 .
Soil pH (1:1 soil-water)
Soil pH was determinedin a 1:1 (w/v) soil-water suspension using a pH meter.
Available trace elements (Fe, Zn & Cu): Extraction with 0.1 M HCl
The oven-dry soil samples are extracted in a 1:10 ratio (w/v) with 0.1 M HCl. The elements are determined using an atomic absorption flame emission spectrophotometer (AAS).
Cation Exchange Capacity (CEC) pH 7.0 and Exchangeable Ca, Mg, K and Na
The soil sample was leached with 1N ammonium acetate buffered at pH 7. The leachate was analyzed for exchangeable Ca, Mg, K and Na. The sample was further leached with 1N KCl, and the leachate is used for the determination of the CEC. Elements such as Na and K were being determined with a flame photometer and Ca and Mg with AAS (atomic absorption spectrophotometer). CEC is determined by distillation, followed by titration with 0.01M HCl [4,5].
Microbial Fuel Cells Construction
Two 1.2 liter containers were prepared as anode and cathode chambers. Two small holes were made on the caps of the containers to insert the wire through. One end of the copper wire was attached to 5.7 cm long and 0.7 cm diameter graphite rod electrodes. A salt bridge was prepared using 2.5 litres of 1M NaCl, 3% agarose solution and lamp wicks. The wicks were boiled in NaCl and 3% agarose solution for 10 minutes after which it was kept in the freezer at -4ºC for solidification. The solidified salt bridge was passed through PVC pipes and attached to the chambers using an adhesive, which makes them leak-proof. The electrodes used in this study were spent battery carbon rods stuck together using a zero-resistance copper wire as shown in figure 1. The carbon rods were obtained from batteries after which they we thoroughly cleaned using water and later scrub using a sand paper. They were then socked in concentrated Sulphuric acid for 24 hours before stacking them together. The electrodes had a 0.00399 m2 operating surface area. The assembly of the H-shaped MFC was done, as shown in figure 1 as earlier described by Kamau et al., 2018. A digital voltmeter was attached to the copper wires from the cathodic and anodic chambers, and the voltage and current were monitored daily [6,7].
Bio-remediation studies
The microbial bio-remediation study involved investigation of efficiency of microbial fuel cells in degradation of lambda cyhalothrin, malathion and chlorpyrifos pesticide residues. The anodic chamber was fed with 750 g loam soil (previously analysed) inoculated with 750 mL bio-slurry from a running biogas digester spiked with 10 mL, of 100 ppm lambda cyhalothrin, malathion and Chlorpyrifos and a mixture solution of lambda cyhalothrin, malathion and Chlorpyrifos [8,9]. The degradation levels were determined by measuring the concentration of the pesticide after every 5 days for 90 days. The Voltage and current generated were recorded on daily basis. The degradation levels were determined by measuring the concentration of the pesticide after every 5 days for 90 days. The pesticides after degradation were extracted using the standard QuEChERS method [10]. The sample extracts were placed onto a tray for automated GC/MS analysis as described by [11]. The Voltage and current generated were recorded on daily basis. The control experiment was run by loading the loam soil into the anodic chamber and reading the daily voltage and current for 90 days.
Results and Discussions
Loam soil properties
The macro and micro properties of the loam soil used in this study is shown in table 1. From the analysis, the soil pH was in the range of 6.5 -6.8 ±0.51 while the electrical conductivity of this soil was 0.03±0.01 ms/cm.
| Profile | Properties | Profile | Properties |
|---|---|---|---|
| Soil depth cm | Top | Calcium milli- equivalent % | 44.4±2.11 |
| Soil pH-H20 (1:2.5) | 6.5±0.51 | Magnesium me % | 3.1±0.09 |
| Elect. Cond. ms/cm | 0.3±0.01 | Potassium me% | 1.5±0.66 |
| Carbon % | 2.7±0.32 | Sodium me% | 3.6±1.11 |
| Sand % | 40±3.56 | Sum me% | 52.6±3.44 |
| Silt % | 40±4.55 | Base % | 100+ |
| Clay % | 20±2088 | ESP | 14.4±6.74 |
| Texture Class | Loam | Total nitrogen % | 0.25±0.08 |
| Cat. Exch. Capacity. me% | 24.8±2.67 | Phosphorns ppm | 44±5.00 |
| Zinc ppm | 62.9±10.22 | Iron ppm | 96.2± 12.90 |
| Copper ppm | 1.22±0. 11 | me is milli- equivalent |
Table 1: The properties of the loam soil
The loam soil was top soil collected about 1-2 cm deep. The organic matter was removed from the surface before sampling. The cation exchange capacity of the loam soil was 24.8±2.67 while carbon levels were 2.70±0.32%. The microbes use carbon as source of nutrient and energy and therefore soil carbon is a very important parameter in soil analysis. Soil quality does not depend just on the physical, physico-chemical and chemical properties of soil but closely linked to the soil microbiological properties [12]. Microorganisms are vital for soil fertility and for the degradation of organic matter and pollutants in soils. Some of the important biosurfactant-synthesizing bacterial species include Pseudomonas sp., Bacillus sp., Acinetobacter sp., Stenotrophomonas sp., and Burkholderia sp. The bacterial biosurfactants increase the rate of biodegradation of hydrophobic (insoluble) organic pollutants such as pesticides and petroleum in the soil or enhance the removal of heavy metals through a series of modes of action such as increasing their mobility, micelle formation, and increasing bioavailability to bacteria-degrading microorganisms [13] Microbial biomass in soil is considered as an important attribute of soil quality [14]. It serves as a measure of potential biological activity and its dynamic changes help in understanding the processes involved in nutrient cycling and ecosystem functioning [15]. Pal et al., (2006) reviewed of the information available in the literature highlighting the various soil properties, which influence the degradation of pesticides. The extent of biodegradability depends upon the chemical structure of the pesticides and the soil physico-chemical properties. They also noted that soil microbial components largely govern pesticide degradation in soil.`
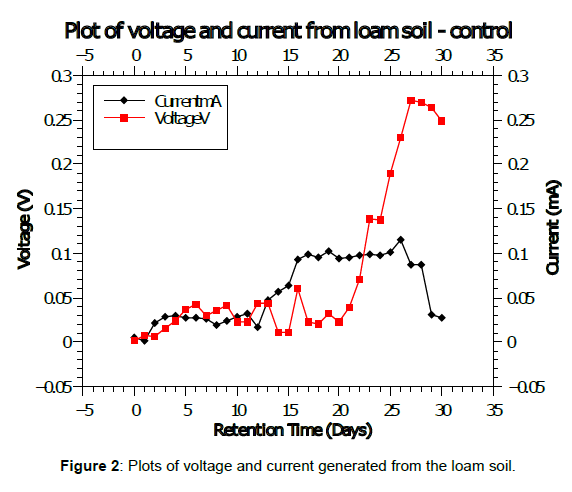
The graph obtained from plots of current and voltage of the control (loam soil) data is shown in figure 2. The daily voltage showed a slow upward trend for the first twenty days with steep increase of voltage for eight days before it started to drop. Similar results were obtained for current since both current and voltage are relates proportionally according to Ohms law.
Current generation means that the soil micro-organisms are breaking down the carbon in the soil thereby generation an electron (current). The slow increase in current and voltage is explained by the fact that the microbes were in aerobic conditions before sampling and therefore takes time to adapt to the anaerobic setup in the anodic chamber [16]. On full adaptation (day 20), the rate of electron increases subsequently increasing the voltage and current generation. On depletion of the available carbon in the soil, the microbes start dying and therefore a voltage and current drop is observed. Similar results had been obtained using market wastes like avocado and tomato by Kamau et al., (2018). In other studies, by Kinyua 2022b and Imwene (2021) using tomato and cabbages as substrates, the voltage and current increased as observed in this study. The voltage and current means were used calculations of power, current and power density using equations 1 to 3, respectively.
The plots of power and current density are shown in figure 3. Similar to plots of current and voltage, the power density increased with retention time. The current and power density shows the current and power per electrode surface area and mostly used to show the efficiency of microbial fuel cell in electricity generation.
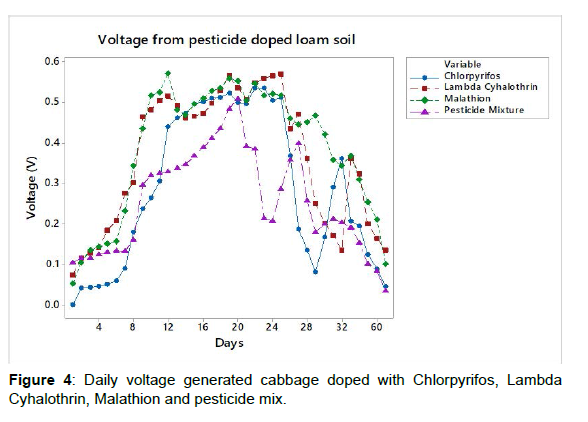
The bio-remediation levels for chlorpyrifos and malathion were 65.80% and 71.32%, respectively while no detectable, lambda cyhalothrin was observed after day 60 of the study (Figure 4). The voltage generated from the pesticide doped loam soil showed an upward trend from day 0 to day 15 in lambda cyhalothrin and malathion and from day 0 to day 20 in chlorpyrifos and MCL mixture after which constant readings were observed for three days with downward trends thereafter. The maximum generated voltage was 0.537 V, 0.571 V, 0.572 V and 0.509 V in chlorpyrifos, lambda cyhalothrin, malathion and MCL respectively.
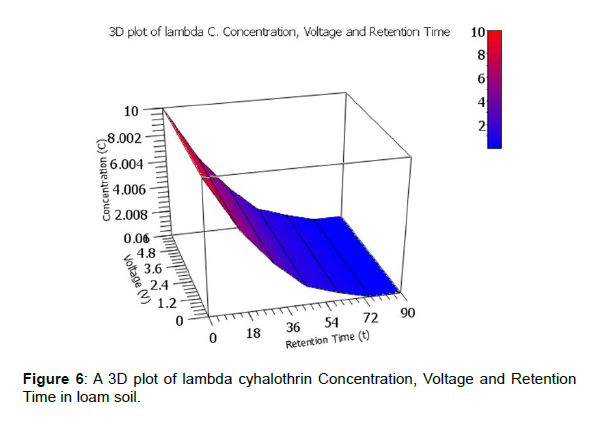
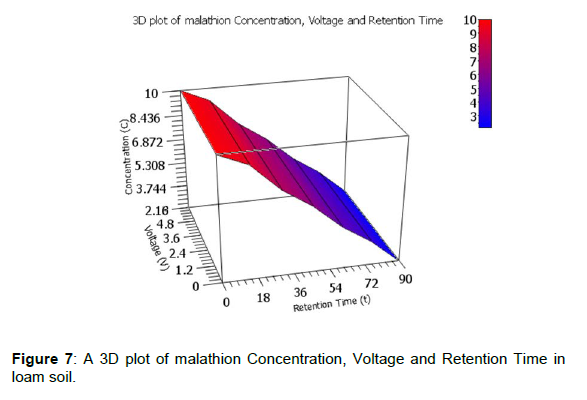
A clearer illustration is shown using 3D plots of voltage generated against degradation levels with retention time for the three pesticides. The 3D plot of pesticides Concentration, Voltage and Retention Time in loam soil is shown by figures 5, 6 and 7.
The observed degradation levels were 79.32%, 99.90% and 78.20% in chlorpyrifos, lambda cyhaolthrin, malathion, respectively as shown in figure 5-7, respectively.
Discussions
Current and voltage generation is an indication of substrate breakdown by microbes in anaerobic anodic chamber of microbial fuel cells. The rate of current generation shows the rate at which microbes are degrading the substrate/carbon releasing electrons [17]. The current generated in this study as per figure showed a slow increase from day 1 to day 27 as microbes are adapting to the experimental environment. As carbon matter in pesticide and soil depletes, the current and voltage generation start to decrease as microbes reaches the death phase. This trend is similar to what had been observed by Kinyua et al., 2022b and Imwene et al., 2021 in vegetable and fruit substrates.
In bio-remediation of pollutants, the pesticide molecule serves as a carbon sources and therefore it’s broken down by micro-organisms in soil [18]. The microbial activity is highly influenced by pesticide properties and environmental factors [20]. From the initial pesticide concentrations of 10 ppm, the observed degradation levels were 79.32%, 99.90% and 78.20% in chlorpyrifos, lambda cyhaolthrin, malathion, respectively as shown in figure 5-7, respectively. This means that the microbes feed on the pesticide molecule and soil organic carbon for their growth and energy. These result are similar to what was previous observed by Kinyua et al., 2022 and Mbugua et al., 2022 on bio-remediation of chlorpyrifos, lambda cyhaolthrin, malathion on on loam soil, cabbage and tomato inoculated with microbe rich rumen waste and by anaerobic digestion bio-slurry, respectively. Similarly, on bio-remediation of chlorpyrifos, lambda cyhaolthrin and malathion inoculated with rumen fluid, 0.312V, 0.572V, 0.364V were recorded in tomato, loam soil and cabbage, respectively [21]. The bio-remediation levels were 79.32%, 99.90% and 78.20% in chlorpyrifos, lambda cyhalothrin, malathion, respectively in loam soil, 65.80% and 71.32% for chlorpyrifos and malathion respectively in cabbage. In tomato setup, 75.60% and 80.10% chlorpyrifos and malathion levels were observed, respectively with undetectable levels of lambda cyhalothrin [22]. In the study by Mbugua et al., 2022, the observed maximum voltage on doping the biogas bio-slurry with the chlorpyrifos, lambda cyhalothrin, malathion and the pesticides mix (CLM) were 0.551, 0.565, 0.538 and 0.533V respectively with bio-degradation levels achieved were 73.40% malathion, 87.70% chlorpyrifos while no lambda cyhalothrin was detected on the 90th day of incubation.
Malathion is degraded by carboxyesterase enzyme and it is detected in several fungi like Aspergillus sp., Penicillum sp. and Rhizoctonia sp [23]. Omar (1998) and Hasan (1999) also demonstrated the same type of fungal utilization and degradation of Malathion. Adhikari, 2010 suggested bio-remediation of malathion from the environment as a pollution control measure. Similarly to the current study, the bioremediation of malathion on contaminated sterile and non-sterile soil showed a degradation levels of 84.81% and 74.11% of malathion, respectively, from malathion concentration of 1.5% kg−1 soil degraded by strain PU after 7 days [24].
In bioremediation study of chlorpyrifos by Jaiswal et al., 2017, the potential degradative microorganisms possess opd (organophosphate degrading) gene which hydrolyses the chlorpyrifos and utilizes it as a sole carbon source. A fungal strain Verticillium capable of utilizing chlorpyrifos as sole carbon and energy sources from soil and degradation of chlorpyrifos in pure cultures and on vegetables has been reported. The 3,5,6-trichloro-2-pyridinol (TCP) and diethylthiophosphate (DETP) as primary products are made when chlorpyrifos is degraded by soil microorganisms which further break into nontoxic metabolites as CO2, H2O, and NH3 [25]. Pseudomonas is a diversified genus possessing a series of catabolic pathways and enzymes involved in pesticide degradation. Pseudomonas putida MAS-1 is reported to be more efficient in chlorpyrifos degradation by a rate of 90% in 24 h among Pseudomonas genus [26]. A bacterial strain C2A1 isolated from soil was found highly effective in degrading chlorpyrifos and its first hydrolysis metabolite 3,5,6-trichloro-2-pyridinol (TCP) [27].
Bacillus thuringiensis ZS-19 has been reported to completely degraded cyhalothrin in minimal medium within 72 h. The bacterium transformed cyhalothrin by cleavage of both the ester linkage and diaryl bond to yield six intermediate products (Chen et al., 2015). Furthermore, strain ZS-19 participated in efficient degradation of a wide range of pyrethroids including cyhalothrin, fenpropathrinn, deltamethrin, beta-cypermethrin, cyfluthrin and bifenthrin. In a study by Kimar and Jahangir, 2018, the strain Rhodococcus erythropolis was proven suitable for the efficient and rapid bioremediation of Lambda cyhalothrin pesticide contaminated environment.
In other pesticides bio-remediation, it has been found that after 21 days 85% carbaryl has been degraded from soil treated with nitrogen source [28]. Pal et al., 2006 investigated the factors influencing the degradation of pesticides in soil, impacts of pesticides on soil microbial biomass, soil ergosterol content, soil respiration, fluorescein diacetate hydrolyzing activity, ecophysiological parameters and the correlation between pesticide transformation and above microbial parameters. Pesticides, which enter the soil environment, are subject to a variety of degradative processes. The overall degradation of a pesticide from soil results from a combination of mechanisms such as microbial degradation, chemical hydrolysis, photolysis, volatility, leaching and surface runoff. The degree to which each mechanism will contribute to the overall degradation of the pesticide is in turn dependent on the physicochemical properties of the pesticide (e.g., water solubility, sorptive affinity), characteristics of the soil (e.g., pH, organic matter content, microbial biomass, redox status), environmental conditions (e.g., temperature, moisture) and management practices (e.g., application rate, formulation type) [29]. The kinetics and pathways of degradation depend on abiotic and biotic factors [30], which are specific to a particular pesticide and therefore find preference. Adverse effect of pesticidal chemicals on soil microorganisms [31], may affect soil fertility [32] becomes a foreign chemicals major issue. Soil microorganisms show an early warning about soil disturbances by foreign chemicals than any other parameters.
The fate and behavior of these chemicals in soil ecosystem is very important since they are degraded by various factors and have the potential to be in the soil, water etc. So it is indispensable to monitor the persistence, degradation of pesticides in soil and is also necessary to study the effect of pesticide on the soil quality or soil health by in depth studies on soil microbial activity [33]. Previous studies have on fipronil bio-remediation in the non-sterile clay loam soil, which resulted in the formation of metabolite, MB45950. The degradation of fipronil in nonsterile clay loam soil was mainly influenced by the soil microbes [34]. The half-lives in non-sterile clay loam soil were 9.72 and 8.78 d at 25 and 35ºC, respectively compared to 33.51 and 32.07 d at 25 and 35ºC, respectively in the sterile soil. The microbial viability test showed that non-sterile clay loam soil had viable microorganisms throughout the experiment. Fipronil did not adversely affect the microbes once soil microbes adapted to the presence of fipronil in the clay loam soil [35].
A close positive correlation between soil microbial biomass, soil respiration and the degradation rate constant of metribuzin [36], linuron and glyphosate [37], alachlor Walker et al., 1992, 2,4-D and dicamba [38] was recorded in agricultural and forest soils. Metalaxyl and propachlor transformation rate constants positively correlated with basal, substrate-induced respiration and physico-chemical (pH, organic C and clay content) properties of soil [39]. In contrast, no correlations were found between microbial biomass and degradation of the pesticides 2,4-D and atrazine [40-42]. It was opined that this relationship might be useful for developing approaches for evaluating and predicting the fate of pesticides in different ecosystems.
The relationship between rimsulfuron [43,44], imazamox and benfluralin [45] degradation and microbial biomass content was studied in a laboratory incubated clay loam soil under different conditions of soil moisture, temperatures and also at different initial dosages. The relationship between pesticide degradation and microbial biomass C content gave parabolic curves (p<0.05 in all cases) under all conditions tested.
Conclusions
The voltage generated from the pesticide doped loam soil showed an upward trend from day 0 to day 15 in lambda cyhalothrin and malathion and from day 0 to day 20 in chlorpyrifos and pesticide mixture after which constant readings were observed for three days with downward trends thereafter. The maximum generated voltage was 0.537 V, 0.571 V, 0.572 V and 0.509 V in chlorpyrifos, lambda cyhalothrin, malathion and pesticide mix (MCL) respectively. The bioremediation levels for chlorpyrifos and malathion were 65.80% and 71.32%, respectively while no detectable, lambda cyhalothrin was observed after day 60 of the study.
References
- Adhikari S (2010) Bioremediation of Malathion from Environment for Pollution Control. Res J Environ Toxicol 4: 147-150.
- Aislabie J, Loydjones G (1995) A review of bacterial-degradation of pesticides. Soil Res 33: 925-942.
- Alexander RT, Terry (1964) Chemical degradation, choliambic (1) stable to hydrolytic degradation. Non sterile soil 10:20-27
- Amirahmadi M, Yazdanpanah H, Shoeibi S, Pirali-Hamedani M, Ostad Gholami M, et al. (2013) Simultaneous Determination of 17 Pesticide Residues in Rice by GC/MS using a Direct Sample Introduction Procedure and Spiked Calibration Curves. Iran J Pharm Res 12: 295-302.
- Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multi-residue method employing acetonitrile extraction/partitioning and “dispersive solid phase extraction” for the determination of pesticide residues in products. JAOAC Int 86: 412-431.
- Anwar S, Liaquat F, Khan QM, KhalidZM, Iqbal S (2009) Biodegradation of chlorpyrifos and its hydrolysis product 3, 5, 6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1. J Hazard Mater 168: 400-405.
- Araujo ASF, Monterio RTR, Abarkeli AB (2003) Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 52:799-804.
- Beigel C, Charnay MP and Barriuso E. (1999). Degradation of formulated and unformulated triticonazole fungicide in soil: effect of application rate. Soil Biol Biochem 31:525-534.
- Boopathy R (2000) Factors limiting bioremediation technologies. Bioresour Technol 74: 63 - 67.
- Chen S, Deng Y, Chang C, Lee J, Cheng Y, et al. (2015) Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Sci Rep 5:8784.
- Chowdhury A, Pradhan S, Saha M, Sanyal N (2008) Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Indian J Microbiol 48:114-127.
- Chowdhury A, Pradhan S, Saha M and Sanyal N (2008) Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Indian journal of microbiology 48: 114-127.
- Cycoń M, Mrozik A, Piotrowska-Seget Z (2017) Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 172: 52-71.
- Das S, Dash HR (2014) Microbial bioremediation: A potential tool for restoration of contaminated areas. Microbial biodegradation and bioremediation. Elsevier.
- Doolotkeldieva T, Konurbaeva M, Bobusheva S (2018) Microbial communities in pesticide-contaminated soils in Kyrgyzstan and bioremediation possibilities. Environ Sci Pollut 25: 31848-31862.
- Doran JW, Parkin TB (1994) Defining and Assessing Soil Quality. Soil Sci Soc Am J 35:3-21.
- https://cir.nii.ac.jp/crid/1570572700962894592
- https://www.researchgate.net/figure/Biodegradation-and-cumulative-retention-of-biodiesel-in-soil-during-the-bioremediation_fig2_350949830
- Ghosal D, Ghosh S, Dutta TK, Ahn Y (2016) Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front Microbiol 7:1369.
- Gilani RA, Rafique M, Rehman A, Munis MF, Rehman SU, et al. (2016) Biodegradation of chlorpyrifos by bacterial genus Pseudomonas. J Basic Microbiol 56:105-119.
- Gilden (2010) Monitoring pesticides residues in food science and pesticide Degradation Patterns: 1-17.
- Gislason EA, Craig NC (2005) Cementing the foundations of thermodynamics: comparison of system-based and surroundings-based definitions of work and heat. J Chem Thermodynamics 37: 954-966.
- Hasan HAH (1999) Fungal utilization of organophosphate pesticides and their degradation by Aspergillus flavus and A. sydowii in soil. Folia Microbial 44: 77-84.
- Hutchinson SL, Schwab A and Banks M (2001) Phytoremediation of aged petroleum sludge: effect of irrigation techniques and scheduling. J Environ Qual 30: 1516-1522.
- Imwene KO, Mbui DN, Mbugua JK, Kinyua AP, Kairigo PK, et al. (2021). Kinetic Modelling of Microbial Fuel Cell Voltage Data from Market Fruit Wastes in Nairobi, Kenya. Int J Sci Res Chem (IJSRCH) 6: 25-37.
- Jaiswal S, Bara JK, Soni R, Shrivastava K (2017) Bioremediation of Chlorpyrifos Contaminated Soil by Microorganism. Int J Envir Agri Bio 2:1624-1630.
- Kamau JM, Mbui DN, Mwaniki JM and Mwaura FB (2018) Characterization of voltage from food market waste: microbial fuel cells. Int J Biotech & Bioeng 4: 1-12.
- Kinyua A, Mbugua JK, Mbui DN, Kithure J, Michira I, et al. (2022a) Bio-Remediation of Lambda Cyhalothrin, Malathion and Chlorpyrifos Using Microbial Fuel Cells. Int J Sci Res Chem (IJSRCH). 7: 22-32.
- Kinyua A, Mbugua JK, Mbui DN, Kithure J, Michira I, et al. (2022b) Voltage Recovery from Pesticides Doped Tomatoes, Cabbages and Loam Soil Inoculated with Rumen Waste: Microbial Fuel Cells. IJSRSET :172-180.
- Kumar TT and JahangirH S (2018) Bio-degradation of lambdacyhalothrin by Rhodococcus erythropolis. Life Science Informatics 4: 192.
- Laurent FM, Devers M, Aymespor, Rourd N, Beguet J, et al. (2021). Potential of preventive bioremediation to reduce environmental contamination by pesticides in an agricultural context: A case study with the herbicide 2, 4-D. J Hazard Mater 416:125740.
- Pisciotta JM, Dolceamore JJ (2016) Bioelectrochemical and Conventional Bioremediation of Environmental Pollutants. J Microb Biochem Technol 8: 327-343.
- Randika JLPC, Bandara PKGSS, Soysa HSM, Ruwandeepika HAD, Gunatilake SK (2022) Bioremediation of Pesticide-contaminated Soil: A Review on Indispensable Role of Soil Bacteria. J Agri Sci 17:19-43.
- Rath AK, Ramakrishnan B, Rath AK, Kumaraswamy S, Sethunathan N (1998) Effect of pesticides on microbial biomass of flooded soil. Chemo 37: 661-671.
- Rodríguez-Eugenio N, McLaughlin M, Pennock D (2018) Soil Pollution: a Hidden Reality. Rome, FAO 142:1-36.
- Sampling and Methods of Analysis: 43-49.
- Schuster E, Schroder D (1990) Side-effects of sequentially applied pesticides on non-target soil microorganisms: field experiments. Soil Biol Biochem 22:367-373.
- Singh B, Kaur J, Kashmir S (2013) Bioremediation of malathion in soil by mixed Bacillus culture. Adv Bio Biotech 4: 674-678.
- Singh A, Ward OP (2004) Biodegradation and bioremediation, Springer Science & Business Media.
- Tran TS, Simard RR (1993) Mehlich III-Extractable Elements. In: M. R. Carter, Ed. Soil.
- Turner RC, Clark JS (1966) Lime potential in acid clay and soil suspensions. Trans Comm II & IV Int Soc Soil Sci: 208-215.
- Vargas JM (1975) Pesticide degradation. J Arboricul 1: 332-333.
- Varshney K (2019) Bioremediation of pesticide waste at contaminated sites. J Emer Techno Inno Res 6:128-134.
- WHO (World Health Organization) (2007) Pesticide residue in food; toxicology and environment protection? P 86/2 in Chem. 1988 No. 756-770.
- Zhu G, Wu H, Guo (2004) Microbial Degradation of Fipronil in Clay Loam Soil. Water Air Soil Pollu 153:35-44.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Kinyua A, Mbugua JK, Mbui DN, Kithure JL, Wandiga SO, et al. (2022) Microbial Fuel Cell Bio-Remediation of Lambda Cyhalothrin, Malathion and Chlorpyrifos on Loam Soil Inoculated With Bio-Slurry. J Bioremediat Biodegrad, 13: 508. DOI: 10.4172/2155-6199.1000508
Copyright: © 2022 Kinyua A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2431
- [From(publication date): 0-2022 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1965
- PDF downloads: 466